-
PDF
- Split View
-
Views
-
Cite
Cite
Curtis L Simmons, Laura K Harper, Kathryn J Holst, Nathan J Brinkman, Christine U Lee, Bargain Hunting for Buffered Lidocaine: A Collaborative Discovery of Cost-saving Strategies That Can Improve Patient Care, Journal of Breast Imaging, Volume 3, Issue 1, January/February 2021, Pages 93–97, https://doi.org/10.1093/jbi/wbaa077
Close - Share Icon Share
Abstract
Buffered lidocaine is a local anesthetic option during percutaneous needle-directed procedures in the breast. At our institution, sodium bicarbonate (the buffer) is dispensed in volumes that frequently lead to medical waste and shortages. In this study, we describe how moving the buffering of lidocaine from the procedure room to our clinical hospital pharmacy results in a reduction in costs and improves satisfaction across the breast radiology department. While cost savings are difficult to tease out in practices that opt for bundled payments, we were able to access pricing and supply data and coordinate with our pharmacy to change our practice. Making these changes saves our practice $26 000 a year and allows us to continue to offer buffered lidocaine even during sodium bicarbonate shortages. This manuscript describes how these changes came about and their economic impact.
Buffering lidocaine with bicarbonate is used for anesthetizing sensitive portions of the body, such as the breasts, and works by decreasing the acidic pH of lidocaine to better match the pH of soft tissues.
Buffering of lidocaine in a clinical hospital pharmacy, rather than in the radiology department, results in reduced medication costs, increased medication availability, and improved breast radiology staff satisfaction.
Departmental cross-collaboration is fertile ground for innovative cost-saving strategies while maintaining high-quality patient care.
Introduction
Buffering lidocaine with sodium bicarbonate has become commonplace for anesthetizing sensitive portions of the body such as the breasts (1,2), with evidence supporting reduced pain and anxiety during breast procedures (3–5).
As a weak acid, unbuffered 1% lidocaine has a pH of approximately 6.1, and unbuffered 1% lidocaine with epinephrine is even more acidic with a pH of 4.24 ± 0.42. Both are orders of magnitude more acidic than soft tissues, which range in pH from 7.38 to 7.62. The closer the pH of the injected solution is to the soft tissues, the less of a “burning” sensation is experienced. The practice of buffering lidocaine with sodium bicarbonate adjusts the pH to a more tolerable range prior to injection (6,7).
Reducing the burning sensation and maintaining the efficacy of lidocaine requires a balance of both pH and chemical stability. For example, while the pH of lidocaine with added epinephrine is considerably more acidic than soft tissues, buffering with sodium bicarbonate quickly oxidizes the epinephrine into a biologically inactive form (8). The stability of lidocaine is likewise dependent on the pH. Excessive alkalization of lidocaine (pH around 12.9) (9) results in precipitation appearing as haziness of the solution and causes the lidocaine to become less biologically active. With proper consideration of pH and chemical stability, lidocaine can be buffered with sodium bicarbonate to match the pH of soft tissues without the loss of efficacy for weeks (10).
Bicarbonate shortages mean that buffering is not always possible due to the lack of supply or prohibitive pricing (11). Shortages of sodium bicarbonate caused by global pandemics, trade tariffs, limited international supply availability, severe weather patterns, and changes in supply chains mean that buffering varies greatly in price and can become impossible if sodium bicarbonate is wholly unavailable.
Buffering lidocaine with bicarbonate can be costly and wasteful, particularly when buffered lidocaine is used for all breast procedures. At our institution, buffering has been performed in procedure rooms by nurses or physicians. The buffered solution is made by mixing 10 mL of 1% lidocaine from a 10 mL vial ($2.60) with 1–2 mL of 8.4% sodium bicarbonate solution from a 50 mL vial ($9.02). The remaining 48–49 mL of sodium bicarbonate is wasted. While not used in our institution, a 5 mL vial of 4.2% sodium bicarbonate is also available at a price of $7.63 but requires twice the volume of fluid to achieve a desirable pH range because of the lower concentration (12).
Developing more economical ways to buffer lidocaine can be challenging given the legislative and safety rules governing medications. The “One and Only One” campaign prevents using the same 50 mL of sodium bicarbonate for 50 separate patients (13). The Joint Commission advises against the reuse of some medications and disposable supplies, like vials of sodium bicarbonate, between patients. Finding solutions that allow for dividing resources to decrease waste is becoming more crucial as health care costs continue to rise and resources become scarcer.
Bundling payments was designed to spur innovations for costly and wasteful situations such as this (14,15). However, a lack of cost transparency for even common supplies makes cost-saving innovation difficult. This lack of transparency is exacerbated with price fluctuations from medication shortages (16–18). The true cost of medications and supplies can be nearly impossible to elucidate, even to physicians.
The aim of this project was to understand our current practice, evaluate the costs and challenges of buffering lidocaine, devise cost- and time-saving solutions via a multidisciplinary team approach, and implement the best solution. Our pricing data used in this article comes with several caveats. The prices depend on individual negotiations, bulk ordering rates, availability in light of medication shortages, and a host of other factors. Additionally, the prices we cite represent a snapshot in time at the commercially available rates.
Project Description
A multidisciplinary team composed of a board-certified radiologist specializing in breast radiology, two radiology residents (one with an MBA degree), a clinical pharmacist, the mammography technologist in charge of the procedural supply chain, and the breast imaging administrative assistant was assembled. Our institution’s clinical pharmacists assisted in aggregating medication prices and selecting several permissible alternatives to our current process of buffering lidocaine. Our pharmacy offered to buffer the lidocaine in-house weekly or to source prebuffered lidocaine syringes from an outside compounding pharmacy.
To forecast the projected medication need and cost savings over the course of a year, we reviewed the anonymized practice volume over the preceding 33 weeks (161 days) by building frequency tables on a daily and weekly basis. The frequency table with the lowest variability was then used to forecast projected savings on an annual basis.
Because the alternatives required changing our process from buffering lidocaine in the procedure room to using a prebuffered lidocaine prepared outside the breast department, we designed a two-week trial period for feasibility of this new process. We choose two weeks with usual projected case volumes and no medical conferences scheduled. We evaluated the trial by assessing cost savings from the supplies used, medical errors and sufficient anesthetic via chart reviews, and staff satisfaction via electronic surveys.
Using the collected data, we determined which supply option would be the most appropriate and implemented it into the practice. After six months, a follow-up survey was conducted. To assess the long-term viability of the innovation, a retrospective evaluation was performed for interval medical errors. Communication alerts from the pharmacy were implemented to relay information about price changes or possible shortages.
Project Outcome
Pricing Assessment
Pricing data for supplies were acquired for our current practice and viable alternatives (Table 1). For each syringe of buffered lidocaine in our practice, approximately 75% of the cost is attributed to the 48–49 mL of wasted sodium bicarbonate (Figure 1). Based on the data of available prices, the price per milliliter of buffered lidocaine decreases when larger volumes of lidocaine are used, from $0.26/mL to $0.056/mL. Our current method of using 10 mL vials of lidocaine and 50 mL vials of sodium bicarbonate is the most expensive option. Moving to pharmacy preparation allows us to use the less expensive, larger lidocaine vials and divide the sodium bicarbonate between multiple patients. Pharmacy compounding of our buffered lidocaine allows us to unlock bulk discounts that we could not achieve otherwise.
| Product . | Commercial Pricing . |
|---|---|
| Fluid dispensing connector | $0.94 |
| 10-mL syringe | $0.18 |
| Lidocaine 1%, 10 mL | $2.60 |
| Lidocaine 1%, 20 mL | $1.42 |
| Lidocaine 1%, 30 mL | $3.19 |
| Lidocaine 1%, 50 mL | $2.79 |
| Sodium bicarbonate, 25 mEq/5 mL | $7.63 |
| Sodium bicarbonate, 50 mEq/50 mL | $9.02 |
| Premixed buffered lidocaine, 10 mL | $6.00–$6.25 |
| Product . | Commercial Pricing . |
|---|---|
| Fluid dispensing connector | $0.94 |
| 10-mL syringe | $0.18 |
| Lidocaine 1%, 10 mL | $2.60 |
| Lidocaine 1%, 20 mL | $1.42 |
| Lidocaine 1%, 30 mL | $3.19 |
| Lidocaine 1%, 50 mL | $2.79 |
| Sodium bicarbonate, 25 mEq/5 mL | $7.63 |
| Sodium bicarbonate, 50 mEq/50 mL | $9.02 |
| Premixed buffered lidocaine, 10 mL | $6.00–$6.25 |
These prices were used to calculate the potential costs of the alternative scenarios.
| Product . | Commercial Pricing . |
|---|---|
| Fluid dispensing connector | $0.94 |
| 10-mL syringe | $0.18 |
| Lidocaine 1%, 10 mL | $2.60 |
| Lidocaine 1%, 20 mL | $1.42 |
| Lidocaine 1%, 30 mL | $3.19 |
| Lidocaine 1%, 50 mL | $2.79 |
| Sodium bicarbonate, 25 mEq/5 mL | $7.63 |
| Sodium bicarbonate, 50 mEq/50 mL | $9.02 |
| Premixed buffered lidocaine, 10 mL | $6.00–$6.25 |
| Product . | Commercial Pricing . |
|---|---|
| Fluid dispensing connector | $0.94 |
| 10-mL syringe | $0.18 |
| Lidocaine 1%, 10 mL | $2.60 |
| Lidocaine 1%, 20 mL | $1.42 |
| Lidocaine 1%, 30 mL | $3.19 |
| Lidocaine 1%, 50 mL | $2.79 |
| Sodium bicarbonate, 25 mEq/5 mL | $7.63 |
| Sodium bicarbonate, 50 mEq/50 mL | $9.02 |
| Premixed buffered lidocaine, 10 mL | $6.00–$6.25 |
These prices were used to calculate the potential costs of the alternative scenarios.
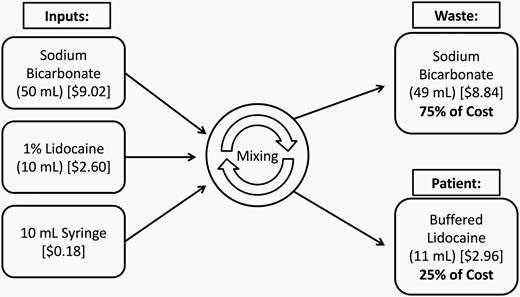
Graphic demonstrating the cost of inputs and the proportion of the cost allocated to waste versus allocated to the patient per procedure. For each syringe of buffered lidocaine in our practice, approximately 75% of the cost was attributed to 48–49 mL of wasted sodium bicarbonate.
Changing our practice from using 50 mL vials of sodium bicarbonate to 5 mL vials decreases costs, but not as significantly as also changing the source of lidocaine. The commercially available 5 mL vials of sodium bicarbonate have a concentration of 4.2% at $7.63 instead of the 50 mL vials with a concentration of 8.4% at $9.02. Because of the differences in concentration, 2 mL of the 4.2% concentration is needed to buffer the lidocaine to the range of soft tissues (12). Switching from the 50 mL to 5 mL vials with a lower concentration would save $1.39 per syringe, but there would be no benefit from the bulk lidocaine savings; utilizing the 5mL vial may be beneficial to smaller institutions, which do not perform a high volume of procedures with buffered lidocaine.
The discounts obtainable from pharmacy compounding of the buffered lidocaine outside of the breast radiology department are considerable when compared with our current practice. Using our in-house hospital pharmacy, we calculate a savings of 84% ($9.94) per syringe of buffered lidocaine for material costs. Moving to an external compounding pharmacy the savings are 47% ($5.55) per syringe of buffered lidocaine. Sterile syringe packaging was considered; however, this would increase costs by shifting the labor of transferring the buffered lidocaine to a sterile syringe from the procedure room to the pharmacy. Instead, a fluid–fluid transfer adapter to move the solution to a sterile syringe is included during the two-week trial and, likewise, included in our cost analysis.
Two-week Trial
The two-week trial using in-house pharmacy preparation tests the feasibility of supplying the buffered lidocaine using this strategy, assesses the ordering and delivery workflow logistics, and may uncover any unexpected problems before changing our practice entirely.
To prepare an adequate number of units of buffered lidocaine for the trial, we analyzed our retrospective data for forecasting. The retrospective review of 161 days over 33 weeks revealed an average daily case load of 13.6 procedures, with a range of 6–20. Over the same period the average weekly case load was 66.2 procedures, with a range of 39–81 (Figure 2). The coefficient of variance for the daily case load was 0.20 and was 0.15 for the weekly case load. Because of the lower coefficient of variance, we used the weekly volumes for forecasting the projected yearly savings. Based on the forecasts, by preparing 90 syringes per week we would have an adequate supply 97% of the time, with only one week (week 33) of our forecast requiring more than 90 syringes. After discussion with our pharmacy, we batched our preparations on a weekly basis. Performing the preparation weekly instead of daily would decrease the amount of time required to prepare the buffered lidocaine for the pharmacy, would allow for splitting the sodium bicarbonate up more efficiently (with only two vials of 50 mL of sodium bicarbonate required instead of five), and would better approximate the usage of the breast imaging department based on the decreased variability of the weekly forecast compared with the daily forecast.
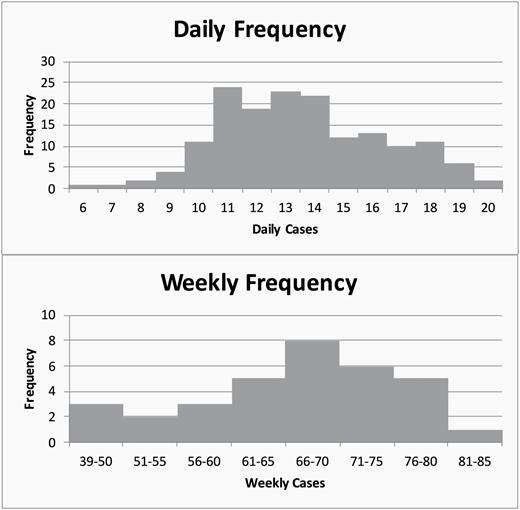
Histograms demonstrating daily and weekly frequency for breast procedures during a 161-day period. The daily frequency has a greater variance than the weekly frequency. The weekly frequencies show 100% service with only 85 units per week, while the daily frequency table shows that 100 units (20*5) are needed for the same period. The potential waste of 15 units per week was avoided during the trial by using a weekly forecast with decreased variability.
The two-week trial demonstrated that the shift to either the in-house pharmacy or external compounding pharmacy for our supply of buffered lidocaine was feasible. During the trial, the practice experienced no medication shortage and used 135/180 prepared units of buffered lidocaine. One MRI-guided breast biopsy was performed with bupivacaine because of a lidocaine allergy. During the two-week trial, no procedures required a second syringe and none of the syringes contained cloudy precipitate, indirectly indicating that sufficient anesthetic was achieved. No medication errors, such as allergic reaction or incorrect medication administration, were reported or uncovered during a subsequent chart review of the two-week trial. The staff satisfaction survey demonstrated positive results and, moreover, universally supported the change as solely a time-saving measure, regardless of cost. Additionally, pharmacy preparation of buffered lidocaine was favored for its accuracy and precision at the time of documentation in the patient’s medical record.
At the conclusion of the trial we chose to source the buffered lidocaine from an external compounding pharmacy, because their solution offered a longer shelf life and was less resource intensive on our in-house pharmacy. The buffered solution from the external pharmacy offered a 30-day refrigerated shelf life, assuming storage at 5°C with protection from light, versus a 10-day beyond-use date (shelf life) from our in-house pharmacy. A longer shelf life would decrease ordering frequency and possibly ensure decreased medication waste from expiring supplies. Additionally, preparing the buffered solutions weekly in our in-house pharmacy placed increased time demands on the pharmacy staff. At the conclusion of the two-week trial it was the pharmacist’s preference that we utilize an external pharmacy.
Unused syringes remaining at the end of the two-week trial period were not accounted for in the calculation of the cost per syringe. During the trial period, only 135/180 prepared syringes were used. This was not allocated into our calculated savings because the goal of the two-week trial was mainly as a feasibility study of switching to a prebuffered syringe from drawing the lidocaine from vials. If we allocate the cost of wasted syringes to the used syringes, then the cost increases by 33%. The calculated savings from an in-house pharmacy supply thus drops from 84% to 79%, and the savings from an external compounding pharmacy supply would drop from 47% to 30%. Even allocating for unused syringes, switching would be a cost-saving opportunity.
Six-month Survey
A follow-up six-month survey of the breast radiologists and procedural staff demonstrated continued, strong support for this change. The external compounding pharmacy was unable to continue producing syringes of buffered lidocaine because they could no longer meet the Food and Drug Administration’s essential copy guidance (19). Fortunately, based on our earlier work and analysis, we were able to seamlessly switch to compounding in our hospital pharmacy. Even with our external supplier no longer producing buffered lidocaine, we continued to be able to offer buffered lidocaine to the breast radiology department.
Discussion
Buffering lidocaine outside of the breast radiology department allowed us to substantially reduce procedural costs; the savings to our department is approximately $26 000 annually by switching to our in-house hospital pharmacy. If we had been able to source from the external compounding pharmacy as initially planned, our savings would have been about $15 000 annually.
Switching to the hospital pharmacy for buffering lidocaine had several significant additional benefits: (1) patients could continue to receive buffered lidocaine in the face of medication shortages, (2) the set-up for procedures was quicker, (3) no safety issues relating to lidocaine occurred since switching, and (4) documentation of medication administration in the electronic health record was more accurate. However, implementing changes such as these will not radically change the finances or strategy of any hospital or department. This can be a part of an overall strategy of interdepartmental collaboration and a starting point for cost saving changes.
This trial demonstrates a few concepts for practice improvement changes. Access to pricing data by physicians, while difficult to obtain, can allow focused collaborative efforts at cost-reducing innovations in the clinical setting. A well-designed proof-of-concept trial should involve multiple stakeholders for a greater chance of success from the beginning. For instance, this project relied heavily on collaboration with pharmacists and administrators from its conception. Continued assessment well past the trial allowed us to implement lessons learned and switch again to our in-house hospital pharmacy for our supply of buffered lidocaine.
Several obstacles exist that prevent widespread adoption of this practice. The 10-day beyond-use date for an in-house pharmacy necessitates a predictable patient load weekly. While the cost of materials works out in favor of in-house compounding for as little as two units, pharmacy costs at each location determine how many units of buffered lidocaine must be produced for a net savings. Breast centers without access to an in-house pharmacy will need to contract with an external pharmacy, which may not be able to consistently provide units of buffered lidocaine. These issues should be addressed by each individual practice before implementing an overhaul to a practice’s source of buffered lidocaine.
While our solution yields considerable cost savings for procedures using buffered lidocaine, care should be taken before applying this to practices that do not routinely buffer lidocaine. Even with the pharmacy buffering the lidocaine, the cost is still about 200% higher compared to instances where lidocaine is not buffered.
Conclusion
Switching to a pharmacy-buffered lidocaine preparation allowed our practice to decrease medication waste, insulate against medication shortages, and substantially decrease procedural costs. This analysis was only possible because of the availability of pricing data and open communication with our pharmacy and administrative colleagues to evaluate potential innovations. Continued close collaboration between departments will be necessary for future innovation.
Funding
None declared.
Conflict of Interest Statement
None declared.
References
Hwang H, Park J, Lee WK, et al. Crystallization of local anesthetics when mixed with corticosteroid solutions. Ann Rehabil Med 2016;40(1):21–27.



