-
PDF
- Split View
-
Views
-
Cite
Cite
Anouk P Meijs, Paul D Hengeveld, Cindy M Dierikx, Catharina B M Maassen, Sabine C de Greeff, Angela de Haan, Thijs Bosch, Engeline van Duijkeren, Prolonged carriage of (livestock-associated) MRSA in individuals without professional livestock contact, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1405–1409, https://doi.org/10.1093/jac/dkaa045
Close - Share Icon Share
Abstract
To investigate prolonged carriage of MRSA in adults from the general population living in a livestock-dense area, using WGS.
A cross-sectional study during 2014–15 among 2492 adults without professional livestock contact identified 14 (0.6%) nasal MRSA carriers, 10 of which carried livestock-associated (LA)-MRSA of multiple-locus variable-number tandem repeat analysis (MLVA) complex (MC) 398. Two years later, 12 MRSA-positive and 88 MRSA-negative participants provided a second nasal swab and filled in a short questionnaire. Isolates from persons who were MRSA positive at both timepoints were compared using MLVA and isolates with the same MLVA type were sequenced. The WGS data were used for core-genome MLST (cgMLST) and resistome analysis, including sequenced isolates from the national MRSA surveillance.
All MRSA-negative persons tested negative again, while 6 of the 12 initially MRSA-positive persons tested positive again. MLVA revealed that isolate pairs from five individuals had the same MLVA type, of which three were LA-MRSA. cgMLST showed that the distance between these isolate pairs ranged between 3 and 13 genes, while the minimum distance to unrelated isolates from the national MRSA surveillance was 38 genes. Moreover, the resistome present in the five isolate pairs was identical within each pair. None of the prolonged carriers was hospitalized during the 3 months before the sampling moment and none of them with LA-MRSA had contact with livestock in this period.
Prolonged carriage of MRSA, including LA-MRSA, can be demonstrated after more than 30 months in persons without professional livestock contact.
Introduction
Worldwide, MRSA causes hospital- and community-associated infections in humans. During the last decade, MLST clonal complex (CC) 398, corresponding to multiple-locus variable-number of tandem repeat analysis (MLVA) complex (MC) 398, has emerged in livestock and persons in contact with livestock in many countries. This type of MRSA is referred to as livestock-associated MRSA (LA-MRSA). In the Netherlands, the prevalence of MRSA carriage in individuals in the population at large is low and has varied between 0.1% and 0.8% in the last 10 years depending on the methods used and the population studied.1–3 In a Dutch study among persons living in a livestock-dense area, but without professional livestock contact, 0.6% carried MRSA and the prevalence of MC398 LA-MRSA was 0.4%.4
Previously, it was suggested that LA-MRSA is a poor persistent colonizer in humans.5 However, two recent longitudinal studies among veterinarians and their family members found that LA-MRSA can be present for up to 2 years.6,7 It is unclear whether prolonged LA-MRSA carriage is caused by persistent carriage or by recontamination by recurrent exposure. Therefore, we investigated the presence of and risk factors for prolonged LA-MRSA carriage in persons without professional livestock contact in the community at large. Furthermore, isolates of persons that tested positive with the same MC twice were analysed using WGS and compared to epidemiologically unrelated MRSA isolates from the national MRSA surveillance.
Methods
From March 2014 to February 2015, a cross-sectional study (T0) assessing the prevalence of and risk factors for MRSA carriage was performed among 2492 adults in a livestock-dense area in the Netherlands as part of the Livestock Farming and Neighbouring Residents’ Health study (Dutch acronym: VGO).4 The VGO study was approved by the medical ethics committee of the University Medical Centre Utrecht (number 13/533). Participants provided signed informed consent.
Included persons were aged between 20 and 72 years and lived in the provinces North-Brabant or Limburg. Only persons not living or working on a livestock farm were allowed to participate and only one person per home address was randomly invited. In this study, 14 persons carried MRSA (prevalence = 0.6%; 95% CI = 0.3–0.9), 10 of whom carried MC398 LA-MRSA (prevalence = 0.4%; 95% CI = 0.2–0.7).4
Following the cross-sectional study, 110 individuals were invited to participate in the longitudinal study (T1), which took place from October 2016 to January 2017. All MRSA-positive individuals who had agreed to be contacted again (n = 13) were invited. For every MRSA-positive person, four to eight MRSA-negative controls (n = 97) were asked to participate. Controls were matched for age and gender to the MRSA-positive person, and the fact that they visited the same research centre for medical examination during the cross-sectional study.4 All participants were asked to provide a nasal swab and fill in a short questionnaire.
Nasal swabs were cultured using pre-enrichment and selective enrichment and were plated on Brilliance MRSA 2 Agar (Oxoid, Germany), as previously described.4 Suspected colonies were confirmed as MRSA by quantitative PCR (qPCR) (for detection of the mecA gene and specific Staphylococcus aureus DNA fragments: nuc and tuf).8 All MRSA isolates were typed by MLVA typing as described previously9 and isolates belonging to MC398 were considered LA-MRSA. All isolates belonging to other MCs were deemed as non-LA-MRSA. Isolates showing the same MLVA type as during the cross-sectional study were sequenced using Illumina HiSeq and subsequently assembled using CLC bio Genomics server/Workbench software version 7.5 (CLC bio, Aarhus, Denmark). Resistance genes were identified by uploading the resulting contigs in the ResFinder software version 2.1.10 The threshold for identification was set at 90% and 60% was selected as minimum length. For comparative analysis, core-genome MLST (cgMLST) using the SeqSphere® software version 2.3.0 (Ridom GmbH, Munster, Germany) and the available cgMLST S. aureus scheme comprising 1861 genes was used. Isolates with a difference of up to 15 genes in cgMLST were considered genetically identical.11 For epidemiological context, we included sequence data of 309 unrelated MRSA isolates comprising multiple MCs from the national MRSA surveillance in healthcare facilities. The isolates were submitted during either the cross-sectional study or the longitudinal study and subjected to WGS as part of the routine MRSA surveillance.
Median time between the cross-sectional study (T0) and the longitudinal study (T1) and the results of the questionnaire were analysed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Results
A total of 100 persons (12 MRSA positive and 88 MRSA negative at T0) submitted a second nasal swab and completed the questionnaire. The median time between T0 and T1 was 24.2 months (range = 20.7–32.9 months). Of the 12 MRSA-positive persons at T0, 6 persons were still MRSA positive after a median of 23.7 months (range = 20.7–30.8 months), while all MRSA-negative persons at T0 remained negative at T1 after a median of 24.3 months (range = 21.0–32.9 months). In five out of six persons, the same MLVA type was identified at T0 and T1. Three of these carried LA-MRSA with MLVA types 398, 555 and 569. The other two persons carried MLVA types 461 and 2104 belonging to the non-LA-MRSA MC22 and MC282, respectively (Table 1). The remaining participant carried different MLVA types at T0 and T1 (398 and 589, respectively) and was therefore excluded from WGS analysis.
MLVA typing results and presence of risk factors in (LA-)MRSA (prolonged) carriers
| . | MRSA-positive persons (T0)a . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | prolonged carriers . | not prolonged carriers . | ||||||||||
| . | 4 . | 5 . | 8 . | 11 . | 12 . | 1 . | 2 . | 6 . | 7 . | 9 . | 13 . | 14 . |
| MC, T0 | 398 | 398 | 398 | 282 | 22 | 398 | 398 | 398 | 398 | 398 | 30 | 30 |
| MLVA type, T0 | 569 | 398 | 555 | 2104 | 461 | 398 | 572 | 572 | 398 | 398 | 211 | 211 |
| MC, T1 | 398 | 398 | 398 | 282 | 22 | — | — | — | — | 398 | — | — |
| MLVA type, T1 | 569 | 398 | 555 | 2104 | 461 | — | — | — | — | 589 | — | — |
| Time between T0 and T1 (months) | 30.8 | 30.5 | 23.2 | 20.7 | 24.2 | 23.3 | 29.3 | 27 | 25.1 | 21 | 23.8 | 23.7 |
| Current job relevant for MRSA | NR | NR | NR | UNK | UNK | penitentiary employee | NR | NR | NR | NR | GP assistant | NR |
| Partner/household member has professional contact with animals | no | no | no | no | no | pig, donkey, horse, rabbit, sheep, chicken, goat | no | pig | no | no | no | no |
| Kept horses during last 3 months | no | no | no | no | yes | yes | no | no | yes | yes | no | no |
| Contact with horses during last 3 monthsb | no | no | no | yes | yes | yes | no | no | yes | yes | no | no |
| Contact with other farm animals during last 3 months | no | no | no | no | poultry, pig | poultry, sheep/goat | no | poultry | no | no | no | no |
| Contact with pet animals during last 3 months | cat, dog | dog | cat, dog | no | rabbit/ rodent, bird | cat, dog, bird | dog | dog, rabbit/ rodent, bird | cat, dog | dog | cat, dog | cat, dog |
| Antibiotic use during last 3 months | no | no | yes | no | no | no | no | no | no | yes | no | no |
| Hospitalized during last 3 months | no | no | no | no | no | no | no | no | no | no | no | no |
| Appointment at outpatient clinic during last 3 months | no | no | no | no | no | no | yes | no | no | yes | yes | no |
| Hospital visit during last 3 months | no | no | yes | yes | no | yes | no | no | yes | no | no | no |
| Travel during last 3 months | W-EU | S-EU, W-Asia | W-EU | no travel | E-EU | E-EU, W-EU | no travel | S-EU, W-EU | no travel | no travel | no travel | E-EU, N-America |
| . | MRSA-positive persons (T0)a . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | prolonged carriers . | not prolonged carriers . | ||||||||||
| . | 4 . | 5 . | 8 . | 11 . | 12 . | 1 . | 2 . | 6 . | 7 . | 9 . | 13 . | 14 . |
| MC, T0 | 398 | 398 | 398 | 282 | 22 | 398 | 398 | 398 | 398 | 398 | 30 | 30 |
| MLVA type, T0 | 569 | 398 | 555 | 2104 | 461 | 398 | 572 | 572 | 398 | 398 | 211 | 211 |
| MC, T1 | 398 | 398 | 398 | 282 | 22 | — | — | — | — | 398 | — | — |
| MLVA type, T1 | 569 | 398 | 555 | 2104 | 461 | — | — | — | — | 589 | — | — |
| Time between T0 and T1 (months) | 30.8 | 30.5 | 23.2 | 20.7 | 24.2 | 23.3 | 29.3 | 27 | 25.1 | 21 | 23.8 | 23.7 |
| Current job relevant for MRSA | NR | NR | NR | UNK | UNK | penitentiary employee | NR | NR | NR | NR | GP assistant | NR |
| Partner/household member has professional contact with animals | no | no | no | no | no | pig, donkey, horse, rabbit, sheep, chicken, goat | no | pig | no | no | no | no |
| Kept horses during last 3 months | no | no | no | no | yes | yes | no | no | yes | yes | no | no |
| Contact with horses during last 3 monthsb | no | no | no | yes | yes | yes | no | no | yes | yes | no | no |
| Contact with other farm animals during last 3 months | no | no | no | no | poultry, pig | poultry, sheep/goat | no | poultry | no | no | no | no |
| Contact with pet animals during last 3 months | cat, dog | dog | cat, dog | no | rabbit/ rodent, bird | cat, dog, bird | dog | dog, rabbit/ rodent, bird | cat, dog | dog | cat, dog | cat, dog |
| Antibiotic use during last 3 months | no | no | yes | no | no | no | no | no | no | yes | no | no |
| Hospitalized during last 3 months | no | no | no | no | no | no | no | no | no | no | no | no |
| Appointment at outpatient clinic during last 3 months | no | no | no | no | no | no | yes | no | no | yes | yes | no |
| Hospital visit during last 3 months | no | no | yes | yes | no | yes | no | no | yes | no | no | no |
| Travel during last 3 months | W-EU | S-EU, W-Asia | W-EU | no travel | E-EU | E-EU, W-EU | no travel | S-EU, W-EU | no travel | no travel | no travel | E-EU, N-America |
NR, not relevant; UNK, unknown; W-EU, Western Europe; S-EU, Southern Europe; E-EU, Eastern Europe; W-Asia, Western Asia; N-America, North America.
The numbers correspond with the subject numbers from the cross-sectional study.4
This includes visits to horse-riding schools and horse stables.
MLVA typing results and presence of risk factors in (LA-)MRSA (prolonged) carriers
| . | MRSA-positive persons (T0)a . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | prolonged carriers . | not prolonged carriers . | ||||||||||
| . | 4 . | 5 . | 8 . | 11 . | 12 . | 1 . | 2 . | 6 . | 7 . | 9 . | 13 . | 14 . |
| MC, T0 | 398 | 398 | 398 | 282 | 22 | 398 | 398 | 398 | 398 | 398 | 30 | 30 |
| MLVA type, T0 | 569 | 398 | 555 | 2104 | 461 | 398 | 572 | 572 | 398 | 398 | 211 | 211 |
| MC, T1 | 398 | 398 | 398 | 282 | 22 | — | — | — | — | 398 | — | — |
| MLVA type, T1 | 569 | 398 | 555 | 2104 | 461 | — | — | — | — | 589 | — | — |
| Time between T0 and T1 (months) | 30.8 | 30.5 | 23.2 | 20.7 | 24.2 | 23.3 | 29.3 | 27 | 25.1 | 21 | 23.8 | 23.7 |
| Current job relevant for MRSA | NR | NR | NR | UNK | UNK | penitentiary employee | NR | NR | NR | NR | GP assistant | NR |
| Partner/household member has professional contact with animals | no | no | no | no | no | pig, donkey, horse, rabbit, sheep, chicken, goat | no | pig | no | no | no | no |
| Kept horses during last 3 months | no | no | no | no | yes | yes | no | no | yes | yes | no | no |
| Contact with horses during last 3 monthsb | no | no | no | yes | yes | yes | no | no | yes | yes | no | no |
| Contact with other farm animals during last 3 months | no | no | no | no | poultry, pig | poultry, sheep/goat | no | poultry | no | no | no | no |
| Contact with pet animals during last 3 months | cat, dog | dog | cat, dog | no | rabbit/ rodent, bird | cat, dog, bird | dog | dog, rabbit/ rodent, bird | cat, dog | dog | cat, dog | cat, dog |
| Antibiotic use during last 3 months | no | no | yes | no | no | no | no | no | no | yes | no | no |
| Hospitalized during last 3 months | no | no | no | no | no | no | no | no | no | no | no | no |
| Appointment at outpatient clinic during last 3 months | no | no | no | no | no | no | yes | no | no | yes | yes | no |
| Hospital visit during last 3 months | no | no | yes | yes | no | yes | no | no | yes | no | no | no |
| Travel during last 3 months | W-EU | S-EU, W-Asia | W-EU | no travel | E-EU | E-EU, W-EU | no travel | S-EU, W-EU | no travel | no travel | no travel | E-EU, N-America |
| . | MRSA-positive persons (T0)a . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | prolonged carriers . | not prolonged carriers . | ||||||||||
| . | 4 . | 5 . | 8 . | 11 . | 12 . | 1 . | 2 . | 6 . | 7 . | 9 . | 13 . | 14 . |
| MC, T0 | 398 | 398 | 398 | 282 | 22 | 398 | 398 | 398 | 398 | 398 | 30 | 30 |
| MLVA type, T0 | 569 | 398 | 555 | 2104 | 461 | 398 | 572 | 572 | 398 | 398 | 211 | 211 |
| MC, T1 | 398 | 398 | 398 | 282 | 22 | — | — | — | — | 398 | — | — |
| MLVA type, T1 | 569 | 398 | 555 | 2104 | 461 | — | — | — | — | 589 | — | — |
| Time between T0 and T1 (months) | 30.8 | 30.5 | 23.2 | 20.7 | 24.2 | 23.3 | 29.3 | 27 | 25.1 | 21 | 23.8 | 23.7 |
| Current job relevant for MRSA | NR | NR | NR | UNK | UNK | penitentiary employee | NR | NR | NR | NR | GP assistant | NR |
| Partner/household member has professional contact with animals | no | no | no | no | no | pig, donkey, horse, rabbit, sheep, chicken, goat | no | pig | no | no | no | no |
| Kept horses during last 3 months | no | no | no | no | yes | yes | no | no | yes | yes | no | no |
| Contact with horses during last 3 monthsb | no | no | no | yes | yes | yes | no | no | yes | yes | no | no |
| Contact with other farm animals during last 3 months | no | no | no | no | poultry, pig | poultry, sheep/goat | no | poultry | no | no | no | no |
| Contact with pet animals during last 3 months | cat, dog | dog | cat, dog | no | rabbit/ rodent, bird | cat, dog, bird | dog | dog, rabbit/ rodent, bird | cat, dog | dog | cat, dog | cat, dog |
| Antibiotic use during last 3 months | no | no | yes | no | no | no | no | no | no | yes | no | no |
| Hospitalized during last 3 months | no | no | no | no | no | no | no | no | no | no | no | no |
| Appointment at outpatient clinic during last 3 months | no | no | no | no | no | no | yes | no | no | yes | yes | no |
| Hospital visit during last 3 months | no | no | yes | yes | no | yes | no | no | yes | no | no | no |
| Travel during last 3 months | W-EU | S-EU, W-Asia | W-EU | no travel | E-EU | E-EU, W-EU | no travel | S-EU, W-EU | no travel | no travel | no travel | E-EU, N-America |
NR, not relevant; UNK, unknown; W-EU, Western Europe; S-EU, Southern Europe; E-EU, Eastern Europe; W-Asia, Western Asia; N-America, North America.
The numbers correspond with the subject numbers from the cross-sectional study.4
This includes visits to horse-riding schools and horse stables.
cgMLST based on 1861 genes showed that the isolates from the same person clustered together with a genetic distance ranging from 3 to 13 genes between isolates belonging to a pair (Figure 1). Isolate pairs 5 and 8 were closely related with a genetic distance of 57 genes, although no epidemiological link could be found between these persons. For the three pairs of isolates belonging to MC398, the genetic distance to the nearest unrelated MC398 isolate from the national MRSA surveillance ranged between 38 and 102 genes, whereas the distance to the closest unrelated isolates for the remaining two pairs differed between 79 and 273 genes. In addition to the limited genetic distance in cgMLST, the resistome of the paired isolates was identical within each of the five pairs. However, each pair had its own unique profile of acquired resistance genes (Table S1, available as Supplementary data at JAC Online). All 10 isolates were mecA positive and carried the β-lactamase gene blaZ. The tetracycline resistance genes tet(M) and/or tet(K) were found in four pairs of isolates, including all LA-MRSA.
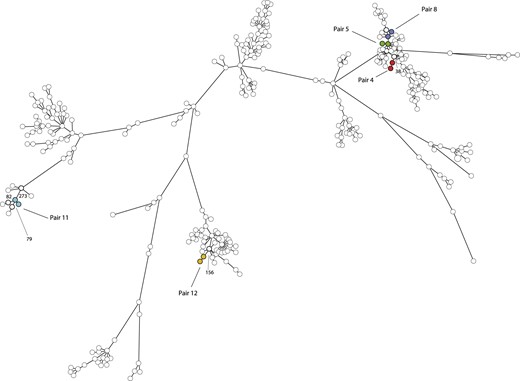
Minimum spanning tree based on WGS of 319 MRSA isolates. The tree was based on cgMLST using 1861 genes and clustering was done using a categorical coefficient. Each circle in the tree represents a single MRSA isolate. Circles with identical colours represent isolates of a single pair, whereas the white nodes represent epidemiologically unrelated isolates from the national MRSA surveillance submitted during the study period. The lines between the circles denote the distance in number of genes. Pair 4 (red): MLVA type 569 and MC398, genetic distance = 13 genes, genetic distance to neighbouring isolate = 38 genes. Pair 5 (green): MLVA type 398 and MC398, genetic distance = 8 genes, genetic distance to neighbouring isolates = 61–87 genes. Pair 8 (blue): MLVA type 555 and MC398, genetic distance = 3 genes, genetic distance to neighbouring isolates = 57–102 genes. Pair 11 (turquoise): MLVA type 2104 and MC282, genetic distance = 6 genes, genetic distance to neighbouring isolates = 79–273 genes. Pair 12 (yellow): MLVA type 461 and MC022, genetic distance = 8 genes, genetic distance to neighbouring isolate = 156 genes. The pair numbers correspond to the subject numbers from the cross-sectional study.4 This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The distribution of known risk factors in all MRSA-positive persons at T0 is shown in Table 1; the subject numbers correspond to the numbers in the cross-sectional study.4 None of the prolonged carriers was hospitalized during the 3 months preceding the sampling moment or worked as a healthcare worker. Furthermore, none of the prolonged LA-MRSA carriers had contact with farm animals in the last 3 months or had a partner/household member with occupational contact with livestock. One person used antibiotics in the last 3 months.
Discussion
In this longitudinal study, we investigated the presence of and risk factors for prolonged (LA-)MRSA carriage in a population without professional livestock contact. We found that 6 of the 12 persons that were colonized during the initial cross-sectional study (T0) were still MRSA carriers approximately 2 years later (T1). Based on MLVA, five of the six persons were prolonged carriers of identical MRSA, of which three were carriers of LA-MRSA. WGS analysis, which has a much higher discriminatory power compared with MLVA, corroborated the MLVA findings by showing a limited genetic distance in cgMLST and an identical resistome for isolate pairs. Furthermore, the genetic distance between isolates of a pair and the epidemiologically unrelated isolates from the MRSA surveillance was high, supporting the conclusion of prolonged MRSA carriage.
The most important risk factor for carriage of LA-MRSA is professional contact with livestock, especially pigs, veal calves and poultry.12 However, none of the prolonged LA-MRSA carriers in our study had contact with livestock in the period preceding sampling or had a partner or household member working with livestock. Lekkerkerk et al.13 found that 15% of persons carrying or infected with LA-MRSA did not report direct contact with pigs, broilers or veal calves. This is in agreement with reports from Germany, where 38% of patients with LA-MRSA CC398 in four hospitals did not report direct contact with livestock.14 The transmission route of these cases remains unknown. From the cross-sectional study preceding the current research, we know that MRSA MC398 carriers lived significantly closer to the nearest farm than non-carriers.4 This suggests that transmission through the environment might occur.15,16 However, transmission through human contact has also been reported for LA-MRSA.6
All initial MRSA-negative persons remained negative at the second sampling moment, which is in agreement with the very low prevalence of MRSA carriage in the Netherlands.1–4 Nevertheless, half of the initial MRSA-positive persons tested positive again approximately 2 years later with the same or another MRSA MLVA type. This indicates that some persons might be more susceptible to becoming colonized with MRSA in the long term. Although knowledge about the underlying mechanism, including host factors and bacterial determinants of S. aureus and MRSA colonization, is increasing, it remains unclear why certain individuals become persistent carriers.17 The other half of the initially MRSA-positive persons tested negative at the second sampling moment. Because only two samples were taken in a 2 year period, we cannot discern whether these persons were only transiently colonized or were MRSA carriers for a longer period.
In conclusion, although the prevalence of MRSA carriage in the Dutch population at large remains very low, we have shown that persons not in professional contact with livestock can become prolonged carriers of (LA-)MRSA, with durations of more than 30 months. The absence of recent contact with livestock of the prolonged LA-MRSA carriers in our study, together with the limited variability of isolates originating from the same person using WGS increases the plausibility that these persons were indeed persistent carriers rather than being recurrently exposed.
Acknowledgements
This research was presented as a poster at the Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2019 (Abstract P2713).
We thank Tizza Zomer (Gelre Hospital, Apeldoorn) for her work on the cross-sectional MRSA study and Lidwien Smit (IRAS, University of Utrecht) for selecting the negative controls. We thank all participants and the steering committee of the Livestock Farming and Neighbouring Residents’ Health (VGO) study.
Funding
The VGO study was funded by the Ministry of Health, Welfare and Sports and the Ministry of Economic Affairs of the Netherlands, and supported by a grant from the Lung Foundation Netherlands (Grant Number: 3.2.11.0211).
Transparency declarations
None to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online



