-
PDF
- Split View
-
Views
-
Cite
Cite
M. T. Neary, J. M. Neary, G. K. Lund, F. B. Garry, T. N. Holt, T. J. Mohun, R. A. Breckenridge, Technical note: A comparison of DNA collection methods in cattle and yaks, Journal of Animal Science, Volume 92, Issue 9, September 2014, Pages 3811–3815, https://doi.org/10.2527/jas.2013-7445
Close - Share Icon Share
ABSTRACT
A variety of biological materials are suitable for the analysis of bovine DNA. The objective of this study was to evaluate the ease of collection, storage, and cost as well as quality and quantity of DNA samples obtained from Bos taurus (European cattle) and Bos grunniens (yak) using 2 different sample types: whole blood sampling and nasal swabs. Hair follicle DNA samples from yaks were also analyzed. Deoxyribonucleic acid samples were collected from 1 herd of Black Angus yearling bulls (n = 166) and 1 herd of yaks (n = 24). A NanoDrop Bioanalyzer ND1000 was used to quantify DNA. To assess DNA purity, absorbance ratios were determined at wavelengths of 260 nm relative to 280 nm and 260 nm relative to 230 nm. Single nucleotide polymorphism genotyping was performed using a competitive allele-specific PCR (KASP) genotyping system and the call rates to 3 specific SNP were compared. Using a commercially available nonautomated ethanol DNA extraction technique, nasal swabs yielded a greater quantity of DNA than blood (P < 0.0001) and a greater quality DNA sample than blood (P < 0.0001). Blood and nasal swab performance in SNP genotyping assays were similar (P = 0.5). The greater expense of nasal swabs was offset by their ease of use: less time, skill, and equipment was needed to obtain a sample and the storage of samples was more convenient (room temperature). In yaks, accessing the coccygeal vein, which is relatively straightforward in cattle, was difficult. Nasal swabbing and hair follicle sampling in yaks was performed relatively easily. Yak hair follicles were a poor source of DNA. In conclusion, DNA collection using nasal swabs was more convenient and provided a greater quantity of DNA and better quality sample than blood collection in both Angus and yak. Notably, yak hair was a poor source of DNA, and yak blood was difficult to obtain.
INTRODUCTION
Several biological materials are suitable for the analysis of bovine DNA, such as blood, semen, hair follicles, and ear tissue. The various materials differ in their relative ease of collection, storage, and shipment conditions such as frozen or cold. Sample quantity and quality can impact the performance of downstream applications such as SNP genotyping for trait characteristics. Rapid and efficient sample collection is important to maximize the quantity and quality of DNA for subsequent use.
New DNA collection methods in humans are compared to gold standard methods on a regular basis (Hansen et al., 2007; Milne et al., 2006; Rogers et al., 2007). There are few independent comparative studies of different DNA collection methods in cattle (Bos taurus) or their close relatives, the Himalayan yak (Bos grunniens). A new technique for the collection of bovine DNA has become available in the form of Performagene nasal swabs (DNA Genotek Inc., Ottawa, ON, Canada). Therefore, the objective of this study was to evaluate the ease of collection, quality, and quantity of DNA obtained from B. taurus and B. grunniens using 2 different methods: whole blood sampling and nasal swabs. The feasibility of hair follicle collection was also assessed in the yak. Collection ease was subjectively described. Time for collection, time for DNA extraction, equipment needed, storage and shipment conditions, and overall cost were recorded. The quality and quantity of DNA obtained was evaluated by nanospectrophotometry (Hansen et al., 2007; Wilfinger et al., 1997) and allele-calling rates in SNP genotyping assays (Hansen et al., 2007).
MATERIALS AND METHODS
Ethical Approval
The Colorado State University Animal Care and Use Committee approved this study (protocol 11-2813A). Export of DNA and RNA from the United States to the United Kingdom was performed under Article 4 of the Importation of Animal Products and Poultry Products Order 1980 (license no. IMP/GEN/2008/03). All other animal samples were transported under license (no. TARP/2011/327) from the United Kingdom's Department for Environment, Food and Rural Affairs (DEFRA) issued by the Animal Health and Veterinary Laboratories Agency.
Study Animals
Deoxyribonucleic acid samples were collected from 1 herd of Black Angus yearling bulls (n = 166) in Wyoming and 1 herd of yaks (n = 24; between 2 mo and 16 yr of age; 4 bulls, 2 steers, 9 cows, and 9 heifers) in Colorado. Blood and nasal swabs were collected from both cattle and yaks. Hair follicles were also collected from yaks but not from the Angus bulls due to availability of personnel. Animals were restrained in a chute during sampling. The ambient temperature varied from 18 to 29°C. The time required for sample collection and DNA extraction was recorded along with equipment needed and the cost of sampling and extraction.
Deoxyribonucleic Acid Collection and Extraction from Nasal Swabs
Nasal swabs were collected from 24 yaks and 142 Angus bulls. The kit contains a sponge applicator and a collection tube containing Performagene solution (DNA Genotek Inc., Ottawa, Canada), which acts as a preservative and cell lysis agent. The sponge is rubbed in the animals' nostril for 5 s, after which the sponge is replaced into the collection tube. Samples were stored and mailed to the United Kingdom at ambient temperature.
Extraction of DNA from nasal swabs was performed 3 wk after sample collection using a solution (PG-L2P) provided with the nasal swabs in accordance with the manufacturers instructions (DNA Genotek Inc.). Briefly, DNA was extracted using a nonautomated ethanol based technique from 500 μL of nasal swab solution and suspended in 100 μL of Tris-EDTA buffer.
Deoxyribonucleic Acid Extraction from Blood
Blood was obtained from the coccygeal vein of the yaks (n = 24) and the jugular vein of the Angus cattle during a procedure to measure their pulmonary arterial pressure (n = 24). This procedure is commonly performed on yearling bulls in the Rocky Mountain region to minimize the risk of congestive heart failure secondary to hypoxia-induced pulmonary hypertension at high altitude. Blood (5 mL) was collected in EDTA vacutainers (BD Biosciences, Franklin Lakes, NJ) by a veterinarian. A 22-gauge, 2.54-cm (1″) hypodermic needle was used for coccygeal venipuncture (in yaks) and a 13-gauge, 8.89-cm (3.5″) needle used for jugular venipuncture (in Angus bulls). Coccygeal venipuncture was performed midline at the level of the tail fold. Samples were stored in an insulated box with ice during sampling (12 h) and then at –20°C. Due to the bulk and fragility of blood samples, DNA was extracted before shipping. The samples of DNA were then shipped on dry ice.
Two days after sample collection, DNA was extracted using a QiaAMP Blood Mini kit (Qiagen, Hilden, Germany) following the manufacturers instructions. Briefly, DNA was extracted from 200 μL of blood using a nonautomated ethanol based technique and eluted into 100 μL of Buffer AE (Qiagen).
Deoxyribonucleic Acid Extraction from Hair Follicles
Hair was taken from the dorsal side of the base of the tail from yaks (n = 24). A pencil-sized tuft of hair was retrieved, containing at least 20 hair follicles, and stored in sterile, sealed falcon tubes in the shade before being stored at 4°C. Hair samples were shipped at ambient temperature in a sealed, dark container.
Three weeks after sample collection, DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen) and a user-developed protocol for the purification of DNA from nails, hair, and feathers (this is a recommended protocol developed by scientists external to the company, that is published on the Qiagen website [Qiagen, 2014]).
Deoxyribonucleic Acid Quantification
Quantification of DNA was performed using a NanoDrop Bioanalyzer ND1000 (Labtech, Uckfield, UK). The NanoDrop Bioanalyzer was cleaned with ethanol, blanked using distilled water, and initialized with the same buffer in which the DNA samples were eluted. Using the same 2-μL calibrated pipette throughout, 1 μL of each DNA sample was placed on the sensor for assessment of concentration and purity. The sensor was blotted between each sample loading.
Deoxyribonucleic Acid Purity
To assess DNA purity, absorbance ratios were determined at wavelengths of 260 nm relative to 280 nm. Pure DNA is recognized as having a ratio of approximately 1.8. A much lower ratio may suggest the presence of phenol, protein, or other contaminants that absorb significantly around 280 nm.
Another assessment of nucleic acid purity is achieved with the 260:230 nm. The ratio should fall in the range of 2.0 to 2.2. A lower than expected ratio due to increased absorbance at 230 nm may indicate the presence of contaminants such as EDTA, carbohydrates, and phenol.
Genotyping
Single nucleotide polymorphism genotyping was performed by LGC Genomics, formerly KBioscience (Teddington, UK), using a competitive allele-specific PCR (KASP) genotyping system. This is a homogeneous fluorescent resonance energy transfer–based system.
Statistical Analysis
The data were analyzed in GraphPad Prism, version 6.0d (GraphPad Software, Inc., La Jolla, CA). Data were evaluated for normal distribution using the D'Agostino and Pearson omnibus normality test. Deoxyribonucleic acid concentration and purity ratios obtained from blood and nasal swabs showed nonparametric; therefore, a Mann Whitney U t test was applied to evaluate significant differences. Similarly, allele-calling rates using DNA obtained from blood and nasal swabs were nonparametric; therefore, a Wilcoxon matched-pairs signed rank test was applied.
RESULTS AND DISCUSSION
This study addresses the ambiguity over the best collection methods to use in cattle and yaks. In this study, the ease, quality, and quantity of DNA obtained from 2 species of the Bos genus B. taurus (European cattle) and B. grunniens (yak) were compared using the gold standard method of whole blood sampling and a new innovation in DNA sampling, Performagene nasal swabs. The feasibility of hair follicle collection was also evaluated in yaks.
Deoxyribonucleic Acid Collection: Sampling, Storage, and Transport
All 3 methods of sampling required the Angus bulls and yaks to be restrained in a chute (Table 1). Nasal swabbing and jugular venipuncture required additional head restraint by an experienced handler, as animals often vigorously withdrew their heads posing an injury risk to the sampler. Obtaining blood samples from the coccygeal vein is relatively easy to perform in B. taurus cattle. However, coccygeal venipuncture of the yak proved to be challenging for 2 reasons: first, access to the coccygeal vein proved to be very difficult using anatomical marks typically used for coccygeal venipuncture of B. taurus and second, the thick, heavy tail of the yak made access and visualization of those anatomical marks difficult. Blood from the Angus bulls was easily obtained from the jugular vein during the pulmonary catheterization procedure. Jugular venipuncture after head restraint may have also been a more effective method of obtaining blood from yaks. However, the thick hair coat would have still posed a challenge. Sampling of hair follicles and nasal swabbing were noninvasive and quicker by comparison to venous blood sampling (Table 1). An inexperienced animal handler could have more easily obtained nasal swabs and hair follicles than blood samples, albeit with head restraint in the former case. Head restraint for the purposes of nasal swabbing may not be necessary in cattle and young calves more accustomed to frequent handling.
| . | DNA collection method . | ||
|---|---|---|---|
| Item . | Blood . | Hair follicle . | Nasal swab . |
| Ease of collection | Invasive | Noninvasive | Noninvasive |
| Time for collection (after animal in chute) | 4–6 min | 3 min | 2 min |
| Storage | Cold/frozen | Cold/room temperature | Room temperature |
| Transport | Frozen: must be shipped on dry ice | Shipped at room temperature | Shipped at room temperature |
| Equipment needed for DNA sampling and extraction (excluding common lab reagents) | Needle and syringe, | Sterile falcon tubes, | Performagene1 nasal swabs + purifier reagent |
| EDTA blood tubes, | Refrigerator, and | ||
| cool box + ice, freezer, and DNeasy kit2 | DNeasy kit2 | ||
| Approximate cost of DNA collection per 50 samples (excluding shipping) | US$300 | $285 | $410 |
| . | DNA collection method . | ||
|---|---|---|---|
| Item . | Blood . | Hair follicle . | Nasal swab . |
| Ease of collection | Invasive | Noninvasive | Noninvasive |
| Time for collection (after animal in chute) | 4–6 min | 3 min | 2 min |
| Storage | Cold/frozen | Cold/room temperature | Room temperature |
| Transport | Frozen: must be shipped on dry ice | Shipped at room temperature | Shipped at room temperature |
| Equipment needed for DNA sampling and extraction (excluding common lab reagents) | Needle and syringe, | Sterile falcon tubes, | Performagene1 nasal swabs + purifier reagent |
| EDTA blood tubes, | Refrigerator, and | ||
| cool box + ice, freezer, and DNeasy kit2 | DNeasy kit2 | ||
| Approximate cost of DNA collection per 50 samples (excluding shipping) | US$300 | $285 | $410 |
1DNA Genotek Inc. (Ottawa, ON, Canada).
2Qiagen, Hilden, Germany.
| . | DNA collection method . | ||
|---|---|---|---|
| Item . | Blood . | Hair follicle . | Nasal swab . |
| Ease of collection | Invasive | Noninvasive | Noninvasive |
| Time for collection (after animal in chute) | 4–6 min | 3 min | 2 min |
| Storage | Cold/frozen | Cold/room temperature | Room temperature |
| Transport | Frozen: must be shipped on dry ice | Shipped at room temperature | Shipped at room temperature |
| Equipment needed for DNA sampling and extraction (excluding common lab reagents) | Needle and syringe, | Sterile falcon tubes, | Performagene1 nasal swabs + purifier reagent |
| EDTA blood tubes, | Refrigerator, and | ||
| cool box + ice, freezer, and DNeasy kit2 | DNeasy kit2 | ||
| Approximate cost of DNA collection per 50 samples (excluding shipping) | US$300 | $285 | $410 |
| . | DNA collection method . | ||
|---|---|---|---|
| Item . | Blood . | Hair follicle . | Nasal swab . |
| Ease of collection | Invasive | Noninvasive | Noninvasive |
| Time for collection (after animal in chute) | 4–6 min | 3 min | 2 min |
| Storage | Cold/frozen | Cold/room temperature | Room temperature |
| Transport | Frozen: must be shipped on dry ice | Shipped at room temperature | Shipped at room temperature |
| Equipment needed for DNA sampling and extraction (excluding common lab reagents) | Needle and syringe, | Sterile falcon tubes, | Performagene1 nasal swabs + purifier reagent |
| EDTA blood tubes, | Refrigerator, and | ||
| cool box + ice, freezer, and DNeasy kit2 | DNeasy kit2 | ||
| Approximate cost of DNA collection per 50 samples (excluding shipping) | US$300 | $285 | $410 |
1DNA Genotek Inc. (Ottawa, ON, Canada).
2Qiagen, Hilden, Germany.
Blood samples had to be kept chilled in the field and frozen as soon as possible on return to the lab. In our study, the EDTA tubes and ice box were not sufficient to prevent 3 out of 48 (6%) samples clotting, making subsequent DNA extraction difficult in 2 of these samples and impossible in 1. Ambient temperature and chilled storage options may determine the feasibility of this method. Hair specimens and nasal swabs are stable are room temperature so samples may be left in the shade during the collection process. Similarly, hair and nasal samples were shipped at ambient temperature; samples, in particular hair specimens, were lightweight and required no specialized packaging. By comparison, blood samples contained in dry ice would have been bulky and expensive to ship and difficult to obtain a U.K. import license for (unless heat treated) and would also run the risk of dry ice evaporation before arrival, and so DNA had to be extracted before shipment to the United Kingdom. Deoxyribonucleic acid was then shipped on dry ice.
For transport from the United States to the United Kingdom, none of the sample types were covered by the general licenses of the DEFRA. As such, a specific license had to be obtained, which took approximately a week to be approved. The Performagene solution used in nasal swabbing contains a cell lysis and preservative agent. As a new technique in DNA collection, Performagene samples need to be evaluated for their potential to harbor pathogens, with a view to potentially incorporating them into a general import license. This would facilitate the United Kingdom's involvement in livestock research on an international level.
Deoxyribonucleic Acid Extraction Time, Equipment, and Cost
Excluding the time for long incubation steps, the DNA extraction time required for the 3 methods was comparable (Table 1). The collection and extraction of DNA was more straightforward using the Performagene nasal swabbing method, because this involved only 1 purchase from DNA Genotek Inc. compared to multiple purchases of reagents and equipment for blood and hair follicle DNA collection (Table 1). Each Performagene nasal swab also has an individual barcode to facilitate animal identification.
A cost estimate for DNA collection for each method took into account the disposable equipment needed to sample, such as syringes, as well as the reagents needed to extract the DNA (Table 1). Nasal swabbing was approximately a third more expensive than hair and blood sampling methods.
Concentration and Purity of DNA Obtained from Blood, Nasal Swabs, and Hair
Concentrations of DNA obtained from yak hair were low (less than 1 ng/μL) and undetectable in more than 10%. Therefore, hair-based DNA collection was eliminated from further evaluation. It was noted that yak hair was fine in comparison to cattle hair and did not contain large follicles, which may have contributed to the surprisingly low DNA output. This method of DNA collection may not be suitable for yaks, unless a much larger hair sample than the one used in this study can be collected. Alternatively, sites other than the tail head, which was used in this study, may yield larger hair follicles. This illustrated a major disadvantage of hair sampling: determining what is a sufficient amount and quality of sample.
Nasal swabbing and blood consistently gave adequate concentrations of DNA for our application, with the exception of 1 clotted blood sample, which was unusable, and 3 further blood DNA samples (6%) on the lower limit of adequate concentration. The concentration of DNA obtained from nasal swabs (574 ng/μL [SEM = ±28.9 ng/μL]; n = 166) was greater than that obtained from blood (11.7 ng/μL [SEM = ±0.7 ng/μL]; n = 47; P < 0.0001; Fig. 1). The DNA obtained from nasal swabs also had greater purity than DNA from blood as indicated by 260:280 and 260:230 ratios (P < 0.0001; Fig. 1). The low 260:230 ratios in the DNA extracted from blood samples may have been due to EDTA contamination from the collection tubes, which absorbs strongly at 230 nm. Purity ratios may also be affected by pH and ionic strength of DNA elutants (Wilfinger et al., 1997). There were no significant differences in concentrations or ratios between DNA extracted from Angus and yak in both nasal and blood samples (P > 0.7).
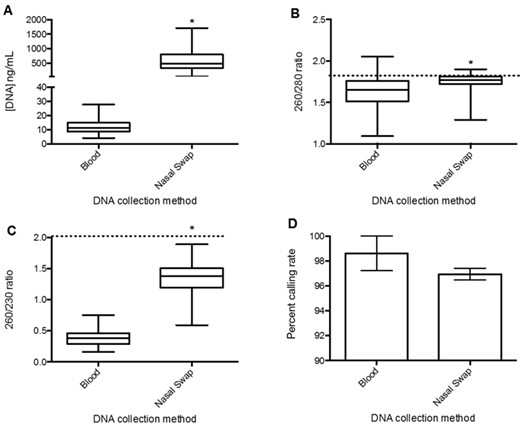
Quantity and quality of DNA obtained from blood (n = 47) and nasal swabs (n = 166) of Angus cattle. (A) DNA quantity expressed as concentration per 100 μL eluted. (B and C) DNA quality determined by nanospectrometry ratios. The dashed lines show the optimal ratios. (D) Mean ± SEM of allele-calling rates from SNP genotyping. *P < 0.0001, where P represents an unpaired Mann Whitney U t test.
Allele Calling
Although purity ratios give an indication of DNA quality (Hansen et al., 2007; Rogers et al., 2007; Wilfinger et al., 1997), performance in downstream applications is of most interest. Single nucleotide polymorphism genotyping is important in trait characteristic selection in cattle and a commonly used measure of DNA quality (Hansen et al., 2007). Therefore, the DNA was assessed for variation in 3 SNP. Although adequate, 3 (6%) blood samples were on the lower limit for the SNP genotyping assay, which required a 3 ng/μg minimum concentration. There was no difference in allele calling between the DNA extracted from blood (98.6% [SEM = ±1.4%]; n = 47) and nasal swabs (97.0% [SEM = ±0.5%]; n = 166; P = 0.5).
Conclusion
In conclusion, nasal swabbing was the most appropriate method of DNA collection in the field using a commercially available nonautomated extraction technique: sample collection, storage, and transport and DNA concentration were superior to blood DNA collection methods in cattle and yaks and DNA obtained from hair follicles in yaks. The main disadvantage of nasal swabbing relative to blood and hair follicle collection is the necessity to restrain the head for safety reasons and the increased cost of DNA collection. We found significant differences in ease of DNA collection between Angus cattle and yak. Further investigation into the performance of nasal swabs is needed in other applications, such as whole genome arrays.
LITERATURE CITED
Author notes
The authors thank Eddy Sanders (Grunniens Ranch, LLC), for making this research possible, and to the Clinical Pathology Laboratory at Colorado State Veterinary Hospital who kindly provided equipment.
These authors contributed equally to the work.



