-
PDF
- Split View
-
Views
-
Cite
Cite
Jinhu Huang, Mengli Wang, Yi Gao, Li Chen, Liping Wang, Emergence of plasmid-mediated oxazolidinone resistance gene poxtA from CC17 Enterococcus faecium of pig origin—authors’ response, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 5, May 2020, Pages 1359–1361, https://doi.org/10.1093/jac/dkaa054
Close - Share Icon Share
Sir,
We have carefully read the letter by Freitas et al.,1 with regard to our published paper on ‘Emergence of plasmid-mediated oxazolidinone resistance gene poxtA from CC17 Enterococcus faecium of pig origin’.2 We really appreciate the comments. Now we gladly take the opportunity to appropriately clarify the issues that were raised.
MLST has been developed to study the epidemiology and genetic relatedness of strains globally from diverse hosts and environments.3 MLST analysis of E. faecium characterized particular clonal complexes (CCs) associated with humans (CC17, CC22 and CC94), swine (CC5), poultry (CC9) or veal calf (CC1).4,5 However, high rates of recombination in this species may lead to clones that diversify rapidly to produce complicated CCs, or even fail to correctly assign STs to CCs.6,7 In our original study, we identified two plasmid-mediated poxtA-carrying CC17 E. faecium isolates belonging to ST29. However, ST29 was clustered into the CC9 group in previous studies.4,8 By using 1640 MLST profiles obtained from the PubMLST database for this response letter, the majority of the STs (1065 STs) are grouped into a large complicated complex (ST17 as primary founder) containing the classical CC17, CC22, CC5, CC9 and CC1 (Figure 1). Interestingly, ST29 and ST123 are not clustered in CC9, but are rather more close to CC17 and CC5, showing the limitation of this typing method. Since ST29 showed four-loci identity to ST17 compared with three-loci identity to ST5, we classified them into CC17. A phylogenetic tree shown in Figure S1 in the original paper supported this point of view. However, due to the low number of strains used to build the phylogenetic tree, the analysis only classified the two ST29 strains QF25-1 and WZ27-2 into hospital-associated clade A, but was unable to further separate them into A1 and A2 subpopulations. For this response letter, we built a core-genome tree using a larger number (n = 93) of genomes from previous studies.9,10 The analysis showed that the two ST29 strains clustered together with ST151, ST437, ST66 and ST210 strains (Figure 2). Among them, ST437, ST66 and ST210 strains were related to the ST117 complex (Figure 1) and were hospital associated, but classified into the clade A2 subpopulation.9 However, in the present phylogenetic tree (Figure 2), the two ST29 strains seem to have evolved as a new subgroup ST123-ST29 complex that belongs to none of the CC17, CC5 and CC9 strains within subclade A2 (Figure 1). Although the two strains did not belong to the hospital-adapted CC17 clones, a risk is still raised because the strains of ST29 and ST57 [single-locus variant (SLV) of ST29] were also reported to be isolated from people in the community, from chickens and from pigs in Brazil, France, Australia and Belgium.8 Hence, the ability of this lineage of strains to colonize both humans and animals might increase the chance to access other enterococci and other Gram-positive bacteria, posing an increased risk of horizontal transfer of poxtA by plasmids.
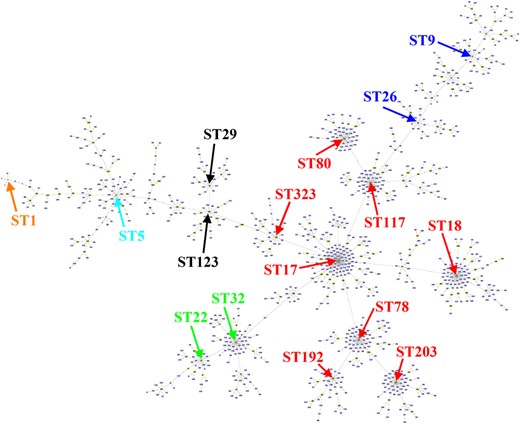
Clustering of E. faecium STs at the SLV level using goeBURST. A total of 1640 MLST profiles were obtained from the PubMLST database and 1065 STs are grouped into a large complex group (ST17 as primary founder). Each ST is represented as a node and lines connect SLVs. Major STs previously identified as CC1, CC5, CC9, CC17 and CC22 are shown in orange, light blue, blue, red and green, respectively. ST29 and ST123 didn’t belong to any of the CC17, CC5 and CC9 groups. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
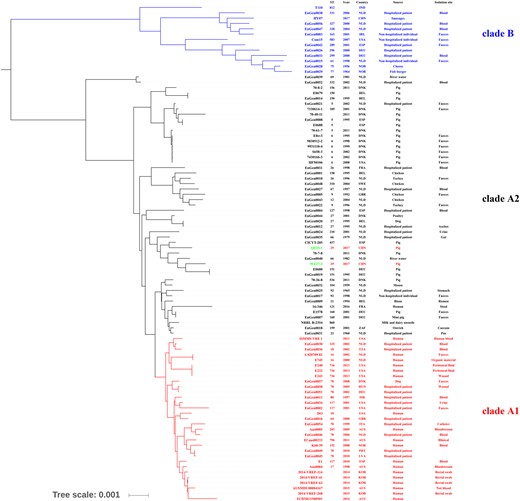
Core-genome tree built from E. faecium isolates QF25-1 and WZ27-2 along with publicly available genomic data for 91 E. faecium. ClustalW was used to align the genome sequences under default parameters and the alignment was checked manually. Maximum-likelihood (ML) methods were performed for the genome-wide phylogenetic analyses using PhyML 3.0. Nucleotide substitution model selection was estimated with jModelTest 2.1.10 and Smart Model Selection in PhyML 3.0. The model GTR+G was selected for ML analyses with 1000 bootstrap replicates to calculate the bootstrap values of the topology. The results were treated with iTOL 3.4.3 and visualized by FigTree with a midpoint root. AUS, Australia; BEL, Belgium; CHN, China; DEU, Germany; DNK, Denmark; ESP, Spain; FRA, France; GBR, Great Britain; HUN, Hungary; IND, India; IRL, Ireland; ISR, Israel; ITA, Italy; KOR, South Korea; LVA, Latvia; NLD, Netherlands; NOR, Norway; PRT, Portugal; SWE, Sweden; TZA, Tanzania; USA, United States of America; ZAF, South Africa. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In addition, please see our associated erratum.11
Funding
This work was supported by the National Key R&D Program of China (2018YFD0500300), the National Natural Science Foundation of China (31572567 and 31702292), the Natural Science Foundation of Jiangsu Province (BK20170710), the China Postdoctoral Science Foundation (2017M611841 and 2018T110515) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Transparency declarations
None to declare.
References
Author notes
J.H. and M.W. contributed equally to this work.



