-
PDF
- Split View
-
Views
-
Cite
Cite
Francesc Corbera-Rubio, Gerben R Stouten, Jantinus Bruins, Simon F Dost, Alexander Y Merkel, Simon Müller, Mark C M van Loosdrecht, Doris van Halem, Michele Laureni, “Candidatus Siderophilus nitratireducens”: a putative nap-dependent nitrate-reducing iron oxidizer within the new order Siderophiliales, ISME Communications, Volume 4, Issue 1, January 2024, ycae008, https://doi.org/10.1093/ismeco/ycae008
Close - Share Icon Share
Abstract
Nitrate leaching from agricultural soils is increasingly found in groundwater, a primary source of drinking water worldwide. This nitrate influx can potentially stimulate the biological oxidation of iron in anoxic groundwater reservoirs. Nitrate-dependent iron-oxidizing (NDFO) bacteria have been extensively studied in laboratory settings, yet their ecophysiology in natural environments remains largely unknown. To this end, we established a pilot-scale filter on nitrate-rich groundwater to elucidate the structure and metabolism of nitrate-reducing iron-oxidizing microbiomes under oligotrophic conditions mimicking natural groundwaters. The enriched community stoichiometrically removed iron and nitrate consistently with the NDFO metabolism. Genome-resolved metagenomics revealed the underlying metabolic network between the dominant iron-dependent denitrifying autotrophs and the less abundant organoheterotrophs. The most abundant genome belonged to a new Candidate order, named Siderophiliales. This new species, “Candidatus Siderophilus nitratireducens,” carries genes central genes to iron oxidation (cytochrome c cyc2), carbon fixation (rbc), and for the sole periplasmic nitrate reductase (nap). Using thermodynamics, we demonstrate that iron oxidation coupled to nap based dissimilatory reduction of nitrate to nitrite is energetically favorable under realistic Fe3+/Fe2+ and NO3−/NO2− concentration ratios. Ultimately, by bridging the gap between laboratory investigations and nitrate real-world conditions, this study provides insights into the intricate interplay between nitrate and iron in groundwater ecosystems, and expands our understanding of NDFOs taxonomic diversity and ecological role.
Introduction
Globally, approximately one-third of the nitrogen applied to agricultural soils is lost via leaching to the surrounding waterbodies [1]. This has led to elevated nitrate (NO3−) levels in anoxic groundwaters, a primary source of drinking water worldwide [2]. Owing to population growth and agriculture intensification, nitrate concentrations in subsurface waters are expected to continue increasing [3]. Besides its direct impact on human health [4], nitrate can significantly alter the biogeochemistry of groundwater reservoirs [5]. Nitrate promotes the oxidation of sulfide and in particular of iron (Fe) – the most prevalent groundwater contaminant – leading to the formation of oxides with high adsorption capacity and the emission of greenhouse gases [6]. Despite these implications, the consequences of nitrate–iron interactions on ecosystems and drinking water production systems remain largely unexplored. A detailed understanding of the underlying principles is paramount for anticipating and mitigating current and future challenges, as well as for exploring potential synergies and biotechnological opportunities.
Nitrate-dependent iron-oxidizing (NDFO) bacteria, also referred to as nitrate-reducing iron-oxidizers (NRFO) [7, 8], couple the anoxic reduction of nitrate to the oxidation of Fe2+ (eq. 1). Since their discovery in 1996 by Straub et al. [9], NDFO microorganisms have been the focus of extensive research both in pure and mixed cultures (reviewed in [10]), and several complete genomes are already publicly available [11, 12]. The metabolic versatility of NDFO bacteria spans from lithoautotrophic to mixotrophic growth [10], to partial denitrification using nitric oxide (NO) [13] and nitrous oxide (N2O) [11] as terminal electron acceptors. At the same time, due to the inherently low energetic yield of iron oxidation, NDFO bacteria live close to the thermodynamic edge [14]. Their fitness is highly dependent on environmental factors such as substrate and product availability, pH and temperature [15]. Chemical reactions – such as the quasi-instantaneous precipitation of the biologically formed Fe3+ − can play a pivotal role by modulating iron and nitrogen concentrations [16]. However, our current understanding is largely based on laboratory settings, and does not necessarily reflect the complexity of natural and engineered ecosystems where several (a)biotic reactions occur simultaneously at temperatures significantly lower than tested to date [17].
To address these knowledge gaps, we established a pilot-scale filter on anoxic groundwater containing both Fe2+ and NO3−. The emulated groundwater conditions allowed for the establishment of a microbial enrichment that simultaneously removed Fe2+ and NO3−. In depth metagenomic analysis of the steady-state community revealed a new order-level NDFO lineage, deepening our understanding of their taxonomic diversity and ecological roles. Overall, our study bridges the gap between laboratory studies and real-world conditions, and offers a nuanced view on the intricate interplay between nitrate and iron in groundwater ecosystems.
Results
Nitrate-dependent iron removal irrespective of the limiting nutrient
A pilot-scale, granular activated-carbon filter was fed with nitrate-rich anoxic groundwater for 120 days. Stable anoxic nitrate and iron removals were achieved after less than 3 weeks of operation and maintained for over 100 days (Fig. 1). With nitrate as the limiting nutrient at both groundwater (8.9 ± 2.8 μM) and nitrate-amended concentrations (13.5 ± 1.5 and 20.2 ± 2.4 μM), effluent nitrate concentrations were consistently below detection limit (1 μM). Throughout the nitrate-limiting period, NO3− and Fe2+ were consumed at a 7.1 ± 1.4 Fe2+:NO3− molar ratio (Fig. S3). Oxygen was always below the quantification limit of 3 μM. Roughly 80 μC-mol dissolved organic carbon (DOC·l−1) was consistently removed from the influent, likely due to Fe2+–DOC complexes formation owing to the non-biodegradable nature of organic matter in the groundwater matrix (<1.2 μC-mol·l−1 assimilable organic matter). Ammonia consumption was negligible (<0.1 μM). Effluent nitrite concentrations stayed below the detection limit (<0.2 μM), while other denitrification intermediates – nitric oxide and nitrous oxide – were not measured. The observed consistent stoichiometric coupling between nitrate and iron removals strongly suggests Fe2+ oxidation to be primarily driven by microbial nitrate-reducing iron oxidation.
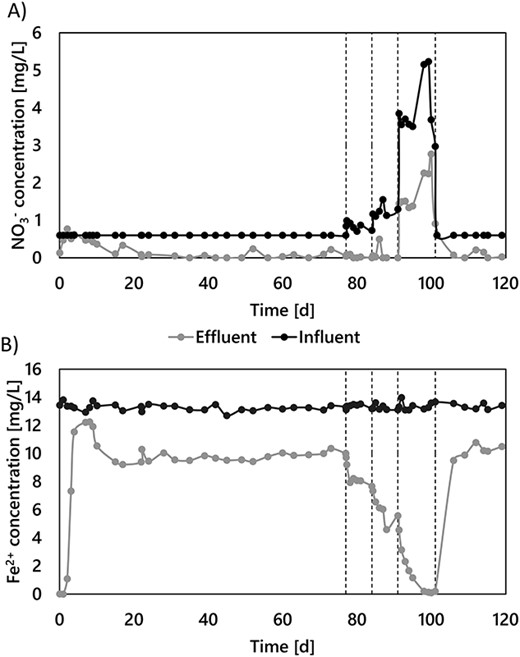
Simultaneous NO3− (A) and Fe2+(B) removals in the groundwater-fed pilot-scale filter during the 120 days of continuous operation. The groundwater Fe2+ concentration was constant throughout the experiment (236 ± 4 μM). NO3− was dosed in the influent to increase the natural groundwater concentration in steps from 8.1 ± 2.1 to 20.2 ± 2.4 μM (NO3− limitation), and up to 83.8 ± 0.6 μM (Fe2+ limitation). Fe2+ and NO3− were proportionally removed throughout the whole experiment, regardless of the limiting nutrient (see Fig. SI 3).
Microbial community dominated by iron oxidizers and denitrifiers
Metagenomic DNA sequencing yielded a total of 107 512 and 8 754 261 quality filtered short and long reads, respectively. After assembly and polishing, this resulted in 19 127 contigs with an N50 value of 15 927. Contigs binning resulted in 13 high (>90% completeness and <5% contamination; containing full-length 23S, 16S, and 5S ribosomal RNA genes and ≥18 transfer RNA genes) and medium (completeness >50% and contamination <10%) quality metagenome assembled genomes (MAGs) as defined by [18] with a relative abundance exceeding 0.5% of the quality filtered long reads. Collectively, these 13 most abundant genomes accounted for 66.9% of the total quality filtered reads, and belonged to four phyla: Proteobacteria (51.6%), Actinobacteria (8.3%), Bacteroidetes (5.6%), and Chloroflexi (1.4%) (Table 1). All genomes in the community contained at least one gene encoding for a denitrifying enzyme, and five featured the genetic potential for iron oxidation. Notably, all putative iron oxidizers also possessed the genetic repertoire for carbon fixation. The most abundant MAG (MAG.13), accounting for 19.3% of the community, could only be taxonomically classified at class level (Gammaproteobacteria). Given its high abundance and potential metabolic relevance, the taxonomy and metabolic potential of MAG.13 was further investigated (Table S1).
General characteristics of the MAGs recovered from the pilot-scale filter. The last three columns indicate the presence/absence of the essential genes for iron oxidation, denitrification, and carbon fixation via the reductive phosphate pentose phosphate cycle. Cov_il and cov_np are the coverage with Illumina short reads and Nanopore long reads, respectively.
| MAG ID . | Phyloegnetic affiliation . | Phylum . | Relative abundance (%) . | Scaffolds . | N50 . | GC (%) . | Completeness (%) . | Contamination (%) . | cov_Il . | cov_np . | Coding genes . | MAG quality . | Iron oxidationa . | Denitrificationb . | Carbon fixationc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAG.13 | **“Candidatus Siderophilus nitratireducens” | Proteobacteria | 19.3 | 9 | 18,97 007 | 56 | 98.59 | 1.73 | 298.27 | 300.86 | 3217 | High | Yes | NO3+ − > NO2− | Yes |
| MAG.26 | f__Gallionellaceae | Proteobacteria | 10.0 | 10 | 5,69 264 | 55 | 95.81 | 0.95 | 234.92 | 155.81 | 3749 | High | Yes | NO3+ − > NO; N2O - > N2 | Yes |
| MAG.18 | f__Anaeromyxobacteraceae | Proteobacteria | 8.6 | 21 | 5,18 414 | 73 | 98.06 | 0.65 | 186.12 | 131.88 | 4018 | High | No | N2O - > N2 | No |
| MAG.19 | g__Devosia | Proteobacteria | 6.5 | 27 | 41,38 465 | 64 | 99.28 | 0.14 | 24.53 | 100.33 | 4029 | High | No | NO2− - > NO | No |
| MAG.10 | f__Chitinophagaceae | Bacteroidetes | 5.6 | 4 | 36,46 631 | 40 | 97.54 | 0.9 | 304.83 | 87.01 | 3193 | High | No | NO - > N2 | No |
| MAG.03 | o__Nanopelagicales | Actinobactiria | 4.3 | 11 | 7,79 605 | 69 | 99 | 2.37 | 140.41 | 67.49 | 4065 | High | No | NO3− - > NO | No |
| MAG.27 | o__Nanopelagicales | Actinobactiria | 3.9 | 9 | 11,66 278 | 71 | 98.3 | 1.98 | 140.56 | 60.77 | 3910 | High | No | NO3− - > NO | No |
| MAG.34 | g__Gallionella | Proteobacteria | 3.5 | 112 | 46 607 | 57 | 95.91 | 3.25 | 64.39 | 52.65 | 2516 | High | Yes | NO3− - > NO | Yes |
| MAG.00 | o__Anaerolineales | Chloroflexi | 1.4 | 100 | 1,14 338 | 55 | 97.27 | 2.91 | 21.93 | 20.04 | 4828 | High | No | NO2− - > NO | No |
| MAG.08 | g__Rhodoferax | Proteobacteria | 1.2 | 121 | 69 171 | 63 | 88.95 | 2.37 | 33.64 | 17.42 | 3870 | Medium | No | NO3− - > NO | Yes |
| MAG.04 | f__Rhizobiaceae | Proteobacteria | 0.9 | 115 | 80 809 | 60 | 95.12 | 4.1 | 25.97 | 13.93 | 4768 | High | No | NO3− - > N2O | No |
| MAG.16 | f__Gallionellaceae | Proteobacteria | 0.9 | 165 | 27 226 | 56 | 88.51 | 3.94 | 52.76 | 13.55 | 3002 | Medium | Yes | NO3− - > N2O | Yes |
| MAG.29 | g__Rhizobacter | Proteobacteria | 0.7 | 257 | 25 511 | 66 | 94.54 | 4.54 | 21.73 | 9.94 | 4225 | Medium | Yes | NO3+ − > NO2− | Yes |
| MAG ID . | Phyloegnetic affiliation . | Phylum . | Relative abundance (%) . | Scaffolds . | N50 . | GC (%) . | Completeness (%) . | Contamination (%) . | cov_Il . | cov_np . | Coding genes . | MAG quality . | Iron oxidationa . | Denitrificationb . | Carbon fixationc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAG.13 | **“Candidatus Siderophilus nitratireducens” | Proteobacteria | 19.3 | 9 | 18,97 007 | 56 | 98.59 | 1.73 | 298.27 | 300.86 | 3217 | High | Yes | NO3+ − > NO2− | Yes |
| MAG.26 | f__Gallionellaceae | Proteobacteria | 10.0 | 10 | 5,69 264 | 55 | 95.81 | 0.95 | 234.92 | 155.81 | 3749 | High | Yes | NO3+ − > NO; N2O - > N2 | Yes |
| MAG.18 | f__Anaeromyxobacteraceae | Proteobacteria | 8.6 | 21 | 5,18 414 | 73 | 98.06 | 0.65 | 186.12 | 131.88 | 4018 | High | No | N2O - > N2 | No |
| MAG.19 | g__Devosia | Proteobacteria | 6.5 | 27 | 41,38 465 | 64 | 99.28 | 0.14 | 24.53 | 100.33 | 4029 | High | No | NO2− - > NO | No |
| MAG.10 | f__Chitinophagaceae | Bacteroidetes | 5.6 | 4 | 36,46 631 | 40 | 97.54 | 0.9 | 304.83 | 87.01 | 3193 | High | No | NO - > N2 | No |
| MAG.03 | o__Nanopelagicales | Actinobactiria | 4.3 | 11 | 7,79 605 | 69 | 99 | 2.37 | 140.41 | 67.49 | 4065 | High | No | NO3− - > NO | No |
| MAG.27 | o__Nanopelagicales | Actinobactiria | 3.9 | 9 | 11,66 278 | 71 | 98.3 | 1.98 | 140.56 | 60.77 | 3910 | High | No | NO3− - > NO | No |
| MAG.34 | g__Gallionella | Proteobacteria | 3.5 | 112 | 46 607 | 57 | 95.91 | 3.25 | 64.39 | 52.65 | 2516 | High | Yes | NO3− - > NO | Yes |
| MAG.00 | o__Anaerolineales | Chloroflexi | 1.4 | 100 | 1,14 338 | 55 | 97.27 | 2.91 | 21.93 | 20.04 | 4828 | High | No | NO2− - > NO | No |
| MAG.08 | g__Rhodoferax | Proteobacteria | 1.2 | 121 | 69 171 | 63 | 88.95 | 2.37 | 33.64 | 17.42 | 3870 | Medium | No | NO3− - > NO | Yes |
| MAG.04 | f__Rhizobiaceae | Proteobacteria | 0.9 | 115 | 80 809 | 60 | 95.12 | 4.1 | 25.97 | 13.93 | 4768 | High | No | NO3− - > N2O | No |
| MAG.16 | f__Gallionellaceae | Proteobacteria | 0.9 | 165 | 27 226 | 56 | 88.51 | 3.94 | 52.76 | 13.55 | 3002 | Medium | Yes | NO3− - > N2O | Yes |
| MAG.29 | g__Rhizobacter | Proteobacteria | 0.7 | 257 | 25 511 | 66 | 94.54 | 4.54 | 21.73 | 9.94 | 4225 | Medium | Yes | NO3+ − > NO2− | Yes |
Based on FeGenie [19]. “Yes” if either cyc2, cyc1, foxABC, foxEYZ, sulfocyanin, pioABC, or mtoAB are present.
Based on GhostKOALA [20] and the presence of napAB or narGHI (K02567, K02568, or K00370, K00371, K00374; NO3− → NO2−), nirK or nirS (K00368, K15864; NO2− → NO), norBC (K04561, K02305; NO → N2O), nosZ (K00376; N2O → N2) from denitrification (M00529).
Based on GhostKOALA [20]. “Yes” if either rbcL (K01601) or rbcS (K01602) and prkB (K00855) from reductive phosphate pentose phosphate cycle (Calvin Cycle) (M00165).
**Initially classified as c_Gammaproteobacteria.
General characteristics of the MAGs recovered from the pilot-scale filter. The last three columns indicate the presence/absence of the essential genes for iron oxidation, denitrification, and carbon fixation via the reductive phosphate pentose phosphate cycle. Cov_il and cov_np are the coverage with Illumina short reads and Nanopore long reads, respectively.
| MAG ID . | Phyloegnetic affiliation . | Phylum . | Relative abundance (%) . | Scaffolds . | N50 . | GC (%) . | Completeness (%) . | Contamination (%) . | cov_Il . | cov_np . | Coding genes . | MAG quality . | Iron oxidationa . | Denitrificationb . | Carbon fixationc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAG.13 | **“Candidatus Siderophilus nitratireducens” | Proteobacteria | 19.3 | 9 | 18,97 007 | 56 | 98.59 | 1.73 | 298.27 | 300.86 | 3217 | High | Yes | NO3+ − > NO2− | Yes |
| MAG.26 | f__Gallionellaceae | Proteobacteria | 10.0 | 10 | 5,69 264 | 55 | 95.81 | 0.95 | 234.92 | 155.81 | 3749 | High | Yes | NO3+ − > NO; N2O - > N2 | Yes |
| MAG.18 | f__Anaeromyxobacteraceae | Proteobacteria | 8.6 | 21 | 5,18 414 | 73 | 98.06 | 0.65 | 186.12 | 131.88 | 4018 | High | No | N2O - > N2 | No |
| MAG.19 | g__Devosia | Proteobacteria | 6.5 | 27 | 41,38 465 | 64 | 99.28 | 0.14 | 24.53 | 100.33 | 4029 | High | No | NO2− - > NO | No |
| MAG.10 | f__Chitinophagaceae | Bacteroidetes | 5.6 | 4 | 36,46 631 | 40 | 97.54 | 0.9 | 304.83 | 87.01 | 3193 | High | No | NO - > N2 | No |
| MAG.03 | o__Nanopelagicales | Actinobactiria | 4.3 | 11 | 7,79 605 | 69 | 99 | 2.37 | 140.41 | 67.49 | 4065 | High | No | NO3− - > NO | No |
| MAG.27 | o__Nanopelagicales | Actinobactiria | 3.9 | 9 | 11,66 278 | 71 | 98.3 | 1.98 | 140.56 | 60.77 | 3910 | High | No | NO3− - > NO | No |
| MAG.34 | g__Gallionella | Proteobacteria | 3.5 | 112 | 46 607 | 57 | 95.91 | 3.25 | 64.39 | 52.65 | 2516 | High | Yes | NO3− - > NO | Yes |
| MAG.00 | o__Anaerolineales | Chloroflexi | 1.4 | 100 | 1,14 338 | 55 | 97.27 | 2.91 | 21.93 | 20.04 | 4828 | High | No | NO2− - > NO | No |
| MAG.08 | g__Rhodoferax | Proteobacteria | 1.2 | 121 | 69 171 | 63 | 88.95 | 2.37 | 33.64 | 17.42 | 3870 | Medium | No | NO3− - > NO | Yes |
| MAG.04 | f__Rhizobiaceae | Proteobacteria | 0.9 | 115 | 80 809 | 60 | 95.12 | 4.1 | 25.97 | 13.93 | 4768 | High | No | NO3− - > N2O | No |
| MAG.16 | f__Gallionellaceae | Proteobacteria | 0.9 | 165 | 27 226 | 56 | 88.51 | 3.94 | 52.76 | 13.55 | 3002 | Medium | Yes | NO3− - > N2O | Yes |
| MAG.29 | g__Rhizobacter | Proteobacteria | 0.7 | 257 | 25 511 | 66 | 94.54 | 4.54 | 21.73 | 9.94 | 4225 | Medium | Yes | NO3+ − > NO2− | Yes |
| MAG ID . | Phyloegnetic affiliation . | Phylum . | Relative abundance (%) . | Scaffolds . | N50 . | GC (%) . | Completeness (%) . | Contamination (%) . | cov_Il . | cov_np . | Coding genes . | MAG quality . | Iron oxidationa . | Denitrificationb . | Carbon fixationc . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAG.13 | **“Candidatus Siderophilus nitratireducens” | Proteobacteria | 19.3 | 9 | 18,97 007 | 56 | 98.59 | 1.73 | 298.27 | 300.86 | 3217 | High | Yes | NO3+ − > NO2− | Yes |
| MAG.26 | f__Gallionellaceae | Proteobacteria | 10.0 | 10 | 5,69 264 | 55 | 95.81 | 0.95 | 234.92 | 155.81 | 3749 | High | Yes | NO3+ − > NO; N2O - > N2 | Yes |
| MAG.18 | f__Anaeromyxobacteraceae | Proteobacteria | 8.6 | 21 | 5,18 414 | 73 | 98.06 | 0.65 | 186.12 | 131.88 | 4018 | High | No | N2O - > N2 | No |
| MAG.19 | g__Devosia | Proteobacteria | 6.5 | 27 | 41,38 465 | 64 | 99.28 | 0.14 | 24.53 | 100.33 | 4029 | High | No | NO2− - > NO | No |
| MAG.10 | f__Chitinophagaceae | Bacteroidetes | 5.6 | 4 | 36,46 631 | 40 | 97.54 | 0.9 | 304.83 | 87.01 | 3193 | High | No | NO - > N2 | No |
| MAG.03 | o__Nanopelagicales | Actinobactiria | 4.3 | 11 | 7,79 605 | 69 | 99 | 2.37 | 140.41 | 67.49 | 4065 | High | No | NO3− - > NO | No |
| MAG.27 | o__Nanopelagicales | Actinobactiria | 3.9 | 9 | 11,66 278 | 71 | 98.3 | 1.98 | 140.56 | 60.77 | 3910 | High | No | NO3− - > NO | No |
| MAG.34 | g__Gallionella | Proteobacteria | 3.5 | 112 | 46 607 | 57 | 95.91 | 3.25 | 64.39 | 52.65 | 2516 | High | Yes | NO3− - > NO | Yes |
| MAG.00 | o__Anaerolineales | Chloroflexi | 1.4 | 100 | 1,14 338 | 55 | 97.27 | 2.91 | 21.93 | 20.04 | 4828 | High | No | NO2− - > NO | No |
| MAG.08 | g__Rhodoferax | Proteobacteria | 1.2 | 121 | 69 171 | 63 | 88.95 | 2.37 | 33.64 | 17.42 | 3870 | Medium | No | NO3− - > NO | Yes |
| MAG.04 | f__Rhizobiaceae | Proteobacteria | 0.9 | 115 | 80 809 | 60 | 95.12 | 4.1 | 25.97 | 13.93 | 4768 | High | No | NO3− - > N2O | No |
| MAG.16 | f__Gallionellaceae | Proteobacteria | 0.9 | 165 | 27 226 | 56 | 88.51 | 3.94 | 52.76 | 13.55 | 3002 | Medium | Yes | NO3− - > N2O | Yes |
| MAG.29 | g__Rhizobacter | Proteobacteria | 0.7 | 257 | 25 511 | 66 | 94.54 | 4.54 | 21.73 | 9.94 | 4225 | Medium | Yes | NO3+ − > NO2− | Yes |
Based on FeGenie [19]. “Yes” if either cyc2, cyc1, foxABC, foxEYZ, sulfocyanin, pioABC, or mtoAB are present.
Based on GhostKOALA [20] and the presence of napAB or narGHI (K02567, K02568, or K00370, K00371, K00374; NO3− → NO2−), nirK or nirS (K00368, K15864; NO2− → NO), norBC (K04561, K02305; NO → N2O), nosZ (K00376; N2O → N2) from denitrification (M00529).
Based on GhostKOALA [20]. “Yes” if either rbcL (K01601) or rbcS (K01602) and prkB (K00855) from reductive phosphate pentose phosphate cycle (Calvin Cycle) (M00165).
**Initially classified as c_Gammaproteobacteria.
“Candidatus Siderophilus nitratireducens” represents a new order within Gammaproteobacteria
Our phylogenomic analysis based on the concatenated amino acid sequences of 120 bacterial single copy conservative marker genes revealed that MAG.13 (98.6% completeness, 1.7% contamination) belongs to a bacterium forming a new order-level lineage Ga0077554 (GTDB release 08-RS214), within the class Gammaproteobacteria, with no known closely related pure-culture representatives (Fig. 2). We propose to name the new species “Candidatus Siderophilus nitratireducens” gen.nov., sp.nov., a member of the Candidate order and family Siderophiliales and Siderophiliaceae, respectively. This lineage, along with several other MAGs from similar groundwater habitats [21], is mostly related to lineages including lithoautotrophic sulfur-oxidizing bacteria from the genera Sulfuriflexus, Thioalbus, and the members of the order Thiohalomonadales, including Thiohalomonas, Sulfurivermis, and Thiohalophilus. Average nucleotide identity and in-silico calculated DNA–DNA hybridization comparison between “Ca. Siderophilus nitratireducens” and its closest relative, a MAG from a drinking water treatment plant (GCA_001464965.1), indicated that the two organisms belong to the same genus but different species (ANI = 89%, DDH = 36.6%).
![Phylogenetic position of the “Ca. Siderophilus nitratireducens” based on sequence analyses of concatenated alignment of 120 single-copy conserved bacterial protein markers [22] – taxonomic designations correspond to the Genome Taxonomy DataBase 207). The trees were built using IQ-TREE2 [23] with approximate likelihood-ratio test for branches [24]. Bootstrap consensus tree is shown with values above 90% placed at the nodes. Bar, 0.10 changes per position.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ismecommun/4/1/10.1093_ismeco_ycae008/2/m_ycae008f2.jpeg?Expires=1748136834&Signature=iO249mSn7CHgNTQrPLr~un4efee9~KM-PFyhW7KUf7JxdU9xPfh~K9oZjb~lKBk-QhHB3Hw1OMkiDr7frteLtlbTw0lG~BW2tbM1HJvB0VwdASv3XqGtUiTwPO09RxfkZYrCFHaRO5OBU4tfCS0S0ao6hJket9heVb1dgpYlelfIgSSwLcWGWhwbxsaJenduEzEYTmvFNkf748h88Uoqkr7syxgyR5V4VfR-3jbhyxgWOyjLVStYxz-zBSLeS3oDqEffGUBI0ZQtmfwa4bbmC580RG7uOsOC7bDs~t9Cg4H~V9VbrK7qsfzINf52eCu9dL8e58RwA4VxL2hUYwT7Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Phylogenetic position of the “Ca. Siderophilus nitratireducens” based on sequence analyses of concatenated alignment of 120 single-copy conserved bacterial protein markers [22] – taxonomic designations correspond to the Genome Taxonomy DataBase 207). The trees were built using IQ-TREE2 [23] with approximate likelihood-ratio test for branches [24]. Bootstrap consensus tree is shown with values above 90% placed at the nodes. Bar, 0.10 changes per position.
Autotrophy in “Ca. Siderophilus nitratireducens”
To resolve the main anabolic and catabolic pathways of “Ca. Siderophilus nitratireducens,” open reading frames (ORFs) were predicted and annotated (Table 2, detailed version in Table S1). The genome contains marker genes coding for two key proteins of autotrophic CO2 fixation via the reductive pentose phosphate (Calvin–Benson–Bassham; CBB) cycle, including the large and small subunits of the ribulose-1,5-bisphosphate carboxylase-oxygenase (rbcLS form I) and the phosphoribulokinase (prk). Genes encoding for carboxysomal shell proteins and carbonic anhydrase were also present, further supporting the inorganic carbon uptake ability of “Ca. Siderophilus nitratireducens”. The absence of phosphofructokinase (pfk) indicates a modified glycolytic pathway initiating at the glyceraldehyde 3-phosphate level. All tricarboxylic acid (TCA) cycle genes were identified except for fumarate hydratase (fh). However, the glyoxylate shunt enzymes malate synthase (glcB) and isocitrate lyase (aceA) were present. Taken together, these findings suggest the capability for full autotrophic growth of “Ca. Siderophilus nitratireducens.”
Key enzyme of the main catabolic and anabolic pathways of “Ca. Siderophilus nitratireducens.” “+” and “–“indicate presence or absence in the genome. CBB, Calvin–Benson–Bassham cycle; TCA, Tricarboxylic acid cycle/Krebs cycle.
| Electron acceptor . | Electron donor . | Carbon fixation . | Carbon metabolism . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element . | Key genes . | Presence . | Element . | Key genes . | Presence . | Pathway . | Key genes . | Presence . | Pathway . | Key genes . | Presence . |
| Nitrogen | napABCD | + | Iron | cyc2 | + | CBB | rbcLS form I | + | Gycolysis | pfk | − |
| narGHI | − | cyc1 | − | prk | + | gpml | + | ||||
| nirKS/norBC/nosZ | − | mtoAB | − | Carboxysome | + | TCA cycle | cs / gltA | + | |||
| Sulfur | aprAB | − | Sulfur | sqr | (+) | sucAB | + | ||||
| soxXABYZ(CD) | − | fccAB | − | fh | − | ||||||
| Oxygen | ccoNOP | + | mdh | + | |||||||
| qoxABCD | − | Glyxoylate shunt | glcB | + | |||||||
| cydABX | − | aceA | + | ||||||||
| Electron acceptor . | Electron donor . | Carbon fixation . | Carbon metabolism . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element . | Key genes . | Presence . | Element . | Key genes . | Presence . | Pathway . | Key genes . | Presence . | Pathway . | Key genes . | Presence . |
| Nitrogen | napABCD | + | Iron | cyc2 | + | CBB | rbcLS form I | + | Gycolysis | pfk | − |
| narGHI | − | cyc1 | − | prk | + | gpml | + | ||||
| nirKS/norBC/nosZ | − | mtoAB | − | Carboxysome | + | TCA cycle | cs / gltA | + | |||
| Sulfur | aprAB | − | Sulfur | sqr | (+) | sucAB | + | ||||
| soxXABYZ(CD) | − | fccAB | − | fh | − | ||||||
| Oxygen | ccoNOP | + | mdh | + | |||||||
| qoxABCD | − | Glyxoylate shunt | glcB | + | |||||||
| cydABX | − | aceA | + | ||||||||
Key enzyme of the main catabolic and anabolic pathways of “Ca. Siderophilus nitratireducens.” “+” and “–“indicate presence or absence in the genome. CBB, Calvin–Benson–Bassham cycle; TCA, Tricarboxylic acid cycle/Krebs cycle.
| Electron acceptor . | Electron donor . | Carbon fixation . | Carbon metabolism . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element . | Key genes . | Presence . | Element . | Key genes . | Presence . | Pathway . | Key genes . | Presence . | Pathway . | Key genes . | Presence . |
| Nitrogen | napABCD | + | Iron | cyc2 | + | CBB | rbcLS form I | + | Gycolysis | pfk | − |
| narGHI | − | cyc1 | − | prk | + | gpml | + | ||||
| nirKS/norBC/nosZ | − | mtoAB | − | Carboxysome | + | TCA cycle | cs / gltA | + | |||
| Sulfur | aprAB | − | Sulfur | sqr | (+) | sucAB | + | ||||
| soxXABYZ(CD) | − | fccAB | − | fh | − | ||||||
| Oxygen | ccoNOP | + | mdh | + | |||||||
| qoxABCD | − | Glyxoylate shunt | glcB | + | |||||||
| cydABX | − | aceA | + | ||||||||
| Electron acceptor . | Electron donor . | Carbon fixation . | Carbon metabolism . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element . | Key genes . | Presence . | Element . | Key genes . | Presence . | Pathway . | Key genes . | Presence . | Pathway . | Key genes . | Presence . |
| Nitrogen | napABCD | + | Iron | cyc2 | + | CBB | rbcLS form I | + | Gycolysis | pfk | − |
| narGHI | − | cyc1 | − | prk | + | gpml | + | ||||
| nirKS/norBC/nosZ | − | mtoAB | − | Carboxysome | + | TCA cycle | cs / gltA | + | |||
| Sulfur | aprAB | − | Sulfur | sqr | (+) | sucAB | + | ||||
| soxXABYZ(CD) | − | fccAB | − | fh | − | ||||||
| Oxygen | ccoNOP | + | mdh | + | |||||||
| qoxABCD | − | Glyxoylate shunt | glcB | + | |||||||
| cydABX | − | aceA | + | ||||||||
Iron oxidation in “Ca. Siderophilus nitratireducens”
The presence of a monoheme c cytochrome cyc2 cluster 3, a primary iron oxidation gene, suggests that “Ca. Siderophilus nitratireducens” can use Fe2+ as an electron donor. Other common Fe2+ oxidases, namely the diheme c cytochrome cyc1 and the multiheme c cytochromes MtoA and MtoB, were not annotated. Despite the close phylogenetic proximity to lithoautotrophic sulfur-oxidizing bacteria, the genes of sulfide dehydrogenases Sqr and FccAB and sulfite dehydrogenases SorAB and SoeABC were not identified.
In terms of potential catabolic electron acceptors, the genes for a periplasmic nitrate reductase (napABCD and its membrane ferrodoxins napGH) and a cbb3-type cytochrome c oxidase (ccoNOP) were annotated. However, genes encoding for other known denitrification reductases, namely membrane-bound nitrate reductase (narGHI) and nitrite, nitric oxide and nitrous oxide reductases, nirK/nirS, norBC, and nosZ respectively, were not found (Table 2, detailed version in Table S1). Additionally, alternative oxidases, such as the cytochrome bd ubiquinol oxidase (cydAB) or the aa3-type cytochrome c oxidase (coxABCD) were not identified. Also, genes of dissimilatory sulfate reduction (aprAB and dsrABC) and the sox complex, responsible for sulfate reduction could not be identified. These findings suggest that “Ca. Siderophilus nitratireducens” relies exclusively on nitrate and oxygen as electron acceptors.
On the thermodynamic feasibility of nap-dependent nitrate-reducing iron-oxidation
Based on its genome, “Ca. Siderophilus nitratireducens” is potentially an autotrophic organism that obtains energy by oxidizing Fe2+ to Fe3+ while reducing NO3− to NO2−. Yet, as iron is a weak electron donor, the standard Gibbs free energy of the reaction is positive under standard biological conditions (pH 7). The process is therefore thermodynamically unfavorable:
However, the exceptionally low solubility constants of iron oxides, ranging from 10−34 to 10−42 [25] result in very low Fe3+ concentrations. Thereby, precipitation creates the conditions for a favorable thermodynamic driving force for the oxidation of Fe2+ to Fe3+ [26]. Under the operational conditions of this study (283 K, <1 μmolNO3−·l−1, <0.2 μmolNO2−·l−1 in the effluent), the reaction becomes thermodynamically favorable owing to the calculated Fe3+/Fe2+ ratio in the order of 10−16 (conservative value, see Section 4.2):
A Gibbs free energy of −66 kJ/e-mol is in principle enough to transport three to four protons over the cytoplasmic membrane, considering a value of 15 kJ·molH+−1 [27], and would yield at least one ATP. If other iron precipitation products are formed, the redox potential of Fe2+/Fe3+ increases further. Even at more conservative Fe3+/Fe2+ ratios and pH, due to non-equilibrium conditions and product gradients, the biological process remains favorable (Fig. 3).
![Gibbs energy dissipated during nitrate-reducing iron oxidation as function of the ratio of substrates and products. As reference, lines represent the ratios at which the minimum energy required to generate pmf (−15 kJ·Mol−1, full lines) and ATP (−45 kJ·Mol−1, dashed lines) is generated at intracellular pH of 6 (green) or 7 (blue) ([27]). Star dots represent the actual ratios based on measured (NO2−/NO3−/Fe2+) and calculated (Fe3+) influent reactor concentrations. Note that NO2− was below detection limit (< 0.2 μmol·l−1), so that the actual reactor conditions were likely even more favorable, i.e. more on the left .](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ismecommun/4/1/10.1093_ismeco_ycae008/2/m_ycae008f3.jpeg?Expires=1748136834&Signature=MfrsEP6lX1RCZD9ShPlU-zgxwl7kVpqRpoumxd0DhWqCZ95knuhsF9ZKaztfwhUlFVrOydDkTgxyX-7cyJOixxl9nIOrhucsUqREbD-WPc7EJi5TCHnR62R9HBmHu3oHdr~mdzItr8~K7DdESV1rx3uJgXy7Ds-ojtEBGbZpASc2PLaLvWwOEl~gN5bJ42SPUHu~BrjWumGlfvefR~9TmA2tUVhdP4dngRngy2WolgLogbUy613aUAuVi7Mbc2Y5Jr5LGGbUvb9DPrjgN0Y2cGedu2cgrICyOgGrbcHlt4iWOxXLz8hKk4ZKBfu3C7P2oYCQDADpDt1OFXfiAyOMvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Gibbs energy dissipated during nitrate-reducing iron oxidation as function of the ratio of substrates and products. As reference, lines represent the ratios at which the minimum energy required to generate pmf (−15 kJ·Mol−1, full lines) and ATP (−45 kJ·Mol−1, dashed lines) is generated at intracellular pH of 6 (green) or 7 (blue) ([27]). Star dots represent the actual ratios based on measured (NO2−/NO3−/Fe2+) and calculated (Fe3+) influent reactor concentrations. Note that NO2− was below detection limit (< 0.2 μmol·l−1), so that the actual reactor conditions were likely even more favorable, i.e. more on the left .
Complete denitrification: a collaborative effort of iron-oxidizing autotrophs and organoheterotrophs
Iron oxidation genes were identified in five MAGs, namely MAG.13 (“Ca. Siderophilus nitratireducens”), MAG.29 (g_Rhizobacter) and MAG.26, MAG.34 and MAG.16 (f_Gallionellaceae, commonly associated with autotrophic iron oxidation). These MAGs also encoded for the central enzymes of the carbon dioxide fixation via the CBB cycle. While all 13 MAGs contained genes encoding for at least one denitrification enzyme (Table 2), none of them possessed a comprehensive gene set to fully reduce nitrate to dinitrogen gas. Dissimilatory nitrate reduction to nitrite, the first denitrification step, was present in nine MAGs, while the final step, nitrous oxide reduction to nitrogen gas, was only found in MAG.26 (g_Rhizobacter), MAG.18 (f_Anaeromyxobacteraceae) and MAG.10 (f_Chitinophagaceae). The five most abundant MAGs (>5%) alone accounted for up to 50% of the community and covered the full denitrification (Fig. 4). All putative iron oxidizers also possessed the genetic potential for aerobic respiration (i.e. they contained cbb3-type terminal oxidases). Interestingly, two distinct potential niches were identified. The autotrophic iron oxidizers, “Ca. Siderophilus nitratireducens” and MAG.26 (f_Gallionelleceae), performed the initial denitrification reductions, while the lower-abundant organoheterotrophs complemented the reduction of (at least) NO to nitrous oxide possibly taking advantage of the autotrophically fixed carbon excreted by the iron oxidizers. Due to the absence of sufficient biodegradable organic matter in the influent, a portion of the biologically generated NO was likely reduced to N2O chemically with Fe2+ [28].
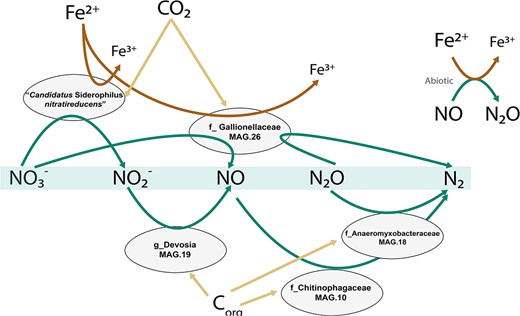
Genome-based conceptual model of substrates fluxes within the microbial community represented by the five most abundant MAGs. Putative autotrophic iron oxidizers perform the upstream part of denitrification, while flanking communities reduce the toxic intermediates to innocuous dinitrogen gas. The putative autotrophic metabolism was inferred based on the presence of ribulose-1,5-biphosphate carboxylase/oxygenase (RuBisCO) and phosphofructokinase (pfk).
Discussion
We established a pilot-scale filter on nitrate-rich anoxic groundwater to elucidate the structure and metabolism of nitrate-reducing iron-oxidizing microbial communities under oligotrophic conditions mimicking natural groundwater. The enriched microbial community stoichiometrically removed iron and nitrate during more than 4 months, and was dominated by a genome belonging to a new Candidate order, named Siderophiliales. The genome of this new species, “Ca. Siderophilus nitratireducens,” encoded the genes for iron oxidation (cytochrome c cyc2) and, within the denitrification pathway, the periplasmic nitrate reductase (nap). The absence of other denitrification genes suggests a short catabolic path, which may offer a kinetic advantage in terms of higher iron oxidation rates [29] especially under nitrogen limiting conditions [30]. In contrast, the majority of NDFO genomes reported so far encode the membrane-bound nitrate reductase (nar) along with other downstream denitrification genes [11, 31–34]. Nar actively translocates protons, whereas nap conserves energy only indirectly by accepting electrons from the quinol pool on the periplasmic side of the membrane, effectively consuming cytoplasmic protons [35]. Recently, novel Zetaproteobacteria genomes possessing nap have been recovered from a complex community, yet they also possessed at least another energy conserving nitrogen oxide [5, 36]. The presence of a cbb3-type cytochrome c oxidase suggests that “Ca. Siderophilus nitratireducens” may also be capable of oxygen respiration. This is consistent with the fact that all reported genomes of anaerobic iron-oxidizing bacteria contain oxygen reductases [31, 34, 37], including the well-studied KS [13] and AG [38] cultures. However, to the best of our knowledge, NDFO growth under (micro)aerophilic conditions has not been reported to date [11]. Although the sporadic detection of traces of oxygen (<3 μM) in our filter does not allow to fully exclude aerobic activity, and in the absence of cultured representatives to confirm it, we posit that nap-driven iron oxidation was the primary catabolic route of “Ca. Siderophilus nitratireducens” under the in-situ restricted availability of alternative substrates. Furthermore, “Ca. Siderophilus nitratireducens” was also identified as a putative autotroph, adding the additional challenge of energy and electrons needs for anabolic CO2 fixation to the growth on iron, a weak electron-donor at standard conditions [15]. Thermodynamic evaluations indicate that nap-dependent iron oxidation can sustain growth at realistic Fe3+/Fe2+ and NO3−/NO2− concentrations ratios. To this end, the quasi-instantaneous precipitation of the biologically formed Fe3+ as iron oxides under circum-neutral pH plays a central role as thermodynamic driving force [39]. The specific mechanisms by which this thermodynamic potential is harnessed for carbon fixation remain to be fully elucidated.
The subsequent reduction of the produced nitrite resulted from the concerted activity of putative autotrophic iron-oxidizers and organoheterotrophs. Within the microbial community, the second most abundant genome, MAG.26 (f_Gallionellaceaea), featured the genetic potential for iron oxidation and most denitrification steps, with the exception of nitrous oxide reductase (nor). MAG.26 also possessed the cytochrome c oxidase cbb3-type ccoNOP for aerobic respiration. Interestingly, this genome contained genes for CO2 fixation, a trait mirrored in all other less abundant genomes with the ability to oxidize iron. This suggests that autotrophy may represent an essential trait for NDFOs in anoxic groundwaters where the dissolved organic carbon is largely non-biodegradable [40]. The three second most abundant genomes, MAG.18 (f_Anaeromyxobacteraceae), MAG.19 (g_Devosia) and MAG.10 (f_Chitinophagaceae) were found to lack the genes for iron oxidation and CO2 assimilation. Yet, these genomes encompassed the full denitrification pathway starting from nitrite. Besides the likely occurrence of chemical reduction of NO to N2O [41], we speculate that these heterotrophs complemented the NDFOs for at least the reduction of NO using autotrophically fixed organic carbon as substrate. A similar metabolic network was also recently observed in mesophilic NDFO communities [13]. Overall, the measured iron and nitrate consumption yield of 7.1 mol Fe2+: mol NO3− is consistent with the expected 5.6, i.e. considering the theoretical catabolism (eq. 1) and the recently estimated 12% of electrons used for growth [7], but higher than the experimentally observed range of 3.8–4.7 [9, 31, 42]. At first, we hypothesized nitrate ammonification to be the reason for the slight excess in iron oxidation, yet none of the putative iron-oxidizing genomes encoded for the common nrf nor for the newly reported octaheme complex [43]. Also, the oxygen sporadically detected in the influent was always below the quantification limit of 3 μM, a conservative concentration that alone would explain less than 15% of the total iron consumption via chemical oxidation. As no Fe3+ was detected in the reactor effluent, all iron necessarily accumulated inside the reactor either as Fe2+ or Fe3+ precipitates. X-ray diffraction and Mössbauer spectroscopy identified over 94% of the Fe in solids as amorphous ferrihydrite, an Fe3+ oxide, with <6% of the solids attributed to magnetite, an Fe2+–Fe3+ oxide typically formed under anaerobic conditions (Figure S4, Table S2 and SI 5). Consequently, the Fe2+ unaccounted for was likely continuously adsorbed onto the newly-formed Fe3+ oxides, a well-studied phenomenon [44], yet the extent to which this occurred was not investigated. In conclusion, pending experimental validation, we surmise that NDFO microorganisms may not only contribute to iron removal by direct oxidation but also by continuously providing newly-formed iron oxides for its adsorption.
Description of “Ca. Siderophilus nitratireducens” gen. nov., sp. nov.
Siderophilus (Si.de.ro’phi.lus Gr. masc.n. sidêros iron; Gr. masc. adj. philos loving; N.L. masc. n. Siderophilus, loving iron).
ni.tra.ti.re.du’.cens (N.L. masc. n. nitras (gen. nitratis), nitrate; L. pres. part. reducens, converting to a different state; N.L. part. adj. nitratireducens, reducing nitrate).
Autotrophic nitrate-reducing iron-oxidizing bacterium isolated from a filtration unit fed with anaerobic groundwater with iron(II) and nitrate. Harbors also have the genetic potential to aerobically oxidize iron.
Materials and methods
Groundwater and pilot-scale filter characteristics
An iron reducing microbial community was enriched anoxically on the granular activated carbon of a 10-L pilot-scale filter in Emmen (the Netherlands) (Figs S1 and S2 and Table S1). The media was devoid of any previously formed biofilm. The anoxic, nitrate-rich groundwater (−75.2 ± 28.4 mV) featured constant Fe2+ and NO3− concentrations, 236 ± 4 μM and 8.1 ± 2.1 μM, respectively (Table 3). Oxygen was consistently below quantification limit (3 μM). The groundwater pH and temperature were 6.7 ± 0.2 and 10.5 ± 0.1°C, respectively. The filter was operated at a filtration flowrate of 3.8 m·h−1 (29.6 L·h−1) during 120 days. After 75 days of steady-state operation, the influent nitrate concentration was manually increased in four steps up to 83.8 ± 0.6 μM, when the system changed from nitrate (NO3− < 1 μM) to iron limiting conditions (Fe2+ < 4 μM).
Influent and effluent water characteristics corresponding to average and standard deviation of daily measurements of Days 21–77, during the nitrate-limited steady-state. Fe3+ was calculated as described in the following section.
| Parameter . | Units . | Value . | Value . |
|---|---|---|---|
| Influent | Effluent | ||
| pH | 6.9 ± 0.4 | 6.7 ± 0.1 | |
| T | °C | 10.5 ± 0.1 | 10.9 ± 0.6 |
| ORP | mV | −64 ± 17 | −58 ± 18 |
| O2 | μmol·L−1 | <3a | <3a |
| NH4+ | μmol·L−1 | 11 ± 1.0 | 11 ± 8.1 |
| NO2− | μmol·L−1 | <0.2a | <0.2a |
| NO3− | μmol·L−1 | 8.1 ± 2.1 | <1 |
| Fe2+ | μmol·L−1 | 236 ± 4 | 178 ± 5 |
| Fe3+ | μmol·L−1 | 2·10−12 | |
| DOC | mg·L−1 | 3.1 ± 0.1 | 2.0 ± 0.2 |
| Parameter . | Units . | Value . | Value . |
|---|---|---|---|
| Influent | Effluent | ||
| pH | 6.9 ± 0.4 | 6.7 ± 0.1 | |
| T | °C | 10.5 ± 0.1 | 10.9 ± 0.6 |
| ORP | mV | −64 ± 17 | −58 ± 18 |
| O2 | μmol·L−1 | <3a | <3a |
| NH4+ | μmol·L−1 | 11 ± 1.0 | 11 ± 8.1 |
| NO2− | μmol·L−1 | <0.2a | <0.2a |
| NO3− | μmol·L−1 | 8.1 ± 2.1 | <1 |
| Fe2+ | μmol·L−1 | 236 ± 4 | 178 ± 5 |
| Fe3+ | μmol·L−1 | 2·10−12 | |
| DOC | mg·L−1 | 3.1 ± 0.1 | 2.0 ± 0.2 |
Below detection limit
Influent and effluent water characteristics corresponding to average and standard deviation of daily measurements of Days 21–77, during the nitrate-limited steady-state. Fe3+ was calculated as described in the following section.
| Parameter . | Units . | Value . | Value . |
|---|---|---|---|
| Influent | Effluent | ||
| pH | 6.9 ± 0.4 | 6.7 ± 0.1 | |
| T | °C | 10.5 ± 0.1 | 10.9 ± 0.6 |
| ORP | mV | −64 ± 17 | −58 ± 18 |
| O2 | μmol·L−1 | <3a | <3a |
| NH4+ | μmol·L−1 | 11 ± 1.0 | 11 ± 8.1 |
| NO2− | μmol·L−1 | <0.2a | <0.2a |
| NO3− | μmol·L−1 | 8.1 ± 2.1 | <1 |
| Fe2+ | μmol·L−1 | 236 ± 4 | 178 ± 5 |
| Fe3+ | μmol·L−1 | 2·10−12 | |
| DOC | mg·L−1 | 3.1 ± 0.1 | 2.0 ± 0.2 |
| Parameter . | Units . | Value . | Value . |
|---|---|---|---|
| Influent | Effluent | ||
| pH | 6.9 ± 0.4 | 6.7 ± 0.1 | |
| T | °C | 10.5 ± 0.1 | 10.9 ± 0.6 |
| ORP | mV | −64 ± 17 | −58 ± 18 |
| O2 | μmol·L−1 | <3a | <3a |
| NH4+ | μmol·L−1 | 11 ± 1.0 | 11 ± 8.1 |
| NO2− | μmol·L−1 | <0.2a | <0.2a |
| NO3− | μmol·L−1 | 8.1 ± 2.1 | <1 |
| Fe2+ | μmol·L−1 | 236 ± 4 | 178 ± 5 |
| Fe3+ | μmol·L−1 | 2·10−12 | |
| DOC | mg·L−1 | 3.1 ± 0.1 | 2.0 ± 0.2 |
Below detection limit
Dissolved Fe3+ estimation
At pH 7, Fe3+ has a markedly low solubility and precipitates as iron oxyhydroxide (Fe(OH)3). Thermodynamically, this phase transition favors the oxidation of Fe2+ to Fe3+, and the resulting low Fe3+ concentration is the primary driving force of equation 1 [45]. In our filter, the dissolved concentration of the Fe3+, resulting from Fe2+ oxidation, was always below detection limit (0.01 mg·l−1). To discuss the thermodynamic feasibility of the NDFO process, we estimated the steady-state [Fe3+]/[Fe2+] ratio following the method proposed by Gorski et al. [39], which assumes thermodynamic equilibrium between [Fe2+] – [Fe3+] – [FeOx] phases based on the fact that the hydroxylation of dissolved Fe3+ is quasi-instantaneous at pH > 3 [46]. Consequently, the following equation can be used to determine the Fe3+ concentration as function of pH and the solid solubility constant.
The most abundant iron oxide in the sand filter was amorphous ferrihydrite (SI 4 and 5), with a Ksp of 10−39 [46]. Therefore:
Analytic procedures
Samples for ammonium, nitrite, and nitrate quantification were immediately filtered through a 0.2 μm nanopore filter and measured within 12 h using photometric analysis (Gallery Discrete Analyzer, Thermo Fischer Scientific, Waltham, MA, USA). Samples for dissolved iron were filtered through a 0.2 μm nanopore filter, acidified to pH < 2 with H2SO4, and quantified by ICP-MS (Analytik Jena, Jena, Germany). Temperature, pH, oxidation–reduction potential (ORP), and dissolved oxygen concentration (DO) were monitored daily using a HI9829-01042 multiparameter analyzer (Hanna Instruments, Smithfield, RI, USA) in the raw water, after nitrate dosage and in the effluent.
Biomass sampling, DNA extraction and quality control
Immediately after the end of the experiment, the filter was emptied and the medium grains were completely mixed. A small volume of grains was used for DNA extraction. Nucleic acid extraction was carried out using DNeasy PowerSoil Pro Kit (QIAGEN, Hilden, Germany) following manufacturer instructions. To improve DNA recovery and avoid the interference of carbon with the extraction, 25 μL of 20 g·l−1 autoclaved (20 min, 121°C, 2 bar) skimmed milk solution (Sigma Aldrich, Saint Louis, MO, USA) were added to the extraction tube. After extraction, DNA was concentrated to 7.68 ng DNA·μl−1 using Microcon centrifugal filter units YM-100 (MilliporeSigma, Burlington, MA, USA) following the manufacturer’s instructions. DNA was quantified with the Qubit 4 Fluorometer and Qubit dsDNA HS assay kit (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. DNA purity was determined using a NanoDrop One Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Library preparation, sequencing, and reads processing
Long-read and short-read DNA sequencing were carried out independently. Long-read library preparation was carried out using the ligation sequencing kit SQK-LSK 109 (Oxford Nanopore Technologies, Oxford, UK). R.9.4.1 flowcells on a GridION were used for sequencing. Raw data were basecalled in super-accurate mode using Guppy v.5.0.16 (https://nanoporetech.com). Raw reads were quality-filtered and trimmed using Filtlong (https://github.com/rrwick/Filtlong) to remove reads below 4000 kb and mean quality score below 80. Adapters were removed using Porechop v.0.2.3 (https://github.com/rrwick/Porechop).
Short-read library preparation was performed using the Nextera XT kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. The libraries were pooled, denatured, and sequenced with Illumina MiSeq (San Diego, CA, USA). Paired end sequencing of 2 x 300 base pairs was performed using the MiSeq Reagent Kit v3 (San Diego, CA, USA) according to the manufacturer’s instructions. Raw sequencing data were quality-filtered and trimmed using Trimmomatic v0.39 (HEADCROP:16 LEADING:3 TRAILING:5 SLIDINGWINDOW:4:10 CROP:240 MINLEN:35) [47]. Sequencing data quality was analyzed using FastQC v0.11.7 before and after trimming [48].
Reads assembly and binning
Reads assembly and binning were done as in [49] with minor modifications. Long-reads were assembled using Flye v. 2.9-b1768 [50] with the “–meta” setting enabled and the “–nano-hq” option. Polishing was carried out with Minimap2 v.2.17 [51], Racon v. 1.3.3 [52], Medaka v.1.4.4 (two rounds) (https://github.com/nanoporetech/medaka). At the end, short-reads were incorporated in a final round of polishing with Racon. Both long- and short-raw reads were independently mapped back to the assembled contigs using BWA-MEM2 [53]. SAMtools v1.14 was used to determine contig coverage and for indexing with default settings [54] .
Automated binning was carried out with the long-reads assembly (polished with short-reads) using MetaBAT2 v. 2.12.1 [55] with “-s 500000,” MaxBin2 v. 2.2.7 [56], and Vamb v. 3.0.2 [57] with “-o C–minfasta 500000.” Additionally, contig coverage from the short-reads assembly was provided as input to the three binners to improve binning. Output integration and refinement was done in DAS Tool v. 1.1.2 [58] . CoverM v. 0.6.1 (https://github.com/wwood/CoverM) was applied to calculate the bin coverage (using the “-m mean” setting) and the relative abundance (“-m relative_abundance”). Additional manual bin polishing was done in R using mmgenome (https://github.com/MadsAlbertsen/mmgenome).
Assembly processing and gene annotation
The completeness and contamination of the genome bins were estimated using CheckM v. 1.1.2 [59]. The bins were classified using GDTB-Tk v. 1.5.0 [60] 202 database. Barrnap v 0.9 (https://github.com/tseemann/barrnap) and structRNAfinder [61] were used to predict 23S, 16S, and 5S ribosomal RNA sequences, and transfer RNA sequences were determined using tRNAscan-SE v.20 [62] with default search mode. Bins were classified using the Minimum Information about a Metagenome-Assembled Genome (MIMAG) standards [18]: high-quality bins were >90% complete and <5% contaminated, and contained full-length 23S, 16S, and 5S ribosomal RNA genes and ≥18 transfer RNA genes. Bins with completeness >50% and contamination <10% were classified as medium-quality, and bins with completeness <50% and <10% contamination as low-quality bins. The remaining ones were considered contamination. 68% of the filtered reads rendered high- and medium-quality bins, 11% low-quality bins and 21.1% were unbinned.
The ORFs of the 10 resulting high-quality and 3 medium-quality bins with relative abundance >0.5% were predicted using Prodigal v2.6.3 [63] and functionally annotated with GhostKoala v2.2 [20] (Kyota Encyclopedia of Genes and Genomes; accessed March 2022). FeGenie [19] was used to improve the annotation of the iron metabolism using the metagenomics (“-meta”) settings. To refine the annotation for MAG.13 (Candidatus Siderophilus nitratireducens), the genome was uploaded to the National Center for Biotechnology Information (NCBI) database Prokaryotic Genome Annotation Pipeline v6.1 [64]. Additionally, manual annotation of genes potentially relevant but not automatically annotated was done by aligning a set of manually selected sequences from UniProtKB against the translated ORFs from MAG.13 with local blastp v2.13 [65]. After annotation, all the predicted genes of interest (manually and automatically annotated) were translated and aligned against the Non-redundant protein sequences (nr) database from NCBI using blastp (accessed June 2022) and accepted only if the coverage was >70% [19] and the identity >35% [66].
RStudio v1.4.1106 was used for data analysis and visualization.
Phylogenetic tree construction
Genome-based phylogenetic reconstruction was done by using 120 bacterial single copy conservative marker genes, as described previously [60]. The trees were built using the IQ-TREE 2 [23] with fast model selection via ModelFinder [67] and ultrafast approximation for phylogenetic bootstrap [68], as well as approximate likelihood-ratio test for branches [24]. Whole genome comparison was conducted by using two different methods: Average Nucleotide Identity (ANI), using JSpeciesWS web server and DNA–DNA Hybridization (DDH) by the Genome-to-Genome Distance Calculator 2.1 online tool (https://ggdc.dsmz.de/ggdc.php) [69].
Acknowledgements
The authors would like to thank Dimitry Sorokin (TU Delft and Russian Academy of Sciences) for the thorough discussions and invaluable insights; Martin Pabst and Dita Heikens (TU Delft) for the yet-unpublished proteomic data; Mantas Sereika, Thomas Nielsen, and Mads Albertsen (Aalborg University) for their help with Nanopore Sequencing and genome assembly and binning during the hands-on Nanopore course; Arjan I. Dugulan (Reactor Institute Delft) for the Mossbauer Analysis; and the anonymous Reviewer #2 for the insightful and constructive comments that significantly improved the manuscript.
Author contributions
Francesc Corbera-Rubio, Jantinus Bruins, Mark C. M. van Loosdrecht, Doris van Halem, and Michele Laureni (conceived the study), Jantinus Bruins and Simon F. Dost (designed the filter and performed the on-site experiments), Francesc Corbera-Rubio (run the metagenomics analysis), and Alexander Y. Merkel (the phylogenetic classification). Francesc Corbera-Rubio and Gerben R. Stouten conducted the thermodynamics calculations. Simon Müller performed the solid characterization. Francesc Corbera-Rubio, Gerben R. Stouten, Mark C. M. van Loosdrecht, Doris van Halem, and Michele Laureni performed data analysis and/or helped interpret the results. Francesc Corbera-Rubio wrote the manuscript, with contributions from Gerben R. Stouten, Mark C. M. van Loosdrecht, Doris van Halem, and Michele Laureni. All co-authors critically reviewed the manuscript and approved the final version.
Conflicts of interest
None declared.
Funding
This work was financed by the NWO partnership program “Dunea–Vitens: Sand Filtration” (project 17830) of the Dutch Research Council (NWO) and the drinking water companies Vitens NV and Dunea Duin & Water. M.L. was supported by NWO (VI.Veni.192.252).
Data availability
Raw reads and MAGs have been deposited in the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/bioproject/) under BioProject PRJNA834785. The BioSample accession numbers for the raw reads and the five most abundant MAGs are SAMN28600298, SAMN28058410 (“Ca. Siderophilus nitratireducens”), SAMN36381704 (f_Gallionelaceae), SAMN36381891 (f_Anaeromyxobacteraceae), SAMN36382736( g_Devosia), SAMN36401011 (f_Chitinophagaceae).



