-
PDF
- Split View
-
Views
-
Cite
Cite
Jordi van Gestel, Franz J Weissing, Oscar P Kuipers, Ákos T Kovács, Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms, The ISME Journal, Volume 8, Issue 10, October 2014, Pages 2069–2079, https://doi.org/10.1038/ismej.2014.52
Close - Share Icon Share
Abstract
In nature, most bacteria live in surface-attached sedentary communities known as biofilms. Biofilms are often studied with respect to bacterial interactions. Many cells inhabiting biofilms are assumed to express ‘cooperative traits’, like the secretion of extracellular polysaccharides (EPS). These traits can enhance biofilm-related properties, such as stress resilience or colony expansion, while being costly to the cells that express them. In well-mixed populations cooperation is difficult to achieve, because non-cooperative individuals can reap the benefits of cooperation without having to pay the costs. The physical process of biofilm growth can, however, result in the spatial segregation of cooperative from non-cooperative individuals. This segregation can prevent non-cooperative cells from exploiting cooperative neighbors. Here we examine the interaction between spatial pattern formation and cooperation in Bacillus subtilis biofilms. We show, experimentally and by mathematical modeling, that the density of cells at the onset of biofilm growth affects pattern formation during biofilm growth. At low initial cell densities, co-cultured strains strongly segregate in space, whereas spatial segregation does not occur at high initial cell densities. As a consequence, EPS-producing cells have a competitive advantage over non-cooperative mutants when biofilms are initiated at a low density of founder cells, whereas EPS-deficient cells have an advantage at high cell densities. These results underline the importance of spatial pattern formation for competition among bacterial strains and the evolution of microbial cooperation.
Introduction
Bacterial cells commonly live in sessile communities known as biofilms (Davey and O’Toole, 2000; Vlamakis et al., 2013). Biofilms are surface-associated aggregations of cells that are surrounded by adhesive extracellular polysaccharides (EPS) (Davey and O’Toole, 2000). Over the past decades, research on bacterial biofilms flourished, partly because of its relevance for medical and industrial problems (Stewart and Costerton, 2001; Davies, 2003; Hall-Stoodley et al., 2004). Biofilm formation is often characterized by properties such as cell-to-cell communication (Davies et al., 1998; Lopez et al., 2009a, 2009b), cell differentiation (Sauer et al., 2002; Lopez et al., 2009c; Lopez and Kolter, 2010) and pattern formation (Branda et al., 2001; Vlamakis et al., 2008; Lopez et al., 2009d; Asally et al., 2012; Kobayashi and Iwano, 2012; Kovács et al., 2012; Dietrich et al., 2013; Vlamakis et al., 2013). Since similar properties are seen in multicellular development, biofilm formation has been viewed by some as a developmental process (Shapiro, 1988, 1998; O’Toole et al., 2000; Bonner, 2001; Kaiser, 2001; Stoodley et al., 2002; Wolpert et al., 2002; Aguilar et al., 2007; Monds and O’Toole, 2009). Besides this analogy, it is known that many cells inhabiting the biofilm secrete products that can protect themselves against environmental stress or contribute to biofilm growth (Costerton et al., 1995; Davey and O’Toole, 2000; Stewart and Costerton, 2001). These can be digestive enzymes, surfactants or structural components like EPS (Costerton et al., 1995; Davey and O’Toole, 2000; Lemon et al., 2008; Lopez et al., 2009c; Marvasi et al., 2010). When these products are costly to produce, a Darwinian dilemma emerges (Nowak, 2006; West et al., 2006). Why should a bacterial cell produce a ‘public good’ (West et al., 2006, 2007) if competing non-producing cells can benefit from this product without having to pay the fitness costs associated with the production of this good?
The question of how cooperation (for example, the costly production of a public good) can evolve in a situation where non-cooperative individuals can reap the benefits without contributing to the costs of cooperation has received a lot of attention in evolutionary biology (Crespi, 2001; Travisano and Velicer, 2004; Kreft, 2004b; Nowak, 2006; West et al., 2006, 2007). Cooperation can only be evolutionarily stable if mechanisms exist that channel the benefits of cooperation to those individuals (or lineages) that contribute to cooperation. Mechanisms leading to a structuring of the population can have such an effect (Nowak and Sigmund, 1992; Nowak et al., 2010). If, for example, cell lineages segregate in space, cooperative cells may find themselves surrounded by other cooperators, while non-cooperative cells are surrounded by non-cooperators. As shown in many theoretical and empirical studies, such assortment may overcome the problem of exploitation and allow the evolution of cooperation (Nowak et al., 1994; Nowak, 2006; West et al., 2006, 2007; Fletcher and Doebeli, 2009; Nowak et al., 2010). In microbiology, these general insights are particularly relevant for biofilms. For example, in surface-attached colonies cell division is often restricted to the edge, since cells at the edge of a colony can access more nutrients than cells in the center. As a consequence, growth is characterized by expanding frontiers of dividing cells (Hallatschek et al., 2007; Korolev et al., 2011). It has been shown—both theoretically and experimentally—that this physical process of biofilm growth can result in strong spatial segregation (Kreft et al., 2001; Kreft and Wimpenny, 2001; Kreft, 2004a, 2004b; Hallatschek et al., 2007; Nadell et al., 2010), which potentially favors the evolution of cooperation (Nadell et al., 2010, 2013; Momeni et al., 2013a).
Here we study the interaction between spatial pattern formation and cooperation in architecturally complex surface-attached colonies of Bacillus subtilis. We show, both experimentally and by mathematical modeling, that colony growth can result in spatial segregation of lineages, and that the degree of segregation is strongly related to the initial density of founder cells. By manipulating the initial cell density, we can therefore examine how spatial pattern formation affects the competitive relationships between lineages that differ in the production of EPS. EPS is a major matrix component and is essential for any form of biofilm formation in B. subtilis (Branda et al., 2001, 2005; Marvasi et al., 2010; Vlamakis et al., 2013). In line with other researchers (Rainey and Rainey, 2003; Nadell and Bassler, 2011), we will argue that EPS qualifies as a ‘public good’ and that EPS production may therefore be viewed as a cooperative trait. The presence of EPS in the local environment does indeed provide various benefits, such as a tighter adhesion to the surface, an improved resistance against antimicrobial compounds and an enhanced surface spreading (Stewart and Costerton, 2001; Marvasi et al., 2010; Seminara et al., 2012; Beauregard et al., 2013). On the other hand, EPS production is costly in that it reduces individual fitness. We will show this by means of competition experiments where EPS-producing and EPS-deficient strains are grown under well-mixed conditions. Next, we will report on similar competition experiments with surface-attached colonies, which were initiated with different densities of founder cells. Finally, we make use of an engineered strain with inducible EPS production, in order to study the interaction between spatial pattern formation and cooperation by varying both the initial cell densities and the amount of EPS production along continuous scales.
Materials and methods
Strains
The B. subtilis epsG knockout strain, eps−, and the IPTG-inducible EPS-producing strain (IPTG=isopropyl β-d-1-thiogalactopyranoside), epsin, were obtained via transformation of a B. subitilis 168 biofilm-proficient lab strain (Kovács and Kuipers, 2011) with genomic DNA originating from B. subtilis NRS1502 and NRS186, respectively. NRS1502 and NRS186 strains (Verhamme et al., 2009) were kindly provided by N. Stanley-Wall. Plasmid phymKATE (encoding for the red-fluorescent protein) and phyGFP (encoding for the green-fluorescent protein) were constructed by replacing the rrnB promoter in front of the fluorescent protein coding genes with the hyper-spank promoter. The hyper-spank promoter was PCR-amplified from pDR111 (D Rudner) with oHYSPANK1 (5′-CACGGAATTCAAACGAAAGGCTCAGTC-3′) and oHYSPANK2 (5′-CGACGCTAGC CATTTCCTCTCCTCCTTAAGC-3′) primers, cleaved with EcoRI and NheI, and ligated into pGFPrrnB (Veening et al., 2009) and pmKATE2rrnB (Genbank Accession number: KF245454) vectors digested with the same restriction enzymes. The resulting constructs express the reporter genes, the red- or green-fluorescent protein, constitutively in B. subtilis due to the lack of the lacI gene in the integrated cassette. The constructs were introduced into B. subtilis strains at the amyE locus. The fluorescent reporters were integrated in eps+, eps− and epsin strains, which allowed us to localize and quantify them in mixed biofilms. Planktonic cells were grown overnight in 3 ml LB broth at 37 °C under constant shaking (200 r.p.m.) and used for further experiments.
Medium and culture conditions
The competition experiments to assess the costs of EPS production were performed in 10 ml of liquid 2 × SG medium (see Kobayashi, 2007), which is a nutrient-rich growth medium. Strains were competed for 5 sequential days, by consecutive inoculation of 50 μl grown culture in 10 ml fresh medium each day (shaken conditions at 37 °C). Colony counts are performed on antibiotic-free and 5 μg ml−1 chloramphenicol or 100 μg ml−1 spectinomycin containing LB plates for epsin or epsG strains, respectively. For biofilm growth, plates were prepared a day before inoculation. Twenty-five milliliters of 2 × SG (1.5% agar) was poured in a Petri dish and left on the bench (without piling them up) for ∼16 h before inoculation. For each biofilm, 0.5 μl of cell culture was spotted on the prepared plate, after which the inoculum was allowed to dry for 15 min. The inoculum size was about 2 mm in diameter. We used five dilution levels of the inoculum for the experiments: 10−1, 10−2, 10−3, 10−4 and 10−5. These dilution levels correspond to approximately the following cell numbers: 105, 104, 103, 102 and 10. After the inoculum dried on the plate, the plates were incubated at 30 °C (humidity level 20–30%, if not otherwise indicated) for 72 h. The biofilms were destructed by shaking them in 1 ml PBS buffer (with 5 μm of d-tyrosine; Kolodkin-Gal et al., 2010) for 4 min in the bead-beater (without adding beads).
Microscopy pictures
Biofilms were imaged using an Olympus MVX10 macrozoom fluorescence microscope (Olympus Corporation, Tokyo, Japan) equipped with a PreciseExcite LED fluorescence illuminator (Olympus Corporation) (470 and 585 nm, for green and red fluorescence, respectively), GFP (U-MGFPHQ/XL) and dual (U-DM-FI/TX2/XL) filter sets, and an Olympus XM10 monochrome camera. Composite images were created by ImageJ (National Institute of Health, Bethesda, MD, USA). The images were processed and analyzed by MatLab R2012a and R version 2.15.1 (MathWorks, Natick, MA, USA).
Quantifying the degree of assortment
In case green- and red-fluorescent cells co-occur in the same biofilm, assortment is defined by the average frequency of red-fluorescent cells that surround a red-fluorescent focal cell minus the average frequency of red-fluorescent cells that surround a green-fluorescent focal cell. Each frequency was calculated within a limited radius surrounding the focal cell. The degree of assortment is a value between −1 and +1. When the degree of assortment is 1, green- and red-fluorescent cells completely segregate in space. When the degree of assortment is 0, green- and red-fluorescent cells are randomly mixed in space. When the degree of assortment is −1, green- and red-fluorescent cells are regularly spaced next to each another. Although the same assortment measure was used in the model and experiments, in the model assortment was calculated with respect to the cell, while for the experiments pixels from fluorescent images were used. The radius used to calculate the level of assortment is 5 grid units in the model and 50 pixels in the image. To ascertain that the radius size did not affect the qualitative outcome of our model and experiments, we examined alternative radius sizes as well. These alternative assortment radii are evaluated in the parameter sensitivity analysis in the Supplementary Information (see Supplementary Figures S3 and S4).
Data analysis
With the exception of a few cases mentioned below, we always applied a two-tailed unpaired t-test and a non-parametric Mann–Whitney U test to compare experimental groups. In all cases, the two tests gave equivalent results. We report the result of the more powerful t-test, unless Shapiro’s test of normality indicated that the experimental data did not follow a normal distribution. The relative fitness of a strain in competition with another strain, as shown in Figure 1, is determined using the frequency of the labeled strain at the onset and end of competition. The frequency of the labeled strain was determined using colony counts on selective (on which only the labeled strain can grow) and non-selective growth plates (see medium and culture conditions). The relative fitness is given by the change in frequency during competition:frequency at the end of competition over the frequency at the onset of competition. When the relative fitness is lesser than 1 the labeled strain has a fitness disadvantage; when it is equal to 1 there is no fitness difference between the competing strains; when it is higher than 1 the labeled strain has a fitness advantage. To test for the differences in relative fitness between the different competition experiments (Figure 1b), we applied Fisher’s exact test, after first having transformed the reported fitness values into the proportions obtained from colony counts. We used logistic regression to quantify and test the effect of IPTG concentration on the relative fitness of an IPTG-inducible EPS-producing strain (Figure 1c). Throughout, we report P-values rather than using arbitrary significance levels (like 0.05 or 0.01). This allows the reader to apply a correction for multiple testing (for example, a Bonferroni correction; Perneger, 1998) when this is considered necessary. All statistical analyses are performed using R version 2.15.1 (The R Foundation for Statistical Computing, www.r-project.org).
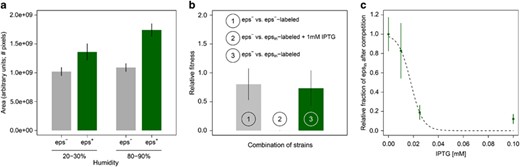
Benefits and costs of EPS production. (a) EPS production facilitates biofilm spreading: on average EPS-producing biofilms (eps+; n=9–10) are bigger than EPS-deficient mutant biofilms (eps−; n=9–10) both at low humidity levels (20%–30%) and at high humidity (80%–90%). (b) EPS-producing cells have a competitive disadvantage when grown in mixed culture with cells that do not produce EPS (n=3 per treatment): when an IPTG-inducible EPS-producing strain and an EPS-deficient mutant compete in the presence of IPTG there is a competitive advantage for the EPS mutant (i.e. the relative fitness of epsin is significantly lower than 1; see treatment 2). When none of the strains produces EPS, because either two EPS mutants compete (treatment 1) or because EPS production was not induced by IPTG (treatment 3), there is no significant change in the frequency of each competitive strain during competition (see Data analysis for a precise description of relative fitness). (c) The competitive disadvantage of the inducible EPS-producing strain epsin increases with the concentration of the inducer IPTG (n=3 per treatment). Bars and dots correspond to the mean and the vertical lines show the associated standard deviation. The dashed line denotes the logistic regression line (y=1/(1+ea+b·x): a=−3.7±1.2 (s.e.), Pa=0.01, b=206±61, Pb=7 × 10−3).
Results
Costly extracellular polysaccharide production facilitates biofilm spreading
Seminara et al. (2012) showed that exopolysaccharide (EPS) production facilitates biofilm spreading via osmotic gradient pressures in a wild isolate strain of B. subtilis. In contrast to Seminara et al. (2012) who used a wild isolate, we use B. subtilis strain 168, a biofilm-proficient lab strain cultivated for many decades under laboratory conditions. Lab strains often experience trait loss due to domestication (McLoon et al., 2011). Therefore, we first examined if the results of Seminara et al. (2012) could be reproduced with our lab strain. Figure 1a shows that EPS facilitates spreading in our lab strain as well (comparing eps− and eps+: low humidity t=−6.37, df=16, P<10−5; high humidity t=−16.10, df=15, P<10−10): the biofilms of EPS-producing strain (eps+; wild type (wt) strain with a functioning eps operon) are larger than those of the EPS mutant (eps−; mutant strain with a genetic deletion in the eps operon disenabling EPS production). Furthermore, the sizes of both eps+ and eps− biofilms depend on the humidity level (comparing high and low humidity: eps+: t=−6.65, df=17, P<10−5; eps−: t=−2.12, df=17, P=0.049). The size difference between eps− and eps+ biofilms is larger for the higher humidity level (Figure 1a).
To investigate whether fitness costs are associated with EPS production, we conducted competition experiments where an EPS-deficient strain, eps−, was grown together with an IPTG-inducible EPS-producing strain, epsin, in a nutrient-rich liquid culture for 5 days (see Materials and methods). epsin increases EPS production in response to higher concentrations of IPTG that can be supplemented to the growth medium. Thereby, we can control EPS production. Furthermore, by using IPTG to induce EPS production we can make sure that all cells of epsin produce EPS, while in the wt only a fraction does so (Vlamakis et al., 2013). In the absence of the inducer, a labeled epsin strain grew as well in competition with eps− cells, as a labeled eps− strain did under the same condition (Figure 1b, treatments 1 and 3). In the presence of the inducer, however, the labeled epsin strain was outcompeted by the eps− cells (Figure 1b). Apparently, the induced EPS production led to competitive disadvantage of the IPTG-inducible EPS-producing strain (comparison of the relative fitness of IPTG-inducible EPS-producing strain in the presence and absence of IPTG: Fisher’s exact test, P<2.2 × 10−12). This disadvantage is closely related to the concentration of IPTG in the medium (Figure 1c): if more IPTG is present in the medium epsin cells produce more EPS, which makes them less competitive against eps− cells (logistic regression, slope=206±61 (SE), P=7 × 10−3). In solid medium, adding 0.025 mm IPTG to the substrate results in epsin biofilms that are indistinguishable from that of eps+ (Figure 4e). Similar results were obtained when competing eps− and wt strains under the same conditions: the eps− strain outcompeted the EPS-producing wt strain (U=0, P=0.03, n=4; data not shown). Thus, in line with the findings in other species (Rainey and Rainey, 2003; Nadell and Bassler, 2011), EPS functions as a ‘common good’ that is costly to produce and facilitates biofilm spreading (Seminara et al., 2012).
Density of founder cells affects spatial pattern formation
Earlier work has shown that spatial segregation can arise spontaneously during biofilm growth, as a consequence of diffusion-limited growth (Kreft and Wimpenny, 2001; Kreft, 2004a; Nadell et al., 2010, 2013; Korolev et al., 2011; Nadell and Bassler, 2011). However, when we mixed two eps+B. subtilis strains, labeled with a green- (GFP) and red-fluorescent protein (RFP), no apparent spatial segregation emerged during biofilm growth (movie S2). These results were obtained under lab conditions that are typically used for culturing biofilms. That is, biofilms were initiated by spotting a small (0.5–2 μl) inoculum droplet containing both strains on a solid growth medium. This droplet often contains a high density of cells. Under natural conditions, it seems unlikely that many cells simultaneously disperse and, subsequently, colonize the same patch.
To investigate whether the initial cell density could affect spatial pattern formation, we first developed an individual-based model allowing the simulation of biofilm growth. Inspired by earlier models (Kreft and Wimpenny, 2001; Kreft et al., 2001; Kreft, 2004a; Xavier and Foster, 2007; Nadell et al., 2010), our model assumes that cells are placed in a two-dimensional space where they can move around by pushing each other away (see Supplementary Information for model description). At every time step, cells consume nutrients that are locally available. After having acquired sufficient nutrients, cells divide, thereby pushing aside the surrounding cells (Supplementary Figure S1). In this way, the biofilm spreads and spatial segregation can potentially emerge (Supplementary Figure S2 and Supplementary movie S1). The simulations clearly reveal that the degree of spatial assortment is closely related to the density of founder cells; the fewer the number of founders, the higher the degree of assortment (Figure 2; Supplementary Figures S3, S5 and S6). At the onset of biofilm growth, founder cells are distributed randomly and separated from each other, where the degree of separation is highest at low initial densities. By means of cell division, the founder cells grow into small cell clusters that only start to merge when getting into contact; getting into contact takes longer when the initial distance between founder cells is larger. Accordingly, biofilms started by a small number of founders will be structured much more in the initial phase of biofilm growth than biofilms started with a high density of founders. At later stages, the differences in spatial patterning might become smaller due to the dynamics of biofilm growth (Nadell et al., 2010). However, since in our model biofilms grow for a limited time (as is the case for the colony biofilms we studied in the lab), the initial signature of spatial patterning is still visible in the mature biofilm.
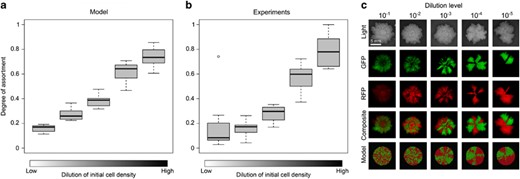
Effect of initial cell density on the spatial segregation of two co-cultured strains in a biofilm. (a) Simulation results. Ten replicate simulations were initiated with 10, 20, 50, 100 or 250 founder cells, respectively. Half of the cells (and their descendants) were marked red and the other half were marked green. At the end of biofilm growth, the degree of assortment with respect to the red and green cells was determined (see Materials and methods). In the box-whisker plots, the median assortment level is indicated by a bold horizontal line, the interquartile range is indicated by the box, and the whiskers include all data except for the outliers. (b) Lab experiments. Biofilms were inoculated by two eps+ strains, each tagged with another fluorescent marker, but otherwise genetically identical, at various initial cell densities. Both strains were equally abundant in the inoculum. Five dilution levels were examined: 10−1, 10−2, 10−3, 10−4, 10−5. Biofilms grew for three days (n=8 per treatment), after which the degree of assortment was determined by analyzing their fluorescence patterns. (c) Visual impression of spatial segregation in biofilms initiated with various initial cell densities. From top to bottom: light images, green-fluorescence images, red-fluorescence images, composite images and simulation results. Dilution levels only apply to the biofilm images and not the simulation results. The scale bar is equal to 5 mm.
Motivated by the computer simulations, we repeated the experiment of growing GFP- and RFP-labeled cells in mixed cultures, but now we systematically varied the initial cell density by diluting the inoculum 10–105 times. Indeed, higher dilution levels and, therefore, lower densities of founder cells strongly increase the degree of assortment that emerges during biofilm growth (Figure 2).
Extracellular polysaccharide production results in competitive benefit under assortment
By manipulating initial densities, we can now examine the interaction between spatial pattern formation and cooperation. Again, we first addressed this question with our theoretical model. In the simulations, we start with a mixture of EPS-producing and EPS-deficient cells. EPS-producing cells allocate part of their nutrients to EPS production. As these nutrients are not available for cell division, it is costly to produce EPS. EPS is secreted in the environment and enhances biofilm spreading (see Materials and methods). Our model makes the assumption that EPS diffuses in space, and cells surrounding the EPS-producing cell can benefit from the EPS as well (see Supplementary Information for model description). The model therefore entails the same cooperative dilemma as described above. We varied the initial cell density and grew the biofilm for a fixed number of time steps. At high cell densities (that is, low initial cell dilution) the EPS-deficient strain outcompetes the EPS-producing strain, while at low cell densities (that is, high initial cell dilution) the opposite result was found (Figure 3a). The relationships between the initial cell density, spatial pattern formation and the competitive advantage of cooperation were consistent under various cost and benefit values of EPS production (Supplementary Figures S7, S8 and S9).
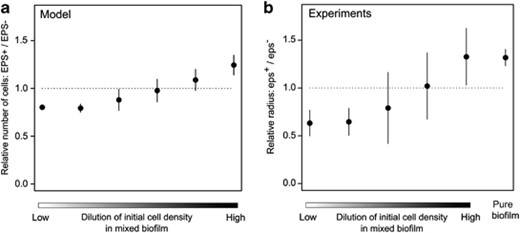
Effect of the density of founder cells on the relative fitness of cooperation. (a) Simulation results (n=10 per treatment). As in Figure 2a, we simulated the growth of two strains in mixed biofilms that were initialized with different numbers of founder cells. Now one strain (‘EPS+’) produced EPS while the other one (‘EPS−’) did not. Starting with equal proportions, the relative frequency of EPS+ cells was determined at the end of the growth period. At high founder densities (i.e. a low dilution level) the EPS− strain had a selective advantage, whereas the EPS+ strain had a selective advantage at low founder densities (i.e. a high dilution level). Dots and error bars represent, respectively, the mean and standard deviation. (b) Lab experiments (n=7–18 per treatment; in total 74 biofilms). At various dilution levels, biofilms were inoculated with eps− and eps+ strains that could be distinguished due to different fluorescent markers. After 3 days of growth, the fitness of each strain was quantified by the distance the strain had grown from the center of the biofilm towards the edge (i.e. the radius). In addition to the relative radius in various mixed cultures of eps− and eps+, also the quotients to the radii of two separate monocultures of eps− and eps+ pure biofilms are shown.
Comparable results were obtained in experiments when an eps− and an eps+ strain were mixed in equal proportion and diluted 10–105 times. For each biofilm, 0.5 μl inoculum was spotted on a nutrient-rich agar medium, after which the biofilm could grow for 3 days. As each strain was tagged with a different fluorescent protein, we could assess the surface occupancies of both strains, which correlate with their relative cell numbers (Supplementary Figure S10 and Aguilar et al., 2010). At low dilution levels, which corresponds to weak or no assortment, eps− has an advantage over eps+, while at high dilution levels the opposite pattern was found (Figure 3b and Supplementary Figure S11a). Thus, the assortative interactions that emerge from the spatial segregation of strains during biofilm growth can indeed result in a selective advantage for the expression of cooperative traits like EPS production. Furthermore, the fitness differences between eps− and eps+ at the strongest assortment level correspond to those of two entirely separated eps− and eps+ biofilms (Figure 3b). In other words, at the highest dilution level eps− cannot exploit eps+. To assure that cells did not assort upon contact in the inoculum (for example, eps+ cells might stick together), the same experiments were repeated with mixtures of eps− and epsin cells. Both strains behave in the same manner in the absence of IPTG. By adding 0.025 mm of IPTG to the agar medium, EPS production was only induced at the start of biofilm growth, after the inoculum dried on the plate. The competition between eps− and epsin gave the same results as that between eps+ and eps− (Supplementary Figure S11b).
Finally, we performed a titration experiment by competing epsin against eps+ at various IPTG concentrations and initial dilution levels. One would expect that epsin has a competitive benefit at low IPTG concentration, because it hardly produces EPS under these conditions in comparison to eps+. However, when increasing the IPTG concentration there should be a tipping point at which epsin and eps+ produce equally much EPSand therefore have the same fitness at all dilution levels. When increasing the IPTG concentration further, there are two possible scenarios. eps+ can benefit from the EPS produced by epsin at low dilution levels, because low levels of assortment allow for exploitation. Alternatively, the induction level in epsin can also become too strong, due to which the costs of EPS production outweigh the benefits. In that case, one would expect that the eps+ would outperform the epsin for all dilution levels. Indeed, our experiments confirm our expectations (Figure 4). epsin performs equally well as eps+ for an IPTG concentration of 0.025 mm. When more IPTG is added epsin overproduces EPS, which results in a fitness disadvantage over all initial dilution levels.
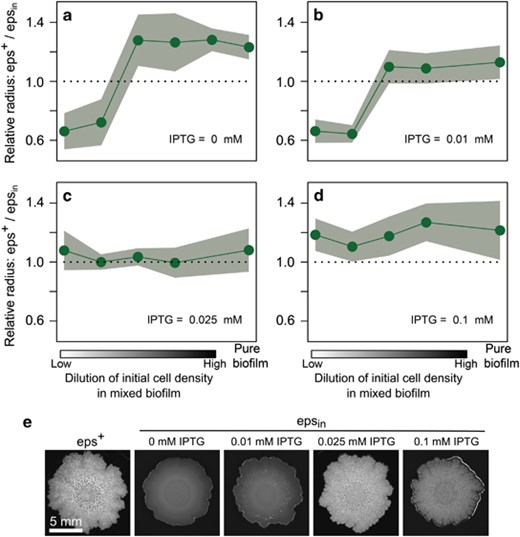
Effects of founder density and level of EPS production on the relative fitness of cooperation. As in Figure 3b, competition experiments were performed at various dilution levels, by co-culturing two strains in the same biofilm, which could be distinguished by different fluorescent markers (n=2–6 per treatment; in total 79 biofilms). After 3 days of biofilm growth, the fitness of each strain was again quantified by the distance the strain had spread from the center to the edge of the biofilm. The competition experiments were performed with epsin and eps+ at various IPTG concentrations: (a) 0 mm; (b) 0.01 mm; (c) 0.025 mm; (d) 0.1 mm. In the absence of IPTG, epsin behaves like eps−, thereby resembling the competition experiment in Figure 3b. By adding IPTG to the medium, epsin cells increase the level of EPS production and, hence, become more cooperative. (e) Light pictures of wild-type and epsin biofilms at various IPTG concentrations.
Discussion
Surfaces confer a great potential for spatial organization (Kolter and Greenberg, 2006). Through local interactions and guided by self-organizing principles, spatial patterns can emerge that affect ecological interactions (Costerton et al., 1995; Tolker-Nielsen and Molin, 2000; Hogeweg, 2007). Here we examined the role of spatial pattern formation on the competition between EPS-producing and EPS-deficient cells in B. subtilis biofilms. EPS production is a cooperative trait that can be exploited by mutants that are deficient in EPS production, because these avoid the costs of producing EPS and benefit from the EPS produced by others. We show that low densities of founder cells result in strong spatial segregation of co-cultured strains, while high densities do not. Spatial segregation results in assortative interactions (Kreft and Wimpenny, 2001; Kreft, 2004a; Nadell et al., 2010; Korolev et al., 2011, 2012; Nadell et al., 2013): cooperative EPS-producing cells are more likely to interact with other cooperators than EPS-deficient cells. Accordingly, cooperative EPS-producing cells only outcompete non-producing cells when biofilms are initiated with low cell densities.
Our results are in agreement with previous studies (Xavier and Foster, 2007; Nadell et al., 2010). For example, Rainey and Rainey (2003) showed that EPS production in Pseudomonas fluorescens can be exploited by non-producing cells when biofilms are grown in air–liquid interface. In contrast to the colony biofilms studied here, biofilms at the air–liquid interface are colonized by cells that swim to the surface and float via EPS production, which presumably does not allow for the same spatial segregation of EPS-producing and EPS-deficient cells (for example, Kobayashi, 2007). In contrast, Nadell and Bassler (2011) examined Vibrio cholerae biofilm formation in flow cells, where strong spatial structuring is apparent. They showed that under these conditions EPS-deficient cells were not able to exploit EPS-producing cells. Our results suggest that the outcomes of these previous studies reflect the opposite extremes of a continuum, in which the degree of assortment that emerges through spatial pattern formation influences the outcome of competition between EPS-producing and EPS-deficient cells.
Spatial pattern formation affects not only cooperation, but also a wide range of ecological interactions (Nielsen et al., 2000; Kerr et al., 2002, 2006; MacLean and Gudelj, 2006; Rickard et al., 2006; Hansen et al., 2007; Kim et al., 2008; Mitri et al., 2011; Elias and Banin, 2012; Momeni et al., 2013a; Momeni et al., 2013b; Allen et al., 2013). A recent study of Momeni et al., 2013a examined the opposite process: how do ecological interactions affect pattern formation? They showed that strong mutualistic interactions can stimulate partner intermixing in microbial communities. This and other studies argue for a reciprocal approach in which both sides of the feedback between spatial pattern formation and ecological interactions are examined (Klausen et al., 2003; Rietkerk and van de Koppel, 2008; Yang et al., 2009; De Jager et al., 2011; Momeni et al., 2013a).
In well-mixed lab conditions, the production of common goods, like EPS, are associated with a fitness disadvantage (West et al., 2007). However, in nature, growth might primarily occur on surfaces (Davey and O’Toole, 2000); as a consequence spatial assortment might be the rule rather than the exception. If that would be the case, cells can simply produce common goods without the risk of exploitation. Alternatively, it might also be plausible that common good production is favorable under some natural settings and not in others. In such case, conditional strategies might evolve that trigger common good production under environmental conditions that favor cooperation (that is, conditions that result in spatial segregation). EPS production is indeed known to be contingent on a number of environmental cues (Lemon et al., 2008; Lopez et al., 2009c; Lopez and Kolter, 2010; Lopez et al., 2010). For example, Beauregard et al., 2013 showed that B. subtilis biofilm formation on Arabidopsis thaliana roots can be triggered by plant polysaccharides. These polysaccharides are as well consumed by B. subtilis in order to produce EPS. EPS-deficient cells are unable to colonize plant roots (Chen et al., 2012; Beauregard et al., 2013). Interestingly, root colonization of these EPS-deficient cells could be recovered by co-culturing them with EPS-proficient cells (Beauregard et al., 2013), thereby showing that also in this experimental context EPS is being shared. These co-cultured strains do, however, show some spatial segregation on the plant root. It would, therefore, be interesting to examine if the EPS-deficient cells can actually exploit (that is, have a net competitive advantage over) EPS-producing cells when grown on plant roots.
Not all cells inhabiting B. subtilis biofilms are committed to EPS production (Vlamakis et al., 2008). In our measure for assortment, we did not account for this phenotypic heterogeneity. It would be interesting to examine how the fraction of EPS-producing cells in a wt biofilm distribute in space, because these might already form some pattern by themselves (Vlamakis et al., 2008). Another limitation of our assortment measure is that we considered only one spatial scale. The relevant spatial scale for measuring assortment depends on the scale at which cells interact and, hence, the cooperative trait that is considered. For example, when the benefits of cooperation are only shared locally, the degree of assortment at the local spatial scale is the only relevant measure, whether or not this correlates with the degree of assortment at the biofilm level. For many cooperative traits the relevant interaction scale is unknown. This is also the case for EPS production in B. subtilis. Even though the eps operon has extensively been studied (Branda et al., 2001, 2006; Guttenplan et al., 2010; McLoon et al., 2011; Terra et al., 2012), the functionality and structure of the polysaccharides that are produced as a result of the expression of this operon are still unknown (Marvasi et al., 2010; Vlamakis et al., 2013).
In line with the emerging field of micro-sociobiology (West et al., 2007; Velicer and Vos, 2009), we have interpreted the properties of microbial cultures from an evolutionary perspective. For this endeavor, it is crucial to map the fitness consequences of the traits under scrutiny in a reliable manner. In lab experiments, the relative growth rates of competing strains are often used as a proxy for fitness. Under natural conditions, however, other fitness components (such as starvation resistance or dispersal ability) might be of similar or even greater importance (for example, Rice et al., 2008). Unfortunately, it is largely unknown for most micro-organisms how ‘overall fitness’ (Metz, 2008) is related to the ecological setting and to environmental conditions. This also holds for the phenotype—biofilm spreading—that is examined in this study, especially if one considers the numerous other functions that have been assigned to EPS production: root colonization(Chen et al., 2012; Beauregard et al., 2013), supporting fruiting body formation (Branda et al., 2001), protection of the cells (for example reducing surface wetting Epstein et al., 2011), and regulating transcription (for example Aguilar et al., 2010; Kovács and Kuipers, 2011). To close this gap, there is a dire need for studies of cooperation in biofilms under natural conditions (Davey and O’Toole, 2000; Webb et al., 2003; Engelberg-Kulka et al., 2006; Earl et al., 2008; Kolter, 2010).
Acknowledgements
We thank N Stanley-Wall for the B. subtilis strains and JW Veening and R Detert for the plasmids with fluorescent reporters. JvG was supported by a Gratama grant and a NWO grant. ATK was supported by an ALW-NWO grant in Groningen, a Marie Curie Career Integration Grant and a startup fund from JSMC (Jena School for Microbial Communication) in Jena.
Competing interests
The authors declare no conflict of interest.
References
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information The online version of this article (doi:10.1038/ismej.2014.52) contains supplementary material, which is available to authorized users.



