-
PDF
- Split View
-
Views
-
Cite
Cite
Fanjie Meng, Chi Zhu, Chan Zhu, Jiaxuan Sun, Dongsheng Chen, Ran Ding, Liyuan Cui, Epidemiology and pathogen characteristics of infections following solid organ transplantation, Journal of Applied Microbiology, Volume 135, Issue 12, December 2024, lxae292, https://doi.org/10.1093/jambio/lxae292
Close - Share Icon Share
Abstract
Solid organ transplantation (SOT) recipients have a heightened risk for infection due to prolonged immunosuppressive drug use following transplant procedures. The occurrence of post-transplant infections is influenced not only by the transplanted organ type but also by varied factors. The kidney is the most common organ in SOT, followed by the liver, heart, and lung. This review aims to provide a comprehensive overview of the current epidemiological characteristics of infections after kidney, liver, heart, and lung transplantation, focusing on bacterial, fungal, and viral infections. The incidence and infection types demonstrated significant variability across different SOTs. Furthermore, this review attempts to elucidate the clinical characteristics of infections across patients following different SOTs and contribute to the development of individualized prevention strategies according to infection incidence, ultimately enhancing the quality of life of transplant recipients.
Introduction
Solid organ transplantation (SOT) has emerged as a highly efficacious therapeutic modality for numerous end-stage diseases. According to the latest statistics from the Global Observatory on Donation and Transplantation, kidney transplants accounted for 64% of the SOTs in 2021, followed by liver, heart, and lung transplants at 24%, 6%, and 5%, respectively (Solid Organ Transplantation Statistics 2021).
In the immediate aftermath of SOT, recipients are placed on a regimen of immunosuppressive drugs to prevent allograft rejection. However, this necessary intervention also renders them more susceptible to infections (Mossad 2018). The susceptibility to infection in individuals with compromised immune systems is influenced by the correlation between exposure to potential pathogens and the extent of immunosuppression necessary for preventing graft rejection. The immunological effects of immunosuppressive medications differ depending on factors such as an individual’s innate and adaptive immune responses, as well as drug metabolism (Fishman 2017). Beyond pharmacological interventions, the physiological state of the recipient, including factors such as age, comorbidities, and nutritional status, also plays a significant role in determining the net state of immunosuppression. Additionally, environmental factors such as hospital-acquired infections, community-acquired pathogens, and travel to regions with high prevalence of certain infections can influence the risk of post-transplant infections (Roberts and Fishman 2021).
Immunosuppressive drugs can be broadly categorized into induction and maintenance therapies. Induction therapy, often administered in the immediate postoperative period, is designed to provide a rapid and potent immunosuppressive effect to prevent early rejection episodes (Szczech et al. 1997). Potent immunosuppressive agents such as monoclonal antibodies are typically used during this phase to rapidly induce a state of immunosuppression (Hashim et al. 2020). Maintenance therapy, on the other hand, is a long-term strategy aimed at achieving a balance between sufficient immunosuppression to ensure graft survival and minimal suppression to reduce the risk of infections and other complications (Tönshoff 2020). As patients transition from the induction to the maintenance phase, the management of immunosuppression becomes more individualized and nuanced. The goal during this phase is to balance the level of immunosuppression sufficiently to ensure graft survival while minimizing the risk of infections. This often involves a combination of calcineurin inhibitors, mTOR inhibitors, and possibly corticosteroids and/or other agents (Wiederrecht et al. 1993, Kirken and Wang 2003).
Transplant rejection is a critical issue in post-transplant patient management, with a complex relationship to the risk of infection. On one hand, episodes of rejection may necessitate an increase in immunosuppressive therapy, potentially elevating the risk of infections. On the other hand, certain infections, such as viral infections, can trigger or exacerbate rejection episodes. Therefore, management of rejection requires a comprehensive approach that considers both infection prevention and treatment strategies (Roberts and Fishman 2021).
The occurrence of infection following transplantation not only impairs the function of the transplanted organ but also leads to systemic infection and even potentially fatal outcomes in severe cases (Timsit et al. 2019). To mitigate the risk of infections in the immunocompromised host, a series of prophylactic measures are implemented. This includes the use of antimicrobial agents for preemptive treatment, vaccination against specific pathogens, and educating patients on avoiding infection risks (Falagas et al. 2007). Additionally, close monitoring of the patient’s immune status allows for the timely detection and management of potential infections (Pascual et al. 2018).
Patients who undergo various types of SOTs are exposed to diverse risk and infection factors. These factors are not solely determined by the transplant organ characteristics, but they are also affected by postoperative immunosuppression regimens, individual patient differences, and nosocomial infection control measures (Fernández-Ruiz et al. 2014, Meesing and Razonable 2018, Allen et al. 2019). Systematic studies of the epidemiological characteristics of infections following different SOTs, such as susceptible sites and specific pathogens, are essential to guide clinical practice and develop personalized prevention and treatment strategies. In recent years, the prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP) has significantly increased, while the detection rate of quinolone-resistant and third-generation cephalosporin-resistant Escherichia coli has exceeded 50% (Herrera et al. 2023). The escalating prevalence of drug- and multidrug-resistant bacterial strains carries a heightened risk of multidrug-resistant bacterial infections among SOT recipients who are in an immunocompromised state. Consequently, the management of post-transplant infections has become inordinately complex and challenging. Related studies have reported that a significant proportion of transplant recipients (∼60%) experience at least one infection within the initial year following transplant surgery. Furthermore, postoperative infections are the primary cause of transplant organ failure and patient death (Gopalakrishnan et al. 2019, van Delden et al. 2020). Therefore, promptly diagnosing the infection type and identifying the causative microorganisms are crucial in the postoperative management of SOT recipients (Fishman 2017).
The current variance observed in the infection status across various transplantation sites is of prominent concern. For example, kidney transplant recipients are at an increased risk of developing urinary tract infections (UTIs) and postoperative infectious complications, including heightened acute graft rejection, delayed graft function recovery, and graft renal insufficiency. These factors can significantly impact the survival rates of kidney transplant recipients and transplanted kidneys (Al-Hasan et al. 2011, Jackson et al. 2021). In the case of liver transplantation, the most prevalent infectious complication during the early post-transplant period is abdominal infection, whereas heart transplant recipients display a heightened susceptibility to pericarditis, endocarditis, and similar infections (Song et al. 2018). In contrast, the incidence of central nervous system infections is relatively low, with rates of ∼1% each among kidney (23 of 2659), liver (8 of 1033), lung (6 of 435), and heart (4 of 361) transplant recipients (van den Bogaart et al. 2022). Moreover, different pathogens exhibit substantially variable effects on infections in organ transplant recipients. The presence of bacteria, viruses, fungi, and parasites in individuals with an immunosuppressed state poses a significant risk for infection, with pathogens such as Staphylococcus aureus, Cytomegalovirus (CMV), and Candida demonstrating distinctive pathogenicity in this particular immune condition (Simon and Levin 2001). Hence, a focus on the diverse pathogens and an epidemiological understanding of their impact on organ transplant recipients are imperative to facilitate early diagnosis and treatment.
The present review systematically examined the epidemiological characteristics, common infection sites, and specific pathogen infections following kidney, liver, heart, and lung transplantation, revealing significant variation in the incidence and infection types among different SOTs (Fig. 1).
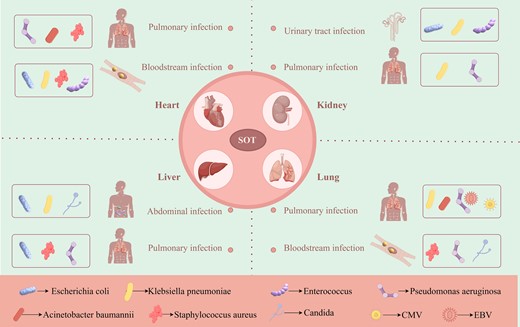
Common infection sites and specific pathogens after organ transplantation.
Solid organ transplantation
In recent decades, remarkable advancements have been achieved in SOT technology, offering hope for numerous patients with end-stage organ failure. However, the persistence of post-transplant infections remains a significant threat to the quality of life and survival rate among these individuals (Mossad 2018). Therefore, gaining a thorough understanding of the epidemiological characteristics related to infections following various types of SOTs, including common infection sites and specific pathogens, is crucial for developing effective prevention and management strategies aimed at improving patient well-being and reducing infection rates (Table 1).
Epidemiological characteristics of infections after SOT according to transplant type.
| Transplant type . | Study years . | Country . | Number of patients . | Follow-up period . | Overall infection rate . | Infection prone site . | Infection incidence . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Lung transplantation | 2009–2016 | USA | 98 | 30 d | 52% | BSI, PI | 47%, 43% | Bae et al. (2020) |
| 2016–2020 | China | 107 | 2 d | 75% | PI | NA | Meng et al. (2022) | |
| 1999–2008 | Chile | 51 | 365 d | 75% | PI | 60% | Liu et al. (2018) | |
| 2012–2014 | Sweden | 135 | 365 d | NA | PI | 85% | Stjärne Aspelund et al. (2018) | |
| 2021–2022 | China | 60 | 7 d | NA | PI | 67% | Torre-Cisneros et al. (2002) | |
| Heart transplantation | 1991–2015 | Spain | 677 | 25 d | 35% | PI | 33% | Gómez-López et al. (2020) |
| 2009–2014 | USA | 172 | 30 d | 30% | PI | 47% | Shultes et al. (2018) | |
| 1993–2014 | USA | 4458 | 1 m/3 m | 18%/39% | BSI | 25% | Rostad et al. (2017) | |
| 2000–2009 | China | 140 | 6 m | 43% | BSI, PI | 35%, 35% | Cervera et al. (2014) | |
| Kidney transplantation | 2017–2019 | China | 315 | 365 d | 46% | PI | 54% | Bucheli et al. (2014) |
| 2008–2017 | Turkey | 195 | 365 d | NA | UTI | 18% | Dubler et al. (2020) | |
| 2013–2019 | China | 209 | 365 d | 34% | PI | 79% | Hidron et al. (2008) | |
| 2011–2015 | USA | 91 | 365 d | 92% | UTI | 30% | Remschmidt et al. (2017) | |
| 2013–2017 | Mexico | 790 | 365 d | 4% | NA | NA | Hefazi et al. (2016) | |
| 1999–2014 | USA | 141661 | 3/12/60 m | 37%/54%/78% | UTI, PI | 47%, 28% | Jackson et al. (2021) | |
| Liver transplantation | 2020–2021 | China | 105 | 2 m | 71% | PI | 36.20% | Wu et al. (2022) |
| 2019–2021 | China | 207 | 2 m | 48% | AI | 29% | Liu et al. (2023) | |
| 2015–2017 | China | 210 | 7 m | 47% | PI | 43% | Ying et al. (2020) | |
| 2015–2016 | South Africa | 30 | 1 m | 31% | PI | 67% | Song et al. (2018) | |
| 2011–2012 | USA | 174 | 1 m | 30% | NA | NA | Mullane et al. (2019) |
| Transplant type . | Study years . | Country . | Number of patients . | Follow-up period . | Overall infection rate . | Infection prone site . | Infection incidence . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Lung transplantation | 2009–2016 | USA | 98 | 30 d | 52% | BSI, PI | 47%, 43% | Bae et al. (2020) |
| 2016–2020 | China | 107 | 2 d | 75% | PI | NA | Meng et al. (2022) | |
| 1999–2008 | Chile | 51 | 365 d | 75% | PI | 60% | Liu et al. (2018) | |
| 2012–2014 | Sweden | 135 | 365 d | NA | PI | 85% | Stjärne Aspelund et al. (2018) | |
| 2021–2022 | China | 60 | 7 d | NA | PI | 67% | Torre-Cisneros et al. (2002) | |
| Heart transplantation | 1991–2015 | Spain | 677 | 25 d | 35% | PI | 33% | Gómez-López et al. (2020) |
| 2009–2014 | USA | 172 | 30 d | 30% | PI | 47% | Shultes et al. (2018) | |
| 1993–2014 | USA | 4458 | 1 m/3 m | 18%/39% | BSI | 25% | Rostad et al. (2017) | |
| 2000–2009 | China | 140 | 6 m | 43% | BSI, PI | 35%, 35% | Cervera et al. (2014) | |
| Kidney transplantation | 2017–2019 | China | 315 | 365 d | 46% | PI | 54% | Bucheli et al. (2014) |
| 2008–2017 | Turkey | 195 | 365 d | NA | UTI | 18% | Dubler et al. (2020) | |
| 2013–2019 | China | 209 | 365 d | 34% | PI | 79% | Hidron et al. (2008) | |
| 2011–2015 | USA | 91 | 365 d | 92% | UTI | 30% | Remschmidt et al. (2017) | |
| 2013–2017 | Mexico | 790 | 365 d | 4% | NA | NA | Hefazi et al. (2016) | |
| 1999–2014 | USA | 141661 | 3/12/60 m | 37%/54%/78% | UTI, PI | 47%, 28% | Jackson et al. (2021) | |
| Liver transplantation | 2020–2021 | China | 105 | 2 m | 71% | PI | 36.20% | Wu et al. (2022) |
| 2019–2021 | China | 207 | 2 m | 48% | AI | 29% | Liu et al. (2023) | |
| 2015–2017 | China | 210 | 7 m | 47% | PI | 43% | Ying et al. (2020) | |
| 2015–2016 | South Africa | 30 | 1 m | 31% | PI | 67% | Song et al. (2018) | |
| 2011–2012 | USA | 174 | 1 m | 30% | NA | NA | Mullane et al. (2019) |
Abbreviations: AI: abdominal infection; BSI: bloodstream infection; d: day; m: month; PI: pulmonary infection; UTI: urinary tract infection.
Epidemiological characteristics of infections after SOT according to transplant type.
| Transplant type . | Study years . | Country . | Number of patients . | Follow-up period . | Overall infection rate . | Infection prone site . | Infection incidence . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Lung transplantation | 2009–2016 | USA | 98 | 30 d | 52% | BSI, PI | 47%, 43% | Bae et al. (2020) |
| 2016–2020 | China | 107 | 2 d | 75% | PI | NA | Meng et al. (2022) | |
| 1999–2008 | Chile | 51 | 365 d | 75% | PI | 60% | Liu et al. (2018) | |
| 2012–2014 | Sweden | 135 | 365 d | NA | PI | 85% | Stjärne Aspelund et al. (2018) | |
| 2021–2022 | China | 60 | 7 d | NA | PI | 67% | Torre-Cisneros et al. (2002) | |
| Heart transplantation | 1991–2015 | Spain | 677 | 25 d | 35% | PI | 33% | Gómez-López et al. (2020) |
| 2009–2014 | USA | 172 | 30 d | 30% | PI | 47% | Shultes et al. (2018) | |
| 1993–2014 | USA | 4458 | 1 m/3 m | 18%/39% | BSI | 25% | Rostad et al. (2017) | |
| 2000–2009 | China | 140 | 6 m | 43% | BSI, PI | 35%, 35% | Cervera et al. (2014) | |
| Kidney transplantation | 2017–2019 | China | 315 | 365 d | 46% | PI | 54% | Bucheli et al. (2014) |
| 2008–2017 | Turkey | 195 | 365 d | NA | UTI | 18% | Dubler et al. (2020) | |
| 2013–2019 | China | 209 | 365 d | 34% | PI | 79% | Hidron et al. (2008) | |
| 2011–2015 | USA | 91 | 365 d | 92% | UTI | 30% | Remschmidt et al. (2017) | |
| 2013–2017 | Mexico | 790 | 365 d | 4% | NA | NA | Hefazi et al. (2016) | |
| 1999–2014 | USA | 141661 | 3/12/60 m | 37%/54%/78% | UTI, PI | 47%, 28% | Jackson et al. (2021) | |
| Liver transplantation | 2020–2021 | China | 105 | 2 m | 71% | PI | 36.20% | Wu et al. (2022) |
| 2019–2021 | China | 207 | 2 m | 48% | AI | 29% | Liu et al. (2023) | |
| 2015–2017 | China | 210 | 7 m | 47% | PI | 43% | Ying et al. (2020) | |
| 2015–2016 | South Africa | 30 | 1 m | 31% | PI | 67% | Song et al. (2018) | |
| 2011–2012 | USA | 174 | 1 m | 30% | NA | NA | Mullane et al. (2019) |
| Transplant type . | Study years . | Country . | Number of patients . | Follow-up period . | Overall infection rate . | Infection prone site . | Infection incidence . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Lung transplantation | 2009–2016 | USA | 98 | 30 d | 52% | BSI, PI | 47%, 43% | Bae et al. (2020) |
| 2016–2020 | China | 107 | 2 d | 75% | PI | NA | Meng et al. (2022) | |
| 1999–2008 | Chile | 51 | 365 d | 75% | PI | 60% | Liu et al. (2018) | |
| 2012–2014 | Sweden | 135 | 365 d | NA | PI | 85% | Stjärne Aspelund et al. (2018) | |
| 2021–2022 | China | 60 | 7 d | NA | PI | 67% | Torre-Cisneros et al. (2002) | |
| Heart transplantation | 1991–2015 | Spain | 677 | 25 d | 35% | PI | 33% | Gómez-López et al. (2020) |
| 2009–2014 | USA | 172 | 30 d | 30% | PI | 47% | Shultes et al. (2018) | |
| 1993–2014 | USA | 4458 | 1 m/3 m | 18%/39% | BSI | 25% | Rostad et al. (2017) | |
| 2000–2009 | China | 140 | 6 m | 43% | BSI, PI | 35%, 35% | Cervera et al. (2014) | |
| Kidney transplantation | 2017–2019 | China | 315 | 365 d | 46% | PI | 54% | Bucheli et al. (2014) |
| 2008–2017 | Turkey | 195 | 365 d | NA | UTI | 18% | Dubler et al. (2020) | |
| 2013–2019 | China | 209 | 365 d | 34% | PI | 79% | Hidron et al. (2008) | |
| 2011–2015 | USA | 91 | 365 d | 92% | UTI | 30% | Remschmidt et al. (2017) | |
| 2013–2017 | Mexico | 790 | 365 d | 4% | NA | NA | Hefazi et al. (2016) | |
| 1999–2014 | USA | 141661 | 3/12/60 m | 37%/54%/78% | UTI, PI | 47%, 28% | Jackson et al. (2021) | |
| Liver transplantation | 2020–2021 | China | 105 | 2 m | 71% | PI | 36.20% | Wu et al. (2022) |
| 2019–2021 | China | 207 | 2 m | 48% | AI | 29% | Liu et al. (2023) | |
| 2015–2017 | China | 210 | 7 m | 47% | PI | 43% | Ying et al. (2020) | |
| 2015–2016 | South Africa | 30 | 1 m | 31% | PI | 67% | Song et al. (2018) | |
| 2011–2012 | USA | 174 | 1 m | 30% | NA | NA | Mullane et al. (2019) |
Abbreviations: AI: abdominal infection; BSI: bloodstream infection; d: day; m: month; PI: pulmonary infection; UTI: urinary tract infection.
Kidney transplantation
Kidney transplantation represents the predominant modality in SOT, accounting for ∼60% of all procedures (Bige et al. 2014). However, post-transplant infection is still a prominent risk factor affecting the prognosis of patients who receive kidney transplants, with infectious complications serving as a leading cause of mortality (Kahwaji et al. 2011). A previous study followed 3249 kidney transplant recipients from 1990 to 2012 and revealed that a cumulative mortality rate of 21% was attributed to infectious complications over the follow-up period (Kinnunen et al. 2018). Another study documented at least one infection in 45%–60% of kidney-transplant recipients during the initial postoperative year (Kosmadakis et al. 2013, Gopalakrishnan et al. 2019). Furthermore, infection incidence has been found to vary significantly across different centers, with UTI being widely acknowledged as the predominant type of infection after kidney transplantation (Silva et al. 2013). The prevalence of UTI following kidney transplantation was reported to be 29%–47% (Mueller et al. 2003, Coussement et al. 2021, Jackson et al. 2021, Santithanmakorn et al. 2022), while the recurrence of UTI following kidney transplantation was also significantly elevated at 72%–86%. The primary causative agents of these infections are predominantly bacteria, particularly Klebsiella spp. (Tawab et al. 2017, Santithanmakorn et al. 2022). Additionally, the increased mortality risk associated with UTIs following kidney transplantation is of considerable concern, as evidenced by a 1.63-fold higher risk of death in a cohort study involving 14 661 recipients of kidney transplants (Jackson et al. 2021). The prevalence of pulmonary infection after kidney transplantation has also been progressively growing in recent years. This prevalence is notable during postoperative hospitalization, with an incidence rate of 17%. A subsequent analysis of infection-related fatalities post-kidney transplantation revealed that pulmonary infections accounted for 45% of the total infection-related deaths (Kinnunen et al. 2018). Most kidney infections (44%) were attributed to bacterial pathogens, and ∼35% of the affected patients required admission to the intensive care unit (Hoyo et al. 2010, Tawab et al. 2017).
The occurrence of bloodstream infections (BSIs) also presents a major concern in the context of kidney transplantation. In a Spanish cohort of 1400 kidney transplant recipients, BSI incidence was found to be 7%. Moreover, UTI-associated bacteremia was detected in ∼39% of these cases, while catheter-associated BSIs accounted for ∼21% (Moreno et al. 2007). Consistent with these findings, another cohort study reported an 8% incidence rate of bacteremia in kidney transplant recipients at 1 year post-transplant (Moreno et al. 2007, Selimoğlu et al. 2020).
Liver transplantation
Compromised immune mechanisms, intestinal flora disturbances, bacterial translocation, and other factors can render patients with liver failure susceptible to secondary bacterial infections, nosocomial infections, and other unexplained infectious conditions. A prior study investigating the incidence of post-liver transplantation infections in pediatric patients revealed an overall infection rate of 67%, wherein bacterial, viral, and fungal infections represented 56%, 34%, and 9% of the cases, respectively (Selimoğlu et al. 2020). Similarly, research on liver transplant recipients in China showed that 71% had an infection in the early postoperative period (Wu et al. 2022). The discrepancy in the infection rates among various centers may be ascribed to the variation in the degree of post-transplant immunosuppression, underlying diseases of the patients, and implemented infection control measures.
Various types of infection can occur after liver transplantation, of which abdominal infection, pulmonary infection, and BSI are the three most common ones. The most prevalent infectious complication during the early post-liver transplantation period is intra-abdominal infection (Liu et al. 2023). The incidence of intra-abdominal infections is frequently associated with surgical procedures, exhibiting a higher prevalence tendency in patients experiencing biliary complications. Other studies have also identified intra-abdominal sepsis as the most prevalent infectious complication, comprising 67% of the reported cases (Song et al. 2018).
In a 30-day follow-up analysis of patients who underwent cadaveric liver transplantation in South Africa, infectious complications were observed in 31%. Furthermore, based on an extended median follow-up time of 214 days, the overall infection incidence reached 47%, encompassing pneumonia (43%), biliary tract infection (22%), peritonitis (21%), and BSI (8%) (Ying et al. 2020). The occurrence of pulmonary infections following liver transplantation has been identified as a significant infectious complication. These infections are a leading cause of mortality and are most prevalent in the first 3 months after the procedure, a period during which the patient’s immune system is considerably suppressed. A prospective single-center study conducted in France revealed an incidence rate of 37% for BSI after liver transplantation. In terms of the pathogen type, Gram-negative bacilli accounted for 52% of these cases, with E. coli being the primary causative pathogen for bacteremia development. Furthermore, the etiology of BSIs can be multifactorial. The main sources contributing to BSI predominantly comprise abdominal infections (28%), catheter-related infections (15%), UTIs (13%), pulmonary infections (9%), biliary tract infections (8%), and wound infections (3%) (Bert et al. 2010). Lastly, the overall incidence of bacteremia surpassed the observed rate of 11% in a Swiss cohort (Neofytos et al. 2023).
Surgical site infection following liver transplantation has also been extensively demonstrated as the primary site for bacterial infections (Jafarpour et al. 2020). However, the incidence of surgical site infection, a frequently encountered complication after liver transplantation, exhibits significant variability across different centers, with an incidence rate ranging from 10% to 36% (Oliveira et al. 2018). This observed heterogeneity could be attributed to the annual volume of liver transplant procedures. Moreover, the development of bacterial infections post-liver transplantation is a frequent complication that significantly contributes to morbidity and mortality in the affected patients. These infections are likely related to the complex nature of the employed surgical procedures and the management process of the transplant recipients, including that of the hepatobiliary tract (Xu et al. 2012, Sanclemente et al. 2014).
All these study findings indicate that the incidence of infectious complications following liver transplantation has significant inter-center variability and may be influenced by factors such as donor sources, surgical techniques, and the duration of study follow-up. Finally, extending the follow-up time can lead to alterations in the observed infection site of infectious complications to some extent.
Heart transplantation
Complexities associated with heart transplantation can result in a heightened infection incidence, potentially manifesting in ∼30%–60% of these recipients (Gurguí and Muñoz 2007, Cove et al. 2012). The prevalence of nosocomial infections following cardiac surgery in patients ranges from 5% to 21%, especially in the first month post-surgery. Additionally, heart transplantation has been shown to have the highest infection rate of all organ transplantations (van Delden et al. 2020). Pulmonary infection is a prevalent form of infection after heart transplantation, and its occurrence is influenced by various factors, including the extent of postoperative immunosuppression, underlying lung disease of the patient, and postoperative care conditions. An observational study of 677 adult patients who underwent heart transplantation revealed that bacteremia, surgical site infections, and intra-abdominal infections accounted for 12%, 7%, and 10% of infections, respectively (Gómez-López et al. 2020). These infection sources are primarily associated with suboptimal surgical wound healing, the colonization of respiratory tract bacteria resulting from tracheal intubation, and urinary catheter utilization. The observational study also highlighted that postoperative infections occurred in 35% of the patients during their hospital stay (Gómez-López et al. 2020). The primary infection sources linked to invasive procedures consist of respiratory tract infections (33%) and UTIs (14%) (Gómez-López et al. 2020). Similarly, another study investigated infections occurring within 30 days following orthotopic heart transplantation and demonstrated that early infections developed in 30% of the patients, with a median diagnosis time of 5 days and pneumonia (47%) as the most common infection. Additionally, Gram-negative bacteria were found to be slightly more prevalent (58%) in these cases (Shultes et al. 2018). Compared to a follow-up period of ≥30 days, a 1-year follow-up of heart transplant recipients reported that the cumulative incidence of postoperative infections, particularly bacterial infections, had risen to 60% (van Delden et al. 2020). In line with these findings, a study involving 4458 patients indicated that bacterial infections formed the predominant type of infection. Furthermore, BSIs were the most prevalent infection sites in that study, wherein coagulase-negative Staphylococcus, Enterobacteriaceae, and Pseudomonas were identified as the primary pathogens (Rostad et al. 2017). BSI incidence in heart transplant recipients was shown to range from 11% to 13%. Moreover, BSI occurrence can lead to rapid progression to systemic infection, creating a severe life-threatening situation for the patients. Gram-positive bacteria represent the predominant pathogens in BSIs, with Staphylococcus spp. being isolated in ∼27% of all cases (Neofytos et al. 2023). The most prevalent source of BSIs is central venous catheters, accounting for 44% of the infections, followed by lungs and surgical wounds at 9% each (Moreno et al. 2007). However, lower respiratory tract infections in heart transplant recipients may be more likely to cause bacteremia than central venous catheter infection (Rodríguez et al. 2006). The occurrence of deep sternal wound infection after heart transplantation is also considered a life-threatening complication and is attributed to 3%–4% of the cases (Fleck et al. 2007), with virulent Gram-negative bacteria as the main etiological agents (Wallen et al. 2019).
Lung transplantation
The lung has a direct interface with the external environment; thus, this vital organ exhibits an increased susceptibility to infections than other organs. The incidence of infection displays an upward trend during the initial 1–12 months following lung transplantation (van Delden et al. 2020). A comprehensive study conducted in Switzerland on 286 lung transplant recipients revealed a cumulative infection incidence rate of 62% during the initial year post-surgery, with bacterial infections prevailing as the most common infection type (van Delden et al. 2020). Moreover, the postoperative infection incidence after lung transplantation in China and Europe ranged from 64% to 75%. Among these cases, the respiratory tract was reported as the predominant infection site, with bacterial pneumonia being the most frequent infectious complication (Stjärne Aspelund et al. 2018, van Delden et al. 2020, Meng et al. 2022). Additionally, Acinetobacter baumannii, Pseudomonas aeruginosa, and K. pneumoniae have been identified as the predominant bacterial pathogens in lung transplant recipients experiencing complications related to lower respiratory tract infections. Candida was found to be the primary fungal infection, while Epstein–Barr virus (EBV), CMV, and human herpesvirus represented the main viral agents (Qiao et al. 2019, Meng et al. 2022).
Along with pulmonary infection, surgical site infection and BSI are frequently observed following lung transplantation. Surgical wound infection is a notable complication in lung transplantation. A previous study showed that ∼16% of the patients who underwent lung transplantation experienced postoperative infections related to surgical wounds (McCort et al. 2021). Furthermore, the researchers found that surgical site infection in the lung transplant recipients was associated with a 35% increase in mortality risk (McCort et al. 2021). Patients who have received lung transplants may also encounter invasive fungal infections from external oral dermatitis and superficial wound infection with herpes simplex virus (Karolak et al. 2017, Klasinc et al. 2019). A retrospective nested multicenter study conducted in Switzerland from 2008 to 2019 reported a 10% incidence rate of bacteremia within the first year after lung transplantation (Neofytos et al. 2023). However, a single-center study in the USA detected BSIs in 25% (44/176) of lung transplant recipients, with S. aureus, P. aeruginosa, and Candida being the predominant pathogens isolated from the bloodstream (Palmer et al. 2000, Danziger-Isakov et al. 2005). Another investigation over an extended follow-up period of 28 months after lung transplantation revealed that the incidence of catheter-related BSIs was highest within the first 6 months, accounting for 30% of the infections (Bae et al. 2020).
The above outlines the four main types of SOT infection, including kidney, liver, heart, and lung transplantation. First, kidney transplantation is the most common type of SOT (Bige et al. 2014); major infection sites include UTIs and lung infections, which are primarily associated with a weakened immune system, making it difficult for patients to effectively defend against bacterial infections (Silva et al. 2013), and the quality of monitoring and care during postoperative hospitalization also directly affects the infection rate. Secondly, liver transplant patients are more susceptible to abdominal infection, lung infection, and BSI due to factors such as impaired immune function, intestinal flora disorder, and bacterial translocation (Liu et al. 2023). In addition, technical problems during surgery and biliary complications may also lead to serious abdominal infection (Oliveira et al. 2018). After heart transplantation, the main types of infection include lung infection and BSI (Rostad et al. 2017, Gómez-López et al. 2020); these infections were closely associated with high postoperative immunosuppression, patients’ pre-existing lung disease, and postoperative care conditions. After lung transplantation, the main manifestations of infection are bacterial pneumonia, surgical site infection, and BSI, mainly due to the lack or insufficient postoperative care (van Delden et al. 2020, McCort et al. 2021). In summary, the risk of infection after organ transplantation is affected by multiple complex factors, including the particularity of the transplanted organ, the immune status of the patient, the level of intraoperative operation, the postoperative immunosuppressive treatment regimen, the implementation of nosocomial infection control measures, and the pathogen carrying status of the donor and recipient. Therefore, the comprehensive consideration of these factors and the optimization and improvement based on them are of vital significance to reduce the infection rate after transplantation.
Impact of pathogen infection on transplant outcomes
Post-transplant patients are at risk of infection with varied pathogens, including bacteria, fungi, and viruses. Bacterial infections are common in the skin, urinary tract, and lungs and can lead to organ dysfunction. In the case of fungal infections, Aspergillus and Candida are the frequent causes, mainly posing a threat to immunosuppressed patients. Moreover, viral infections, such as CMV and herpes simplex virus, may be activated or recur, severely affecting graft function and prognosis. Therefore, such pathogen infections following organ transplantation should be diagnosed and promptly treated to ensure adequate functioning of the transplanted organs and optimal patient health (Table 2).
Risk and prognostic effects of different pathogen infections after transplantation.
| Types of infection . | Types of pathogens . | Transplant organ . | Status of infection . | Clinical prognosis . | Reference . |
|---|---|---|---|---|---|
| Bacterial infection | Staphylococcus | Liver | Accounted for 42%–69% of BSIs, followed by pneumonia, intra-abdominal, and surgical site infections | Bacteremia with pneumonia and abdominal infection were associated with mortality | Dudzicz-Gojowy et al. (2022) |
| Heart | Accounted for 26% of the pericarditis infections | Linked to a higher risk of death | Smedbråten et al. (2014) | ||
| Enterococcus | Liver | Approximately 20% occurred as digestive tract infections in liver transplant recipients, with a high incidence in the first 180 days | Vancomycin resistance did not influence outcome in patients with Enterococcus faecium bacteremia after liver transplantation | Pouch et al. (2015) and van Delden et al. (2020) | |
| Clostridioides difficile | Liver/kidney/pancreas/heart/lung | CDI incidence was 3%–7% in the liver, 3%–16% in the kidney, 1.5%–7.8% in the pancreas-kidney, 15% in the heart, and 7%–31% in lung recipients | Correlated with graft loss | Linares et al. (2007), Abad and Razonable (2016), and Subramanian et al. (2019) | |
| Acinetobacter baumannii | Liver | Median time of CRAB bacteremia emergence was 56 days after transplantation, with 72% of the cases occurring within 6 months post-transplant | Patients with CRAB bacteremia after liver transplant showed an unfavorable outcome, leading to an overall mortality of 50% in 14 recipients with CRAB bacteremia | Kim et al. (2018) | |
| Klebsiella pneumoniae | Heart/liver/kidney | Infection prevalence in heart, liver, and kidney transplant recipients was 17%, 13%, and 26%, respectively, with a median time to infection of 20 days after transplantation | CRKP infection had a mortality rate of >40% | Bergamasco et al. (2012), Clancy et al. (2013), and Righi (2018) | |
| Kidney | The most common isolated pathogen in UTI (53%) | Associated with severe invasive infections, graft loss, and mortality | Silva et al. (2013) | ||
| Escherichia coli | Liver | ESBL-positive E. coli had a higher rate of drug resistance than ESBL-negative E. coli | Bacteremia caused by ESBL-producing Enterobacteriaceae in liver transplant recipients had a 30-day mortality rate of up to 41% | Men et al. (2013) | |
| Kidney | One of the leading causes of UTIs (>60%) | Linked to graft loss and higher mortality | Alevizakos et al. (2017b) and Kinnunen et al. (2018) | ||
| Fungal infection | Candida spp. | Liver | Invasive Candida spp. accounted for 62%–91% of all invasive fungal infections | Often associated with delayed diagnosis and high mortality rates | Righi (2018) |
| Collins et al. (1994) | |||||
| Nieto-Rodriguez et al. (1996) | |||||
| Kidney | Accounts for 90% of fungal infections | Mortality rate of this fungal infection was 53% | Singh (2000) | ||
| Sahin et al. (2015) | |||||
| Aspergillus spp. | Liver | Invasive Aspergillus spp. infection occurred in 1%–8% of patients, and the median time for infection following liver transplantation was shorter (109 days) than that after lung transplantation (486 days) | Mostly all liver transplant recipients with invasive Aspergillus spp. infection had significant liver and/or renal insufficiency, along with a mortality rate of 64% | Fisher et al. (1999) and Husain et al. (2017) | |
| Lung | Aspergillus spp. was detected in airway specimen cultures of 9%–68% (mean positivity, 29%) of patients, with 13% experiencing progression to invasive aspergillosis or even death. | Mortality rate of 68% for lung transplant recipients with invasive Aspergillus spp. infection | Hamacher et al. (1999) and Sahin et al. (2015) | ||
| Heart | Incidence of invasive Aspergillus spp. infection varies substantially (1%–15%), with an overall incidence of 5% | Associated with a significantly higher fatality (75%) than that from bacterial infection (36%) | Schulman et al. (1988), Cisneros et al. (1998), and Echenique et al. (2017) | ||
| Viral infection | Cytomegalovirus | Liver | A common conditional pathogenic virus after transplantation | Linked to an increased graft loss within 1 year post-transplant | Burak et al. (2002) and Bosch et al. (2011) |
| Kidney | Infection and pathogenicity rates of 40%–100% and 67%, respectively, with the highest infection risk in the first 3 months | Correlated with a higher risk of death and graft loss as well as an elevated risk of fungal infections | Helanterä et al. (2006), Smedbråten et al. (2014), and Huang et al. (2018) | ||
| Lung | Asymptomatic CMV infection was observed in 13% of patients, while 10% developed CMV disease | Associated with increased mortality after transplantation | Beam et al. (2016) | ||
| Epstein–Barr virus | Kidney | Multiple organ transplantation and small bowel transplant recipients exhibited the highest PTLD rate, followed by lung, heart, pancreas, liver, and kidney transplant recipients | Linked to worsened graft outcomes and escalated opportunistic infections | Opelz and Döhler (2004), Bamoulid et al. (2013), and Dierickx et al. (2013) | |
| SARS-CoV-2 | SOT | Average viral load of SOT recipients with severe infection was 60 times that of mild patients, while the median duration of viral shedding was longer and even extended to >3 months | Death rate of kidney transplant recipients infected with the SARS-CoV-2 is 8.1 times that of the general population, along with a low response rate after vaccination and greater vulnerability to reinfection | Hippisley-Cox et al. (2021), Marinelli et al. (2021), and Jefferson et al. (2023) |
| Types of infection . | Types of pathogens . | Transplant organ . | Status of infection . | Clinical prognosis . | Reference . |
|---|---|---|---|---|---|
| Bacterial infection | Staphylococcus | Liver | Accounted for 42%–69% of BSIs, followed by pneumonia, intra-abdominal, and surgical site infections | Bacteremia with pneumonia and abdominal infection were associated with mortality | Dudzicz-Gojowy et al. (2022) |
| Heart | Accounted for 26% of the pericarditis infections | Linked to a higher risk of death | Smedbråten et al. (2014) | ||
| Enterococcus | Liver | Approximately 20% occurred as digestive tract infections in liver transplant recipients, with a high incidence in the first 180 days | Vancomycin resistance did not influence outcome in patients with Enterococcus faecium bacteremia after liver transplantation | Pouch et al. (2015) and van Delden et al. (2020) | |
| Clostridioides difficile | Liver/kidney/pancreas/heart/lung | CDI incidence was 3%–7% in the liver, 3%–16% in the kidney, 1.5%–7.8% in the pancreas-kidney, 15% in the heart, and 7%–31% in lung recipients | Correlated with graft loss | Linares et al. (2007), Abad and Razonable (2016), and Subramanian et al. (2019) | |
| Acinetobacter baumannii | Liver | Median time of CRAB bacteremia emergence was 56 days after transplantation, with 72% of the cases occurring within 6 months post-transplant | Patients with CRAB bacteremia after liver transplant showed an unfavorable outcome, leading to an overall mortality of 50% in 14 recipients with CRAB bacteremia | Kim et al. (2018) | |
| Klebsiella pneumoniae | Heart/liver/kidney | Infection prevalence in heart, liver, and kidney transplant recipients was 17%, 13%, and 26%, respectively, with a median time to infection of 20 days after transplantation | CRKP infection had a mortality rate of >40% | Bergamasco et al. (2012), Clancy et al. (2013), and Righi (2018) | |
| Kidney | The most common isolated pathogen in UTI (53%) | Associated with severe invasive infections, graft loss, and mortality | Silva et al. (2013) | ||
| Escherichia coli | Liver | ESBL-positive E. coli had a higher rate of drug resistance than ESBL-negative E. coli | Bacteremia caused by ESBL-producing Enterobacteriaceae in liver transplant recipients had a 30-day mortality rate of up to 41% | Men et al. (2013) | |
| Kidney | One of the leading causes of UTIs (>60%) | Linked to graft loss and higher mortality | Alevizakos et al. (2017b) and Kinnunen et al. (2018) | ||
| Fungal infection | Candida spp. | Liver | Invasive Candida spp. accounted for 62%–91% of all invasive fungal infections | Often associated with delayed diagnosis and high mortality rates | Righi (2018) |
| Collins et al. (1994) | |||||
| Nieto-Rodriguez et al. (1996) | |||||
| Kidney | Accounts for 90% of fungal infections | Mortality rate of this fungal infection was 53% | Singh (2000) | ||
| Sahin et al. (2015) | |||||
| Aspergillus spp. | Liver | Invasive Aspergillus spp. infection occurred in 1%–8% of patients, and the median time for infection following liver transplantation was shorter (109 days) than that after lung transplantation (486 days) | Mostly all liver transplant recipients with invasive Aspergillus spp. infection had significant liver and/or renal insufficiency, along with a mortality rate of 64% | Fisher et al. (1999) and Husain et al. (2017) | |
| Lung | Aspergillus spp. was detected in airway specimen cultures of 9%–68% (mean positivity, 29%) of patients, with 13% experiencing progression to invasive aspergillosis or even death. | Mortality rate of 68% for lung transplant recipients with invasive Aspergillus spp. infection | Hamacher et al. (1999) and Sahin et al. (2015) | ||
| Heart | Incidence of invasive Aspergillus spp. infection varies substantially (1%–15%), with an overall incidence of 5% | Associated with a significantly higher fatality (75%) than that from bacterial infection (36%) | Schulman et al. (1988), Cisneros et al. (1998), and Echenique et al. (2017) | ||
| Viral infection | Cytomegalovirus | Liver | A common conditional pathogenic virus after transplantation | Linked to an increased graft loss within 1 year post-transplant | Burak et al. (2002) and Bosch et al. (2011) |
| Kidney | Infection and pathogenicity rates of 40%–100% and 67%, respectively, with the highest infection risk in the first 3 months | Correlated with a higher risk of death and graft loss as well as an elevated risk of fungal infections | Helanterä et al. (2006), Smedbråten et al. (2014), and Huang et al. (2018) | ||
| Lung | Asymptomatic CMV infection was observed in 13% of patients, while 10% developed CMV disease | Associated with increased mortality after transplantation | Beam et al. (2016) | ||
| Epstein–Barr virus | Kidney | Multiple organ transplantation and small bowel transplant recipients exhibited the highest PTLD rate, followed by lung, heart, pancreas, liver, and kidney transplant recipients | Linked to worsened graft outcomes and escalated opportunistic infections | Opelz and Döhler (2004), Bamoulid et al. (2013), and Dierickx et al. (2013) | |
| SARS-CoV-2 | SOT | Average viral load of SOT recipients with severe infection was 60 times that of mild patients, while the median duration of viral shedding was longer and even extended to >3 months | Death rate of kidney transplant recipients infected with the SARS-CoV-2 is 8.1 times that of the general population, along with a low response rate after vaccination and greater vulnerability to reinfection | Hippisley-Cox et al. (2021), Marinelli et al. (2021), and Jefferson et al. (2023) |
Abbreviations: BSI: bloodstream infection; CDI: Clostridioides difficile; CMV: Cytomegalovirus; CRAB: carbapenem-resistant A. baumannii; CRKP: carbapenem-resistant K. pneumoniae; ESBL: extended-spectrum beta-lactamase; PTLD: post-transplant lymphoproliferative disease; SOT: solid organ transplantation; UTI: urinary tract infection.
Risk and prognostic effects of different pathogen infections after transplantation.
| Types of infection . | Types of pathogens . | Transplant organ . | Status of infection . | Clinical prognosis . | Reference . |
|---|---|---|---|---|---|
| Bacterial infection | Staphylococcus | Liver | Accounted for 42%–69% of BSIs, followed by pneumonia, intra-abdominal, and surgical site infections | Bacteremia with pneumonia and abdominal infection were associated with mortality | Dudzicz-Gojowy et al. (2022) |
| Heart | Accounted for 26% of the pericarditis infections | Linked to a higher risk of death | Smedbråten et al. (2014) | ||
| Enterococcus | Liver | Approximately 20% occurred as digestive tract infections in liver transplant recipients, with a high incidence in the first 180 days | Vancomycin resistance did not influence outcome in patients with Enterococcus faecium bacteremia after liver transplantation | Pouch et al. (2015) and van Delden et al. (2020) | |
| Clostridioides difficile | Liver/kidney/pancreas/heart/lung | CDI incidence was 3%–7% in the liver, 3%–16% in the kidney, 1.5%–7.8% in the pancreas-kidney, 15% in the heart, and 7%–31% in lung recipients | Correlated with graft loss | Linares et al. (2007), Abad and Razonable (2016), and Subramanian et al. (2019) | |
| Acinetobacter baumannii | Liver | Median time of CRAB bacteremia emergence was 56 days after transplantation, with 72% of the cases occurring within 6 months post-transplant | Patients with CRAB bacteremia after liver transplant showed an unfavorable outcome, leading to an overall mortality of 50% in 14 recipients with CRAB bacteremia | Kim et al. (2018) | |
| Klebsiella pneumoniae | Heart/liver/kidney | Infection prevalence in heart, liver, and kidney transplant recipients was 17%, 13%, and 26%, respectively, with a median time to infection of 20 days after transplantation | CRKP infection had a mortality rate of >40% | Bergamasco et al. (2012), Clancy et al. (2013), and Righi (2018) | |
| Kidney | The most common isolated pathogen in UTI (53%) | Associated with severe invasive infections, graft loss, and mortality | Silva et al. (2013) | ||
| Escherichia coli | Liver | ESBL-positive E. coli had a higher rate of drug resistance than ESBL-negative E. coli | Bacteremia caused by ESBL-producing Enterobacteriaceae in liver transplant recipients had a 30-day mortality rate of up to 41% | Men et al. (2013) | |
| Kidney | One of the leading causes of UTIs (>60%) | Linked to graft loss and higher mortality | Alevizakos et al. (2017b) and Kinnunen et al. (2018) | ||
| Fungal infection | Candida spp. | Liver | Invasive Candida spp. accounted for 62%–91% of all invasive fungal infections | Often associated with delayed diagnosis and high mortality rates | Righi (2018) |
| Collins et al. (1994) | |||||
| Nieto-Rodriguez et al. (1996) | |||||
| Kidney | Accounts for 90% of fungal infections | Mortality rate of this fungal infection was 53% | Singh (2000) | ||
| Sahin et al. (2015) | |||||
| Aspergillus spp. | Liver | Invasive Aspergillus spp. infection occurred in 1%–8% of patients, and the median time for infection following liver transplantation was shorter (109 days) than that after lung transplantation (486 days) | Mostly all liver transplant recipients with invasive Aspergillus spp. infection had significant liver and/or renal insufficiency, along with a mortality rate of 64% | Fisher et al. (1999) and Husain et al. (2017) | |
| Lung | Aspergillus spp. was detected in airway specimen cultures of 9%–68% (mean positivity, 29%) of patients, with 13% experiencing progression to invasive aspergillosis or even death. | Mortality rate of 68% for lung transplant recipients with invasive Aspergillus spp. infection | Hamacher et al. (1999) and Sahin et al. (2015) | ||
| Heart | Incidence of invasive Aspergillus spp. infection varies substantially (1%–15%), with an overall incidence of 5% | Associated with a significantly higher fatality (75%) than that from bacterial infection (36%) | Schulman et al. (1988), Cisneros et al. (1998), and Echenique et al. (2017) | ||
| Viral infection | Cytomegalovirus | Liver | A common conditional pathogenic virus after transplantation | Linked to an increased graft loss within 1 year post-transplant | Burak et al. (2002) and Bosch et al. (2011) |
| Kidney | Infection and pathogenicity rates of 40%–100% and 67%, respectively, with the highest infection risk in the first 3 months | Correlated with a higher risk of death and graft loss as well as an elevated risk of fungal infections | Helanterä et al. (2006), Smedbråten et al. (2014), and Huang et al. (2018) | ||
| Lung | Asymptomatic CMV infection was observed in 13% of patients, while 10% developed CMV disease | Associated with increased mortality after transplantation | Beam et al. (2016) | ||
| Epstein–Barr virus | Kidney | Multiple organ transplantation and small bowel transplant recipients exhibited the highest PTLD rate, followed by lung, heart, pancreas, liver, and kidney transplant recipients | Linked to worsened graft outcomes and escalated opportunistic infections | Opelz and Döhler (2004), Bamoulid et al. (2013), and Dierickx et al. (2013) | |
| SARS-CoV-2 | SOT | Average viral load of SOT recipients with severe infection was 60 times that of mild patients, while the median duration of viral shedding was longer and even extended to >3 months | Death rate of kidney transplant recipients infected with the SARS-CoV-2 is 8.1 times that of the general population, along with a low response rate after vaccination and greater vulnerability to reinfection | Hippisley-Cox et al. (2021), Marinelli et al. (2021), and Jefferson et al. (2023) |
| Types of infection . | Types of pathogens . | Transplant organ . | Status of infection . | Clinical prognosis . | Reference . |
|---|---|---|---|---|---|
| Bacterial infection | Staphylococcus | Liver | Accounted for 42%–69% of BSIs, followed by pneumonia, intra-abdominal, and surgical site infections | Bacteremia with pneumonia and abdominal infection were associated with mortality | Dudzicz-Gojowy et al. (2022) |
| Heart | Accounted for 26% of the pericarditis infections | Linked to a higher risk of death | Smedbråten et al. (2014) | ||
| Enterococcus | Liver | Approximately 20% occurred as digestive tract infections in liver transplant recipients, with a high incidence in the first 180 days | Vancomycin resistance did not influence outcome in patients with Enterococcus faecium bacteremia after liver transplantation | Pouch et al. (2015) and van Delden et al. (2020) | |
| Clostridioides difficile | Liver/kidney/pancreas/heart/lung | CDI incidence was 3%–7% in the liver, 3%–16% in the kidney, 1.5%–7.8% in the pancreas-kidney, 15% in the heart, and 7%–31% in lung recipients | Correlated with graft loss | Linares et al. (2007), Abad and Razonable (2016), and Subramanian et al. (2019) | |
| Acinetobacter baumannii | Liver | Median time of CRAB bacteremia emergence was 56 days after transplantation, with 72% of the cases occurring within 6 months post-transplant | Patients with CRAB bacteremia after liver transplant showed an unfavorable outcome, leading to an overall mortality of 50% in 14 recipients with CRAB bacteremia | Kim et al. (2018) | |
| Klebsiella pneumoniae | Heart/liver/kidney | Infection prevalence in heart, liver, and kidney transplant recipients was 17%, 13%, and 26%, respectively, with a median time to infection of 20 days after transplantation | CRKP infection had a mortality rate of >40% | Bergamasco et al. (2012), Clancy et al. (2013), and Righi (2018) | |
| Kidney | The most common isolated pathogen in UTI (53%) | Associated with severe invasive infections, graft loss, and mortality | Silva et al. (2013) | ||
| Escherichia coli | Liver | ESBL-positive E. coli had a higher rate of drug resistance than ESBL-negative E. coli | Bacteremia caused by ESBL-producing Enterobacteriaceae in liver transplant recipients had a 30-day mortality rate of up to 41% | Men et al. (2013) | |
| Kidney | One of the leading causes of UTIs (>60%) | Linked to graft loss and higher mortality | Alevizakos et al. (2017b) and Kinnunen et al. (2018) | ||
| Fungal infection | Candida spp. | Liver | Invasive Candida spp. accounted for 62%–91% of all invasive fungal infections | Often associated with delayed diagnosis and high mortality rates | Righi (2018) |
| Collins et al. (1994) | |||||
| Nieto-Rodriguez et al. (1996) | |||||
| Kidney | Accounts for 90% of fungal infections | Mortality rate of this fungal infection was 53% | Singh (2000) | ||
| Sahin et al. (2015) | |||||
| Aspergillus spp. | Liver | Invasive Aspergillus spp. infection occurred in 1%–8% of patients, and the median time for infection following liver transplantation was shorter (109 days) than that after lung transplantation (486 days) | Mostly all liver transplant recipients with invasive Aspergillus spp. infection had significant liver and/or renal insufficiency, along with a mortality rate of 64% | Fisher et al. (1999) and Husain et al. (2017) | |
| Lung | Aspergillus spp. was detected in airway specimen cultures of 9%–68% (mean positivity, 29%) of patients, with 13% experiencing progression to invasive aspergillosis or even death. | Mortality rate of 68% for lung transplant recipients with invasive Aspergillus spp. infection | Hamacher et al. (1999) and Sahin et al. (2015) | ||
| Heart | Incidence of invasive Aspergillus spp. infection varies substantially (1%–15%), with an overall incidence of 5% | Associated with a significantly higher fatality (75%) than that from bacterial infection (36%) | Schulman et al. (1988), Cisneros et al. (1998), and Echenique et al. (2017) | ||
| Viral infection | Cytomegalovirus | Liver | A common conditional pathogenic virus after transplantation | Linked to an increased graft loss within 1 year post-transplant | Burak et al. (2002) and Bosch et al. (2011) |
| Kidney | Infection and pathogenicity rates of 40%–100% and 67%, respectively, with the highest infection risk in the first 3 months | Correlated with a higher risk of death and graft loss as well as an elevated risk of fungal infections | Helanterä et al. (2006), Smedbråten et al. (2014), and Huang et al. (2018) | ||
| Lung | Asymptomatic CMV infection was observed in 13% of patients, while 10% developed CMV disease | Associated with increased mortality after transplantation | Beam et al. (2016) | ||
| Epstein–Barr virus | Kidney | Multiple organ transplantation and small bowel transplant recipients exhibited the highest PTLD rate, followed by lung, heart, pancreas, liver, and kidney transplant recipients | Linked to worsened graft outcomes and escalated opportunistic infections | Opelz and Döhler (2004), Bamoulid et al. (2013), and Dierickx et al. (2013) | |
| SARS-CoV-2 | SOT | Average viral load of SOT recipients with severe infection was 60 times that of mild patients, while the median duration of viral shedding was longer and even extended to >3 months | Death rate of kidney transplant recipients infected with the SARS-CoV-2 is 8.1 times that of the general population, along with a low response rate after vaccination and greater vulnerability to reinfection | Hippisley-Cox et al. (2021), Marinelli et al. (2021), and Jefferson et al. (2023) |
Abbreviations: BSI: bloodstream infection; CDI: Clostridioides difficile; CMV: Cytomegalovirus; CRAB: carbapenem-resistant A. baumannii; CRKP: carbapenem-resistant K. pneumoniae; ESBL: extended-spectrum beta-lactamase; PTLD: post-transplant lymphoproliferative disease; SOT: solid organ transplantation; UTI: urinary tract infection.
Bacteria
Staphylococcus
A total of 24% of all Staphylococcus infections occur as bacteremia caused by S. aureus, especially methicillin-resistant S. aureu s (MRSA). This infection complicates the clinical course of liver transplantation and is associated with high morbidity and mortality (Hashimoto et al. 2008, Liu et al. 2018). Torre-Cisneros et al. reported a fatality rate of 21% from S. aureus BSIs at 30 days after liver transplantation, whereas this rate nearly doubled to 42.9% in the case of MRSA BSIs, possibly due to MRSA having more potent virulence than S. aureus (Torre-Cisneros et al. 2002). Nevertheless, MRSA infections have been declining in SOT patients worldwide in recent years (Cervera et al. 2014). Additionally, the most common cause of infective endocarditis in heart transplant recipients was found to be S. aureus (26.3%), followed by Aspergillus fumigatus in 19.3% (11/57 patients).
Enterococcus
Enterococci were considered harmless inhabitants of the human gastrointestinal tract, with rarely reported incidences of opportunistic infections in critically ill patients. The Swiss transplant cohort study failed to demonstrate a significant association between enterococcal infections and increased mortality (Bucheli et al. 2014). However, the prevalence of vancomycin-resistant Enterococcus (VRE) in SOT patients has been growing worldwide owing to multiple hospitalizations, prolonged hospital stays, and treatment with broad-spectrum antibiotics (Dubler et al. 2020). A US study showed that up to 80% of Enterococcus faecium isolates exhibited resistance to vancomycin (Hidron et al. 2008). Furthermore, the presence of VRE was associated with increased BSI morbidity, as demonstrated by the increase in vancomycin resistance in enterococcal BSI from 5.9% in 2007 to 16.7% in 2016 in a German study involving patients admitted to intensive care units (Remschmidt et al. 2017). Nonetheless, this outcome did not affect post-transplant survival outcomes (Dubler et al. 2020), consistent with the findings observed in SOT patients (Hefazi et al. 2016).
Clostridioides difficile
Clostridioides difficile infection (CDI) is among the most common hospital-acquired infections (Mullane et al. 2019). During a 5-year follow-up study of patients with C. difficile infection after kidney transplantation, 6.8% required hospitalization due to the infection, with the annual incidence displaying an upward trend (Hosseini-Moghaddam et al. 2021). CDI occurrence is also significantly elevated following SOT, including liver, heart, and lung transplants (Dudzicz-Gojowy et al. 2022), and varies according to the type and number of transplanted organs. A previous meta-analysis revealed that postoperative CDI occurred in ∼0.51% of the general surgical population. In contrast, the prevalence of CDI among SOT recipients varied significantly, ranging from 3.2% in patients undergoing pancreatic transplantation to 12.7% in those receiving multiple organ transplants (Riddle and Dubberke 2008).
Klebsiella pneumoniae
CRKP is increasingly recognized as one of the most common pathogens causing infection after kidney transplantation (Silva et al. 2013). In China, CRKP isolates primarily (70%) produce carbapenemase that can decompose almost all β-lactam antibiotics, such as carbapenem antibiotics, ultimately presenting an extremely challenging problem for treating CRKP infection (Han et al. 2023). Correspondingly, the fatality rate of CRKP infection in SOT recipients has been reported to be as high as 43% (Xu et al. 2017). Additionally, mortality in kidney recipients with CRKP infection was about three times higher than that in those who were uninfected; however, this pattern was not associated with graft failure (Pouch et al. 2015). Moreover, infection with K. pneumoniae subspecies after liver transplantation displayed an increasing annual trend from 2017 to 2020, accounting for more cases than E. coli (Han et al. 2023).
Escherichia coli
The widespread use of carbapenem antibiotics has led to the increased prevalence of bacterial infections with carbapenem-resistant strains in clinical practice. Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) is one such strain that has been suggested to cause severe infections. A systematic review reported an overall prevalence of 18% for ESBL-PE colonization, with stratification by transplant type revealing prevalence rates of 17% and 24% in liver and kidney transplant recipients, respectively (Alevizakos et al. 2017a). Furthermore, ∼1 in 10 kidney transplant recipients with colonization by ESBL-producing bacteria will experience a UTI caused by these pathogens (Alevizakos et al. 2017b). In a Spanish study of kidney (n = 142), liver (n = 98), and kidney/pancreas (n = 7) transplant recipients, the most isolated multidrug-resistant Enterobacterales was E. coli (46%), followed by K. pneumoniae (35%) (Fernández-Martínez et al. 2021). Infections arising from such drug-resistant bacterial pathogens are associated with an increased risk of recurrent infection, allograft dysfunction, and heightened mortality compared to those from non-drug-resistant pathogens (Linares et al. 2007).
Mycobacterium tuberculosis
The incidence of active tuberculosis (TB) among SOT patients is 20–74 times higher than that in the general population (Rashid et al. 2021). Tuberculosis typically develops during the first year after SOT, with a large proportion of cases being attributed to extrapulmonary and disseminated TB (Chiang et al. 2023). In terms of transplant type, individuals who have undergone lung transplantation are the most susceptible to post-transplant tuberculosis (Subramanian et al. 2019). Additionally, active TB incidence in kidney recipients can vary from 0.3% to 15.2%, contributing to patient mortality (Anand et al. 2017, Sorohan et al. 2022).
Nontuberculous mycobacteria
The estimated rates of nontuberculous mycobacteria (NTM) infections range from 0.16% to 0.55% among kidney transplant recipients, with slightly higher rates in heart (2.8%) and lung (0.46%–4.4%) recipients and a lower rate of 0.04% in those who underwent liver transplant. Mycobacterium kansasii is a frequent and predominant NTM species detected in patients with heart transplants (Abad and Razonable 2016). Of all the populations of SOT recipients, lung transplant recipients are at a heightened risk for NTM disease. This increased susceptibility may be explained by the direct exposure of the transplanted lung to the environment and structural abnormalities in lung transplant recipients that potentially impede the host defenses against inhaled microorganisms (Shah et al. 2016). A Korean study involving 215 lung transplant recipients who were followed for more than 1 year revealed that NTM infection was diagnosed in 6.5% of the patients at an average duration of 11.8 months post-transplant, with Mycobacterium abscessus identified as the primary pathogen (Park et al. 2022). Lastly, among SOT recipients with NTM infections, ∼40% and 10% of kidney and heart/lung transplant recipients, respectively, were affected by rapidly growing mycobacteria (Abad and Razonable 2016).
Fungi
Fungi are ubiquitous microorganisms, among which Aspergillus and Candida are the most common pathogens in the surrounding environment. These two fungi species represent the most common invasive fungal infections after SOT. Invasive fungal infection is a severe infection caused by fungal invasion in subcutaneous tissues, mucous membranes, and internal organs and their subsequent spread in the blood.
Candida spp.
Invasive Candida infection is one of the most common fungal infections observed in patients with SOT. According to the epidemiological data on invasive Candida infections in North America, liver (41.1%) and kidney transplant patients (35.3%) form the major susceptible groups. The most common sites of Candida infections are the blood (44%) and abdominal cavity (14%). Compared with 26.5% of all-cause mortality within 90 days, Candida tropicalis infection had the highest mortality rate (44%), followed by Candida glabrata infection (35.2%) (Andes et al. 2016). Another prospective study of invasive Candida infections in 2010–2011 and 2016–2018 in Spain showed that liver (30.9%) and kidney transplant patients (38.2%) were the most vulnerable, exhibiting a 30-day mortality rate of 27.3% (Fernández-Ruiz et al. 2019).
Aspergillus spp.
Although Candida spp. infections are a significant complication in liver and pancreas transplant recipients, Aspergillus spp. infections have been demonstrated to have the greatest impact on liver and lung transplants. Considering that the lung is an organ exposed to ambient air, it is more likely to develop fungal infections. Fungal infections occur in 15%–35% of patients after lung transplantation, with more than 80% of cases attributed to Aspergillus and Candida infections and an overall risk of death close to 60% (Hamacher et al. 1999, Phoompoung et al. 2022). Additionally, the decreasing incidence of invasive Candida infections has resulted in Aspergillus emerging as the most influential fungal pathogen postoperation. Consequently, evaluating the likelihood of Aspergillus colonization in the respiratory tract of lung SOT recipients is required to avoid unnecessary treatment (Unterman et al. 2020). Among SOT patients with invasive Aspergillus infection, lung transplant recipients have a median time of 486 days to present with invasive Aspergillus infections, whereas liver transplant recipients have the shortest median time (109 days) and the highest mortality rate (64%) (Husain et al. 2017). However, the incidence of invasive Aspergillus spp. infection varies considerably (1%–15%), displaying an overall incidence of 5% in heart SOT recipients (Echenique et al. 2017, Jordan et al. 2022). Furthermore, a 1-year study investigating pneumonia causes in 307 heart transplant recipients indicated that Aspergillus spp. infection is an independent factor for poor pneumonia prognosis (relative risk, 7.4; 95% confidence interval, 5.8–9.1) (Cisneros et al. 1998).
Invasive mold infections (IMIs) pose a significant threat to SOT recipients due to their compromised immune systems. A study describing IMI after SOT showed a cumulative incidence of IMI in the range of 1.2%–18.8% over 1 year (Farges et al. 2020). Liver transplant recipients had the lowest median time to IMIs, the highest rate of disseminated disease, and the highest mortality. Lung transplant recipients had the highest median time to IMIs and the lowest mortality (Husain et al. 2017). Given the high mortality associated with IMIs, a high index of suspicion and a low threshold for initiating antifungal therapy are warranted.
Other fungi
There has been a noted increase in non-Aspergillus mold infection (NAMI) in SOT recipients in recent years, particularly in infections caused by non-Candida albicans and non-Aspergillus species. These infections, caused by molds such as Mucorales, Scedosporium/Lomentospora spp., Fusarium spp., Scopulariopsis/Microascus, and Paecilomyces spp., present unique challenges due to their intrinsic resistance patterns and the limited availability of effective antifungal therapies (Elhaj Mahmoud et al. 2024). In a prospective surveillance of NAMI in the USA from 2001 to 2006, 26.6% were SOT recipients. The cumulative incidence of mucormycosis in SOT recipients was 0.07% (Park et al. 2011). Lung transplant recipients are particularly at risk for NAMI, likely due to inhalation of fungal spores from the environment (Elhaj Mahmoud et al. 2024). In lung transplant recipients, Mucorales infections may occur as early as the first month post-transplant, while in other SOT recipients, such infections may occur 3–6 months or later (Elhaj Mahmoud et al. 2024).
Virus
Cytomegalovirus
Human CMV is a double-stranded DNA virus from the herpes virus family. Although CMV infection is the most common viral infection after SOT, the incidence of CMV tissue invasive disease is low (1%–2%). However, breakthrough infections have been observed in 4%–34% of the patient population during CMV prophylaxis (Dulek 2023).
CMV infection exerts diverse indirect effects, encompassing heightened vulnerability to other infectious complications such as bacteremia and invasive fungal disease, along with increased rejection, graft failure, and mortality rates (Roman et al. 2014, Razonable 2020). The risk of CMV disease can vary based on transplant type, with lung transplant recipients exhibiting a higher disease susceptibility than those undergoing kidney and liver transplants (Beam et al. 2016). However, clinical practice data show that a greater number of kidney and liver recipients develop CMV infection and disease because they are the most frequently performed transplants worldwide (Alhefzi et al. 2016, Beam et al. 2016, 2018, Dulek 2023). The incidence of liver transplant failure among hepatitis C transplant recipients was found to be significantly higher in CMV-positive patients than in those who were CMV-negative (52% vs. 19%, P = .002) (Burak et al. 2002). CMV infection and disease were also significantly associated with graft loss within 1 year post-liver transplantation (Bosch et al. 2011). In a long-term survival study with a median follow-up of 13.7 years, early CMV infection in the recipients predicted increased overall mortality after kidney transplantation (Smedbråten et al. 2014). Correspondingly, Helantera et al. also demonstrated that CMV infection significantly reduced the survival rate and kidney graft function, implying its role as an independent risk factor for declining graft survival in kidney transplant recipients (Helanterä et al. 2006). Another study reported a 21.9% incidence rate of CMV infection among 3065 kidney transplant recipients across 17 transplant centers in Iran, with a higher occurrence observed within the first 6 months post-transplant (Einollahi 2012). Additionally, the implementation of prevention strategies in transplant centers has altered the epidemiology of CMV infection, ultimately decreasing the incidence of CMV disease to 8% (Roman et al. 2014). However, a large cohort of heart transplant recipients showed that the presence of CMV infection did not significantly correlate with heart transplant rejection (Boutolleau et al. 2021).
COVID-19
A UK study during the epidemic period of the Delta variant found that the fatality rate in kidney transplant recipients infected with the novel coronavirus was 8.1 times that in the general population (Hippisley-Cox et al. 2021). In particular, the average viral load of SOT recipients having severe infection was 60 times that of mild patients, and the median duration of viral shedding was also longer, with the median duration of viral shedding primarily being <25 days, while it was >30 days in a few and even >3 months in some. In contrast, the median time of mild patients was 10 days (Marinelli et al. 2021, Jefferson et al. 2023). Additionally, individuals with suppressed immune systems who have undergone SOT may continue to exhibit heightened susceptibility to COVID-19 and limited responsiveness toward currently available vaccines (Safa and Kotton 2022).
Epstein–Barr virus
EBV is a linear double-stranded DNA virus that typically causes no or only mild symptoms in individuals with a normal immune system. However, the use of immunosuppressive drugs after organ transplantation suppresses the recipient’s immune system and diminishes its ability to combat EBV, thus increasing the risk of contracting EBV infection. Post-transplant lymphoproliferative disease (PTLD) is a lymphatic system disease observed in SOT recipients, wherein immunosuppression induces the proliferation of lymphocytes or plasma cells from benign tissue to malignant tumors. The relationship between EBV infection and PTLD accounts for more than 70% of the PTLD cases. In adult SOT, multiple organ transplantation and small bowel transplant recipients have the highest PTLD rate of up to 20%, followed by lung (3%–10%), heart (2%–8%), pancreas (1%–5.5%), liver (0.5%–5%), and kidney transplantations (0.8%–2.5%) (Opelz and Döhler 2004, Dierickx et al. 2013). Furthermore, other researchers have reported on the occurrence of autoimmune disease with neural tissue reactivity as a consequence of EBV infection (Masajtis-Zagajewska et al. 2012, Cooper et al. 2017).
Parasitic infection
The incidence of parasitic infection following organ transplantation is relatively low, primarily occurring in developing countries and regions, and is often reported as cases (Ye 2022). In Africa, where the majority of the global malaria burden occurs, SOT recipients are at heightened risk for severe malaria due to their immunosuppressed state. A recent study conducted in Sudan revealed a concerning incidence of malaria, with 25 cases documented among a cohort of 55 renal transplant recipients over the course of a 1-year follow-up period (Elsharif et al. 2012). Chagas disease, caused by the parasite Trypanosoma cruzi, is a leading cause of heart disease (Chin-Hong et al. 2011, Benvenuti et al. 2017). Among SOT recipients in Ceará State, Brazil, a recent study identified the prevalence of T. cruzi infection among these potential donors was 1.3% (Costa et al. 2018). In sub-Saharan Africa, leishmaniasis is highly prevalent. A report from Madrid, Spain, indicated that SOT recipients residing in leishmaniasis-endemic areas faced a 10.3% increased risk of developing the disease, which significantly affected both graft function and patient survival (Carrasco-Antón et al. 2017).
Toxoplasmosis after organ transplant is more commonly observed in heart transplant recipients, with an incidence of 57%–75% in seronegative patients who receive a seropositive donor heart and are not administered preventive treatment within 3 months after surgery (Ramanan et al. 2020). A previous study involving 15 800 organ transplant recipients identified 22 patients with toxoplasmosis disease. Moreover, 90% of these recipients were seronegative for toxoplasmosis before transplantation, while the mortality rate was high at 13.6% (Fernàndez-Sabé et al. 2012).
Discussion
The risk of infections following SOT is not uniform across the post-transplant period. It is influenced by the timing of immunosuppressive therapy, the patient’s exposure to pathogens, and the natural recovery of the immune system. Early Infections (first month post-transplant): During this period, recipients are at the highest risk for surgical site infections, wound healing complications, and infections from the donor organ (donor-derived infections). Gram-negative bacterial infections, including those from P. aeruginosa, A. baumannii, and Enterobacteriaceae family members such as E. coli and K. pneumoniae, accounted for 50%–80% of the lower respiratory tract infections in the first month after the SOT procedure (Youhua and Wenyu 2023). The intense immunosuppression required to prevent early rejection makes them particularly vulnerable. Intermediate infections (1–6 months post-transplant): As immunosuppression is weaned to maintenance levels, the risk of opportunistic infections, such as those caused by Pneumocystis jirovecii, CMV, and Aspergillus species, becomes more pronounced (Youhua and Wenyu 2023). Late infections (after 6 months post-transplant): Beyond the first 6 months, the risk of community-acquired and chronic infections, such as tuberculosis, viral hepatitis, and certain types of cancer, starts to dominate (Fishman 2017). The long-term use of immunosuppressive drugs can lead to a gradual decline in immune surveillance, increasing the risk of these infections.
UTI is a common complication following kidney transplantation, often caused by Klebsiella spp., E. coli, and Enterococcus (Tawab et al. 2017, Santithanmakorn et al. 2022). These bacteria typically reside in the gastrointestinal tract of the patient. The transplant procedure and subsequent immunosuppressive therapy attenuate the patient’s immune response, making them more susceptible to these opportunistic pathogens (Silva et al. 2013).
Intra-abdominal infection is frequently observed after liver transplantation, with Enterobacteriaceae, particularly E. coli and Klebsiella spp., serving as the primary causative agents (Liu et al. 2023). Additionally, extensive antibiotic and immunosuppressive therapy can lead to the prevalence of fungal infections from Candida spp. among liver transplant recipients (Andes et al. 2016). Common sites of infection after liver transplantation include the abdominal region, respiratory system, and circulatory system. Intra-abdominal infections tend to be more prevalent in the early postoperative phase, while respiratory infections become more prominent during long-term follow-up (Ying et al. 2020). Furthermore, heart transplantation has the highest infection rate among all organ transplantations (van Delden et al. 2020).
Pulmonary infection is the most prevalent infection following SOT, where the respiratory tract is the predominant infection site and bacterial pneumonia is the most frequent infectious complication (Meng et al. 2022). In terms of bacterial species, A. baumannii, P. aeruginosa, and K. pneumoniae have been identified as the primary bacterial pathogens in lung transplant recipients having complications related to lower respiratory tract infections (Qiao et al. 2019). Pulmonary infection is a prevalent form of infection following heart transplantation. Additionally, bacteremia, surgical site infections, and intra-abdominal infections represent significant postoperative complications after heart transplantation (Cove et al. 2012, van Delden et al. 2020).
Although this review extensively analyzed the epidemiological characteristics of infections following various types of SOTs, including common infection sites and specific pathogen infections, certain limitations and deficiencies should be acknowledged. First, this review primarily relied on retrospective data analysis, potentially limiting this analysis due to issues related to data collection completeness and accuracy. Second, the patients with SOT included in this review were obtained from multiple centers, possibly introducing variability owing to the different medical practices. Nevertheless, this approach may have provided a broader perspective on the relevant data. Finally, the analysis of the specific pathogen infections focused solely on bacterial, fungal, and viral infections and overlooked other factors that could have affected the risk for post-transplant infections. This limitation may have hindered our comprehensive understanding of the dynamics of post-transplant infections.
Conclusions
In the present review, the epidemiological characteristics, common infection sites, and specific pathogen infections following kidney, liver, heart, and lung transplantation were systematically investigated. This review revealed significant variations in the incidence and types of infections among different SOTs, thereby providing essential reference information for clinicians in managing post-transplant infections. Given the continued advancements in transplantation medicine, effective infection management strategies will be pivotal in enhancing patient prognosis in the future.
Author contributions
Fanjie Meng (Investigation, Writing – original draft), Chi Zhu (Writing – original draft), Chan Zhu (Writing – original draft), Jiaxuan Sun (Writing – review & editing), Dongsheng Chen (Formal analysis, Writing – review & editing), Ran Ding (Writing – review & editing), and Liyuan Cui (Writing – review & editing)
Conflict of interest
None declared.
Funding
None declared.
Data availability
No new data were generated or analyzed in support of this research.
References
Author notes
Contributed equally.



