-
PDF
- Split View
-
Views
-
Cite
Cite
Xiaoyu Pan, Ling Chen, Chunyan Shan, Lisha Cai, Yue Wang, Yue Chen, Ming Gu, Kaiping Liu, Pihong Li, Jianchun Pan, Inhibition of Phosphodiesterase 2 Ameliorates Post-Traumatic Stress–Induced Alcohol Intake Disorder by Regulating cAMP/cGMP Signaling, International Journal of Neuropsychopharmacology, Volume 25, Issue 11, November 2022, Pages 936–945, https://doi.org/10.1093/ijnp/pyac064
Close - Share Icon Share
Abstract
Post-traumatic stress disorder (PTSD) is the prevalent psychiatric disorder that induces alcohol use disorders (AUD) such as abnormal alcohol intake and anxiety. However, little is known about whether phosphodiesterase 2 (PDE2)-cAMP/cGMP signaling is involved in PTSD-induced AUD.
The present study used single-prolonged stress (SPS) to mimic PTSD that induced increases in ethanol intake and preference (2-bottle choice test) and anxiety-like behavior (elevated-plus maze test and novelty suppressed feeding test). PDE2 inhibitor Bay 60-7550 (Bay) was administered to the mice and protein kinase A (PKA) inhibitor H89 and PKG inhibitor KT5823 were micro-injected into dorsolateral striatum (DLS) and central amygdala (CA) of mice to determine whether the effects of Bay on anxiety-like behavior in SPS mice are brain region dependent.
PDE2 inhibitor Bay rescued SPS-induced decreases in open arm entries and open arm time exposure in elevated-plus maze test and reversed increased latency to feed in the novelty suppressed feeding test. Moreover, SPS-induced ethanol use disorder was reversed by Bay as evidenced by decreased ethanol intake and preference without changing total fluid intake in the SPS mice after treatment with Bay. However, Bay did not change the ethanol metabolism or sucrose or quinine intake and preference. The locomotor activity was not affected after treatment with Bay. Interestingly, microinjection of PKA or PKG inhibitor H89 or KT5823 into DLS prevented the effects of Bay on alcohol intake and preference and cAMP-response element binding proteins phosphorylation and brain derived neurotrophic factor expression in DLS but not on the anxiety-like behavior in SPS mice. Microinjection of these inhibitors into CA prevented Bay-induced anxiolytic-like effects and cAMP-response element binding proteins phosphorylation and brain derived neurotrophic factor levels in CA but did not affect ethanol intake in SPS mice, indicating that the effects of Bay on different behaviors are brain region dependent.
These findings support the hypothesis that PDE2-cAMP/cGMP signaling may differentially mediate PTSD-induced AUD and anxiety-like behavior.
The present study determined that SPS-induced PTSD-like behavior was reversed by treatment of PDE2 inhibitor Bay 60-7550 for 2 weeks. The results showed that Bay 60-7550 treatment induced increased open arm entries and time spent in open arms in the EPMT, reduced latency to catch food in the NSFT, and reduced alcohol intake in the 2-bottle choice test. Microinjection of PKA and PKG inhibitors into dorsolateral striatum or central amygdala prevented Bay 60-7550–induced alcohol intake and anxiety-like behavior, respectively, suggesting that PDE2-mediated behavioral changes were brain region specific. The findings suggest that the effects of Bay 60-7550 on anxiety-like behavior and alcohol intake may be involved in differentially activating PDE2-dependent cAMP/cGMP signaling in the amygdala and striatum.
Introduction
Post-traumatic stress disorder (PTSD) is characterized by a series of psychological distresses such as sleep disturbances and aggressive behavior and psychiatric symptoms such as anxiety, major depressive disorder, and strong craving for alcohol (Ralevski et al., 2020). Individuals with PTSD are more vulnerable to developing alcohol use disorder (AUD), with occurrence rates ranging from 28% to as high as 85% (Ralevski et al., 2014; Flanagan et al., 2018). PTSD comorbid with psychiatric disorders such as major depressive disorder, anxiety, and AUD will increase suicide rates by 2–3 times compared with these diseases alone. However, the pathological relationship between these disorders remains unknown as well as whether the damaged neurons from different brain regions mediate these different psychiatric symptoms. The complicated pathogenesis of co-occurrence results in the scarcity of effective treatments. Thus, it is imperative to identify novel targets and effective therapeutics to treat comorbid PTSD and psychiatric symptoms such as anxiety, depression, and AUD.
Recent studies, including ours, found that regulation of cAMP and cGMP activities ameliorates PTSD-induced fear memory deficits and nociceptive response abnormalities, which are associated with rescue of the neuronal atrophy in the hippocampus and amygdala (Harvey et al., 2005; Shi et al., 2019; Chen et al., 2021). Phosphodiesterase 2 (PDE2 or PDE2A) is one of the super-family members of cyclic nucleotide-degrading enzymes that catalyzes the hydrolysis of cAMP and cGMP (Harvey et al., 2005). PDE2 is highly expressed in the prefrontal cortex, hippocampus, amygdala, hypothalamus, and pituitary and adrenal glands, regions vulnerable to stress-related disorders (Chen et al., 2021). Some studies show that changes of cGMP levels in the striatum might participate in the processes of chronic alcohol consumption and withdrawal in rats (Uzbay et al., 2004; Werner et al., 2004). The central nucleus of amygdala is involved in conversion of emotionally relevant sensory information into behavioral responses associated with anxiety (Davis, 2000). PDE2 regulates cAMP and cGMP to transmit messages between neuronal cells, making it an attractive target for treatment of PTSD-related anxiety and AUD (Chen et al., 2021). Some studies suggest that overexpression of PDE2 is involved in many neurological diseases such as anxiety and its related cognitive disorders (Xu et al., 2013; Ding et al., 2014). Inhibition of PDE2 upregulates cAMP and cGMP and increases protein kinase A and G (PKA and PKG) expression, which ultimately activate the downstream cAMP- and cGMP-response element binding proteins phosphorylation (pCREB) and brain derived neurotrophic factor (BDNF) expression (Xu et al., 2013). The activation of BDNF in brain regulates synaptic growth and development and improves neuronal remodeling and behavior (Lu et al., 2014). However, traumatic stress can induce contrasting patterns of neuroplasticity based on the function of the neurons from different brain regions (Vyas et al., 2002), which indicate that the cAMP- and cGMP-dependent BDNF signaling in different brain regions may play different roles and mediate different behavior. Thus, we hypothesized that PDE2-related cAMP/cGMP signaling in striatum and amygdala may differentially mediate PTSD-induced AUD and anxiety/depression-like behavior, respectively.
Materials and Methods
Animals
Male C57BL/6J mice, 20–25 g, were obtained from Shanghai Center of Experimental Animals, Chinese Academy of Sciences, Wenzhou, zhejiang, China . Mice were raised in a constant temperature (22°C ± 1°C) and constant humidity (50% ±10%) environment with a 12-hour-light/-dark cycle, and they were allowed to freely obtain food that met the standards as well as water. Before the behavioral test, the mice were allowed to have a 7-day adaptation period. All procedures in these experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80-23, revised 1996) and were approved by the Committees of Wenzhou Medical University on Animal Care and Use.
Drugs and Treatment
The selective PDE2 inhibitor Bay 60-7550 (Bay) and rolipram (Rol) were obtained from MedChem Express (USA) and Sigma Chemical Co. (USA). Bay and Rol were dissolved in the vehicle containing 95% saline and 5% dimethyl sulfoxide, respectively, and were given via i.p. injection for 14 days. The PKA inhibitor H89 was purchased from Sigma-Aldrich (USA), and the PKG inhibitor KT5823 was purchased from Gayman Chemical (USA). H89 and KT5823 were dissolved in artificial cerebrospinal fluid and bilaterally microinjected into the different brain regions of mice 30 minutes before treatment of Bay every day.
Experimental Design
Surgery
The mice were anesthetized by i.p. injection with ketamine/xylazine mixtures (100 mg/kg and 10 mg/kg). The hairs of the anesthetized mice were removed and fixed to the stereotaxic instrument. Using Bregma as the 0 point, we located the central amygdala (AP, −1.5 mm from the bregma, ML, ±2.8 mm from the midline, DV, −2.0 mm from the dura) and dorsolateral striatum (AP, 0.5 mm from the bregma; ML, ±2.5 mm from the midline; DV, −3.0 mm from the dura mater), then inserted a guide cannula (No. 30) into each hole and fixed it in place. The injection speed was 0.1 µL/min. After the infusion was completed, the catheter remained at the needle insertion site for 10 minutes to allow the drug to fully enter the tissue. The mice were allowed to recover 7 days after the operation and then proceeded to follow-up experiments.
Single-Prolonged Stress (SPS) Procedure
SPS was performed as previously described, which induces PTSD-like behavior (Chen et al., 2021). Mice were placed on a metal board by taping the limbs with surgical tape and restricting motion of the head and were immobilized for 2 hours. They were subjected to a forced swimming for 20 minutes in a glass cylinder (25-cm height, 10-cm diameter) filled with 24°C fresh water (two-thirds of the glass cylinder). Mice were allowed to recuperate for 15 minutes and exposed to ether vapor until loss of consciousness. Figure 1A illustrates the experimental procedure for SPS and behavioral tests. One cohort of mice were for behavioral tests such as the elevated plus maze test (EPMT), novelty suppressed feeding test (NSFT), and 2-bottle choice test (TBC). The other cohort of mice were for the EPMT, ethanol-induced sedation test, locomotor activity test, sucrose intake experiment, ethanol metabolism test, and quinine intake experiment.
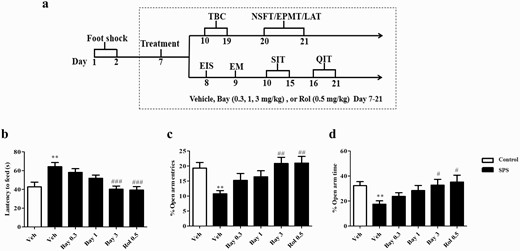
The experimental schedule of single-prolonged stress (SPS) model to mimic post-traumatic stress disorder (PTSD)-like syndrome. Treatment schedule and test order for mice (A). The effects of Bay 60-7550 (Bay) on the latency to feed time (B) in the novelty suppressed feeding test (NSFT) and the percentages of open arm entries (C) and time spent in the open arms entries (D) in the elevated plus maze test (EPMT). Bay and rolipram (Rol) were injected (i.p.) through the whole experiment for 14 days. EIS, ethanol-induced sedation test; EM, ethanol metabolism test; LAT, locomotor activity test; QIT, quinine intake experiment; SAC, sacrifice; SIT, sucrose intake experiment; TBC, 2-bottle choice test. Values are the mean ± SEM. **P < .01 vs. vehicle-treated control group; #P < .05, ##P < .01, and ###P < .001 vs. vehicle-treated SPS group.
Elevated Plus Maze Test
The EPM device includes 2 open arms and 2 closed arms (30 × 5 cm), and the height of all the arms are 15 cm from the ground. All the mice were placed in the central area of the maze facing the open arm, and each of them was placed in the same position thereafter. At the same time, the camera monitor was turned on to record the number of times the mouse entered the open arm and the closed arm and the time of entering each arm within 5 minutes (all 4 paws). During the experiment, the experimenter needed to be 1 m away from the maze. At the same time, the maze was cleaned and wiped to eliminate the influence of animal odor on subsequent experimental animals.
Novelty Suppressed Feeding Test
The mice were placed in a corner of a white plastic open arena (35 × 28 × 16 cm) after being deprived of food for 24 hours. A grain of experimental food (regular chow) was placed in the center of the venue. The latency of the mice starting to eat and the ratio of the amount of food (milligrams) the mice ate were recorded within 5 minutes.
Two-Bottle Choice Test.
—Two glass centrifuge tubes (50 mL) were placed in each squirrel cage; one bottle was 7% alcohol solution and the other was drinking water for 4 consecutive days. Mice were singly housed in cages with caps for food and water/ethanol bottles. The ethanol concentration was then increased to 9% for 3 days and further increased to 12% for 3 days. During this period, the mice were free to drink 2 bottles of water, and the positions of the 2 bottles were exchanged every 24 hours to eliminate the influence of position preference. Every morning from 8:30 to 9:30, fresh alcohol solution and water were prepared. During the test, the mice were treated with corresponding medications at 9 am and 6 pm every day. The consumption of alcohol and water was recorded at 6 pm every day.
Sucrose or Quinine Intake Test)
To determine whether the effect of PDE2 on ethanol intake was related to taste preference, sucrose intake experiment or quinine intake experiment was performed by the same 2-bottle choice paradigm. Mice in each cage were provided with 2 bottles: one bottle was 2% sucrose solution and the other was water. The sucrose concentration increased to 4% for the following 3 days. Similarly, the quinine concentration increased to 0.1 mM for the following 3 days. Average daily intake of sucrose or quinine was calculated from all 3 days of drinking.
Blood Ethanol Metabolism
Blood ethanol concentrations (BECs) were examined to evaluate whether Bay affected alcohol metabolism. The second batch of mice were injected with vehicle or Bay (3 mg/kg) or Rol (0.5 mg/kg) 30 minutes before ethanol (2 g/kg) treatment. After 15, 30, 60, and 120 minutes of alcohol treatment, blood was collected from the tail vein of the mice. BECs were determined with yeast alcohol dehydrogenase, which is a reliable enzyme assay for ethanol contents. The yeast alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde with the simultaneous reduction of nicotinamide adenine dinucleotide to reduced form of nicotinamide adenine dinucleotid. BECs were determined using a custom EtOH assay kit (BioAssay Systems, Hayward, CA, USA) following the kit instructions. The absorbance at 340 nm was measured by spectrophotometry to the ethanol concentration in the sample.
Locomotor Activity
This locomotor activity experiment mainly took from 6 am to 11 pm. The interruption of the infrared beam was recorded using an automatic activity detection system. On the first day, the mice were allowed to adapt for 20 minutes. The duration of time that each mouse spent in the room was set for 2 hours at intervals of 20 minutes, and the camera located above the chamber was used to record the total distance of horizontal locomotor activity.
Western-Blot Analysis
Western-blot analysis was performed as previously described (Chen et al., 2021). The brains were removed after decapitation, and the striatum and amygdala tissues were rapidly separated on normal saline ice and stored at −80°C for standby. The sample was separated for approximately 2 hours and transferred onto polyvinylidene fluoride membrane by transfer membrane method, sealed with 0.5% defatted milk at room temperature. The primary antibody (rabbit anti-phosphorylated CREB, 1:1000, rabbit anti-CREB, 1:1000, rabbit anti-BDNF, 1:1000, rabbit anti-GAPDH, 1:5000) was incubated overnight at a low temperature of 4°C. After washing, the secondary antibody (HRP-Convoluted Monkey Anti-Rabbit IgG 1: 10 000) was added and incubated for 1 hour at room temperature, and the membrane was washed. Color exposure was measured with ECL kit of bands and caching by the ImageLab gel imaging analysis system. The imaging system carries out statistical analysis on the optical density of the strip.
Data Analysis
All data are expressed as means ± SEM and were statistically analyzed by 2-way repeated-measures ANOVA followed by post hoc Dunnett’s t tests using GraphPad Prism5.0. In the ethanol intake and preference tests, the ethanol concentration, Bay treatment, and their interaction (drug treatment × ethanol concentration) could be determined. All the test results that yielded a P value < .05 were considered statistically significant.
Results
Effects of Bay on SPS-Induced Anxiety-Like Behavior
The experimental procedures of SPS and behavioral tests are shown in Figure 1A. In the SPS group, treatment began on day 7, and vehicle, Bay (0.3, 1, 3 mg/kg), or Rol (0.5 mg/kg) were i.p. injected for 2 weeks. In the sham group, mice received only the vehicle. As shown in Figure 1B, the SPS procedure induced increased latency to food in the NSFT (P < .01). Treatment with Bay at a dose of 3 mg/kg for 2 weeks significantly decreased the latency to food compared with the vehicle-treated SPS group (F (4,45) = 8.555, P < .001). As shown in Figure 1C–D, the decreased percentages of open arm entries and the time spent in the open arms induced by the SPS were significantly reversed by treatment with Bay at a dose of 3 mg/kg for 2 weeks in the EPMT (F (4,45) = 3.061, P < .01; F (4,45) = 4.747, P < .05) compared with vehicle-treated SPS groups. These effects were similar to those of the PDE4 inhibitor Rol (0.5 mg/kg), which was used as the positive control drug in the study.
Effect of Bay on SPS-Induced Ethanol Intake and Preference Changes
Two-way ANOVA revealed that drug treatment and ethanol concentration significantly differed during the ethanol intake test (drug treatment: F (4,115) = 6.810, P < .001; ethanol concentration: F (2,230) = 53.39, P < .001; Figure 2A). SPS induced a significant increase in the involuntary drinking of 7%, 9%, and 12% ethanol (P < .001, P < .001, P < .01). Bay at a dose of 3 mg/kg decreased 7% ethanol drinking (P < .001). Bay at doses of 1 and 3 mg/kg decreased involuntary ethanol drinking at 2 concentrations of 9% (P < .01; P < .001) and 12% (P < .05; P < .01) compared with the vehicle-treated SPS group. These effects were similar to those of treatment with the PDE4 inhibitor Rol (Ps < .001).
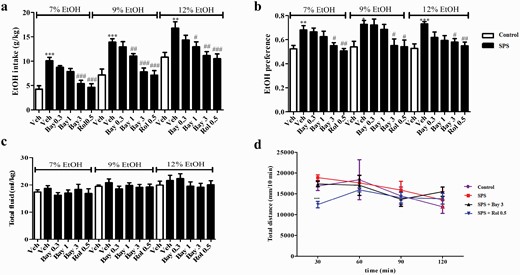
Bay 60-7550 (Bay) prevented single-prolonged stress (SPS)-induced increases ethanol intake (A) and preference (B) in the 2-bottle choice test (TBC). SPS and Bay did not affect total fluid consumption (C) in the TBC and locomotor activity (D) in open-field test in mice. Values are mean ± SEM. *P < .05, **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated SPS group.
Similarly, the SPS procedure caused a significant increase in ethanol preference compared with the vehicle-treated control groups at concentrations of 7% (P < .01), 9% (P < .05), and 12% (P < .001). However, Bay at 3 mg/kg significantly decreased the preference of mice to different concentrations of ethanol (F (4,45) = 14.21, P < .05 for 7%; F (4,45) = 15.20, P < .05 for 9%; F = (4,45) 14.01, P < .05 for 12%, respectively; Figure 2B). The subsequent tests suggested that neither Bay nor Rol changed the total fluid intake compared with the vehicle-treated control mice, regardless of 3 ethanol concentrations (Figure 2C). As shown in Figure 2D, the total distance of horizontal locomotor activity was gradually decreased over time during the 2-hour test in the vehicle-treated mice, which was similar to SPS-treated mice. Bay at 3 mg/kg did not affect the locomotor activity. In contrast, Rol reduced locomotor activity during the first 30 minutes (P < .001), but it was no longer effective after 60 minutes.
PKA or PKG Inhibitor Into Basolateral Striatum Reversed Effects of Bay on Ethanol Intake in SPS Mice
The experimental procedure of drug treatment and behavioral tests is shown in Figure 3A. Microinjection of H89 or KT5823 into dorsolateral striatum did not prevent the effects of Bay (3 mg/kg) on the latency to feed in the NSFT (Figure 3B) or on the percentages of open arms entries (P < .01) (Figure 3C) or time spent in open arms (P < .05; Figure 3D) in the EPMT in SPS mice. However, this treatment prevented Bay’s effects on alcohol intake and voluntary drinking of ethanol at 3 concentrations: 7% (P < .001; P < .01), 9% (P < .01; P < .01), and 12% (P < .01; P < .01) (Figure 4A). Bay-induced decrease in ethanol preference was also reversed by microinjection of H89 (for 7%, P < .01; for 9%, P < .01; for 12%, P < .01) or KT5823 (for 7%, P < .01; for 9%, P < .05; for 12%, P < .05; Figure 4B) into dorsolateral striatum. The subsequent tests suggested that all groups did not change the total fluid intake (Figure 4C).
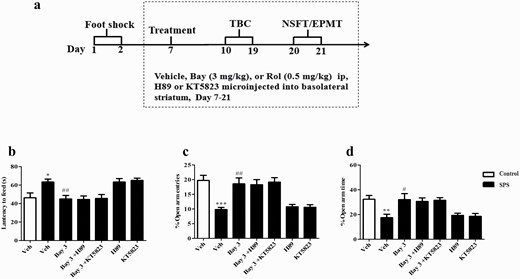
Microinjection of PKA or PKG inhibitor (H89 or KT5823) into basolateral striatum did not affect anxiolytic-like effects of Bay 60-7550 (Bay) in single-prolonged stress (SPS) mice. (A) Treatment schedule and test order for mice. The effects of Bay on the latency to feed in the novelty suppressed feeding test (NSFT) (A), the percentages of open arm entries (C), and time spent in the open arms entries (D) in the elevated plus maze test were not prevented by microinjection of H89 or KT5823 into basolateral striatum. EPMT, elevated plus maze test; TBC, 2-bottle choice test. Values are mean ± SEM. *P < .05, **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated SPS group.
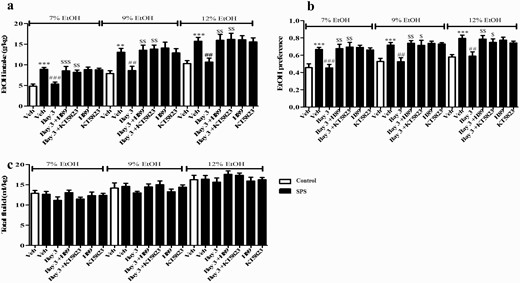
Microinjection of H89 or KT5823 into basolateral striatum reversed the effects of Bay 60-7550 on ethanol intake (A) and preference (B) in the 2-bottle choice test in single-prolonged stress (SPS) mice. Drug treatment did not affect total fluid consumption (C). Values are mean ± SEM. **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated SPS group. $P < .05, $$P < .01, $$$P < .001 vs. Bay 3-treated SPS group.
Microinjection of H89 or KT5823 Into Basolateral Striatum Reversed Bay-Induced Increased pCREB and BDNF Levels
As shown in Figure 5A, SPS did not impact the total CREB expression but significantly decreased the ratio of pCREB to CREB in the striatum (P < .01). However, treatment of Bay reversed the decreased ratio of pCREB/CREB induced by SPS (P < .001). This increased pCREB/CREB by Bay was prevented by microinjection of H89 or KT5823 into basolateral striatum (P < .05; P < .01).
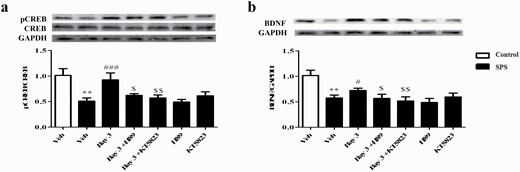
Microinjection of H89 or KT5823 into basolateral striatum reversed Bay 60-7550 (Bay)-induced increase in pCREB (A) and BDNF (B) levels in this brain region. Values are mean ± SEM (n = 8). **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated single-prolonged stress (SPS) group. $P < .05, $$P < .01 vs. Bay 3-treated SPS group.
As shown in Figure 5B, further results suggested that the BNDF level was decreased in striatum of SPS-treated mice compared with the vehicle group (P < .01). Treatment of mice with Bay significantly reversed this decrease in BDNF level in striatum (P < .05). However, this effect was prevented by pretreatment with H89 or KT5823 into basolateral striatum (P < .05; P < .01). Neither H89 nor KT5823 used alone induces any change in pCREB and BDNF expression in the striatum.
PKA or PKG Inhibitor Into Central Amygdala Reversed Effects of Bay on Anxiety-Like Behavior in SPS Mice
PKA inhibitor H89 or PKG inhibitor KT5823 was micro-injected into central amygdala of mice, and the experimental procedure of drug treatment and behavioral tests is shown in Figure 6A. Microinjection of H89 or KT5823 into central amygdala prevented the effects of Bay (3 mg/kg) on the latency to feed in the NSFT (Figure 6B), the percentages of open arms entries (P < .001) (Figure 6C), and time spent in open arms (P < .05) (Figure 6D) in the EPMT in SPS mice. However, this treatment did not affect Bay-induced alcohol intake and preference (Figure 7A–B). The total fluid intake did not differ between any of the groups (Figure 7C).
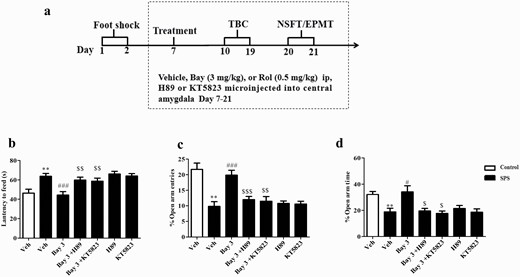
Microinjection of PKA or PKG inhibitor (H89 or KT5823) into central amygdala prevented the anxiolytic-like effects of Bay 60-7550 (Bay) in single-prolonged stress (SPS) mice. (A) Treatment schedule and test order for mice. (B) The effects of Bay were prevented by treatment of H89 or KT5823 as shown by the increased latency to feed time in the novelty suppressed feeding test (NSFT), the decreased percentages of open arm entries (C), and time spent in the open arms entries (D) in the elevated plus maze test (EPMT). TBC, 2-bottle choice test. Values are mean ± SEM (n = 8). *P < .05, **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated SPS group. $P < .05, $$P < .01, $$$P < .001 vs. Bay 3-treated SPS group.
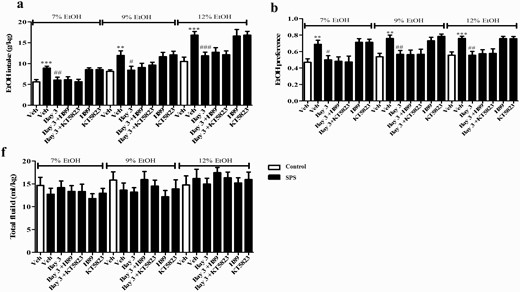
Microinjection of H89 or KT5823 into central amygdala did not affect the effects of Bay 60-7550 on ethanol intake (A) and preference (B) in the 2-bottle choice test in single-prolonged stress (SPS) mice. Drug treatment did not affect total fluid consumption (C). Values are mean ± SEM (n = 8). **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated SPS group.
Microinjection of H89 or KT5823 Into Central Amygdala Reversed Bay-Induced Increases in pCREB and BDNF Levels
The subsequent study suggested that SPS exposure significantly decreased the ratio of pCREB to CREB in the amygdala compared with the control group (P < .01; Figure 8A). However, this decreased pCREB/CREB caused by SPS was reversed by Bay (P < .01). Pretreated mice with either H89 or KT5823 in the central amygdala prevented the increase in the ratio of pCREB/CREB (P < .05; P < .05). Further results suggested that the increased BDNF levels in Bay–treated groups (P < .05; P < .05) were prevented by pretreatment of H89 or KT5823 in the central amygdala of SPS mice (P < .01; P < .05; Figure 8B). However, neither H89 nor KT5823 used alone induced any change in pCREB and BDNF expression in the amygdala.
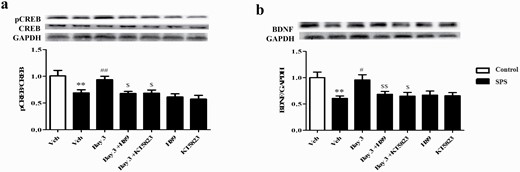
Microinjection of H89 or KT5823 into central amygdala reversed Bay 60-7550 (Bay)-induced increases in pCREB (a) and BDNF (b) levels in this brain region. Values are mean ± SEM (n = 8). **P < .01, ***P < .001 vs. vehicle-treated control group; #P < .05, ##P < .01, ###P < .001 vs. vehicle-treated single-prolonged stress (SPS) group. $P < .05, $$P < .01 vs. Bay 3-treated SPS group.
Discussion
The present study determined that SPS-induced PTSD-like disorders, such as increases in ethanol intake and preference, were reversed by treatment of PDE2 inhibitor Bay for 2 weeks. The results showed that Bay treatment induced increases in open arm entries and time spent in open arms in the EPMT, reduced latency to catch food in the NSFT, and reduced alcohol intake and preference in the TBC. However, Bay did not change the ethanol metabolism, which was consistent with the results from the PDE4 inhibitor Rol (supplementary Figure 1). We also found that Bay did not affect the sucrose or quinine intake and preference, indicating that Bay did not change the taste preference of mice (supplementary Figure 2). The locomotor activity was not affected after treatment with Bay, suggesting that the above-mentioned behavioral changes did not seem to relate to stimulating or inhibiting the central nervous system. A further pharmacological interaction analysis suggested that the effects of Bay on anxiety- and depression-like behavior and increased alcohol preference may be involved in differentially activating PDE2-dependent cAMP/cGMP signaling in the amygdala and striatum, respectively.
Previous studies, including ours, suggested that PDE2 is predominately expressed in the limbic hypothalamus-pituitary-adrenal axis that involves anxiety, depression, AUD, and drug addiction behavior (Ding et al., 2014; Zhang et al., 2015). Recent studies showed that inhibition of PDE2 by Bay reversed PTSD-like fear memory and neuropathic pain (Chen et al., 2021). The significance of this study was to determine whether Bay is effective on SPS-induced anxiety/depression-like and alcohol drinking behavior. SPS-induced behavioral changes due to increases of the hypothalamic-pituitary-adrenal gland axis and other brain structure changes, including decreased hippocampal neurogenesis and neuronal remodeling, are consistent with those observed in PTSD patients (Battle, 2013). Our present study found that a single prolonged stress procedure induced PTSD-like anxiety-like behavior and fear memory deficits, as evidenced by the fact that SPS-treated mice reduced the time spent in the center zone of arena in the open field test, spent less time in the open arms, and had fewer entries into the open arms in the EPMT (Chen et al., 2021). However, this anxiety-like behavior was reversed by treatment of the PDE2 inhibitor Bay for 14 days. Moreover, SPS also caused increases in alcohol intake and preference in mice in the present study. Indeed, phenotypes related to alcohol addiction can be modeled by the TBC in which mice intermittently drink escalated concentrations of alcohol within 9 days of testing (Carnicella et al., 2014). Our study found that C57BL/6J mice drank more ethanol when we provided escalated concentrations of alcohol at 7%, 9%, and 12% after they were exposed to the SPS procedure. This increase in ethanol drinking disappeared after treatment of Bay for 14 days, which is consistent with a previous study that demonstrated that the cAMP- and cGMP-dependent signaling pathway is related to alcohol drinking, withdrawal, craving, and relapse in the animal models of drug abuse (Koob and Volkow, 2010). However, Bay did not affect either sucrose or quinine water consumption, indicating its potential to inhibit SPS-induced increases in ethanol intake and preference.
Some studies suggest that chronic alcohol drinking behavior is related to neuroadaptive alterations by regulation of cAMP and cGMP levels in different brain regions (Yang et al., 1996; Osterndorff-Kahanek et al., 2013). It should be noted that among 11 PDE family members, PDE2 is predominantly expressed across the human brain. The most prevalent PDE2 is found in the frontal cortex and hippocampus, while enriched PDE2 expression is also expressed in the amygdala and striatum (Lakics et al., 2010). The specific distributions of PDE2 in the brain indicate that targeted inhibition of PDE2 and its dependent cAMP/cGMP signaling may mediate amygdala- and striatum-related behavioral changes (Wen et al., 2018).
Our previous study suggested that traumatic stress–induced increased expression of PDE2 and subsequent decreased levels of cAMP and cGMP in the hippocampus and amygdala may be involved in both emotional change and alcohol-drinking behavior (Xu et al., 2013; Chen et al., 2021). However, the causal link of neuronal function between different brain regions such as amygdala and striatum and behavioral changes remains unknown. The present study showed that Bay rescued anxiety-like and alcohol intake behaviors, but these effects were brain region dependent, as evidenced by the fact that microinjection of PKA or PKG inhibitor H89 or KT5823 into dorsolateral striatum prevented the effects of Bay on alcohol intake; similar effects were not observed when either inhibitor was microinjected into central amygdala. However, blockade of PKA or PKG in the central amygdala, but not dorsolateral striatum, reversed Bay-induced anxiolytic-like effects. These interesting results indicate that the brain region–specific cAMP/cGMP-PKA/PKG signaling may mediate different psychiatric behavior caused by post-traumatic brain injury (or stress). Indeed, upregulation of cAMP- and cGMP-BDNF signaling in the hippocampus regulates neuroplasticity and ameliorates depression-like behavior (Ding et al., 2014; Chen et al., 2021), while the upregulated neuroprotective factors in the amygdala may be involved in excessive growth of neurons and deterioration of stress-related anxiety-like behavior (Vyas et al., 2002). Unlike the hippocampus and amygdala, relatively little is known about how stress and PTSD affect the striatum and the nature of its role in the PTSD-induced psychiatric symptoms. Some studies showed that the changes of cGMP levels in the striatum might participate in the processes of chronic alcohol consumption and withdrawal in rats (Uzbay et al., 2004; Werner et al., 2004). More recent work found that activating cAMP signaling in the striatum is a molecular transducer to inhibit the development of excessive alcohol consumption (Ehinger et al., 2021). Our previous work suggested that Bay reversed PTSD-like fear memory and neuropathic pain by regulation of cAMP and cGMP levels in the brain (Chen et al., 2021), supporting that inhibition of PDE2-induced upregulation of cyclic nucleotide levels may treat PTSD-related symptoms, including alcohol-drinking behavior. Considering that PDE2 is a dual substrate enzyme that catalyzes both cAMP and cGMP hydrolyses, inhibition of PDE2 exhibits a dual inhibitory effect that should be more effective for treatment of AUDs than those of other PDE inhibitors such as PDE4 inhibitors, which only inhibit cAMP hydrolysis.
Our subsequent study suggested that Bay did not affect ethanol metabolism after treatment of ethanol within a 120-minute test period, supporting that Bay reversed SPS-related alcohol-drinking behavior is independent of ethanol metabolism. Our previous studies only focused on memory enhancing and antidepressant- and anxiolytic-like effects of PDE2 inhibition (Ding et al., 2014; Xu et al., 2015). The present study found that inhibition of PDE2 is also effective for treatment of SPS-induced alcohol abuse. As the main downstream targets of PDE2-related signaling, pCREB and BDNF are recognized as the 2 molecular biomarkers in the development of alcohol abuse and treatment (Alberini, 2009). Our findings extended the previous results that suggest that cAMP/cGMP-dependent pCREB and BDNF were increased both in the amygdala and striatum after treatment of Bay. However, updated cAMP/cGMP signaling in different brain regions may correlate with different behavioral responses, respectively.
In summary, the present study demonstrated that inhibition of PDE2 by Bay reversed SPS-induced anxiety-like behavior, alcohol intake, and preference without affecting sweet and bitter taste. Moreover, the effects of Bay on these behavioral changes were brain region specific, that is, PDE2-cAMP/cGMP signaling in the striatum was involved in alcohol intake and preference, while this signaling in the amygdala was linked to anxiety-like behavior. Overall, our findings demonstrate that regulation of PDE2-cAMP/cGMP-pCREB-BDNF signaling in the amygdala and striatum may be potential treatment of SPS-related psychiatric disorders such as anxiety-like behavior and AUDs.
Acknowledgments
This project was supported by the Natural Science Foundation of Zhejiang Province (no. LY21H310004) and Science and Technology Development Project of Wenzhou (no. Y20180818) to Jianchun Pan and the Zhejiang Provincial Public Welfare Research Project (no. 2018C37082) and special scientific research fund project for Hospital Pharmacy of Zhejiang Pharmaceutical Association (no. 2021ZYY05) to Ling Chen.
Statement Conflict of Interest
The authors declare no conflicts of interest included in this manuscript, as applicable to each author, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials.
References
Author notes
X.P., L.C., and C.S. contributed equally to this work.
P.L. and J.P. co-directed this study.
- anxiety
- physical activity
- signal transduction
- cyclic gmp
- alcohol drinking
- brain-derived neurotrophic factor
- corpus striatum
- microinjections
- neostriatum
- post-traumatic stress disorder
- sucrose
- arm
- brain
- cyclic amp
- mice
- quinine
- stress
- current good manufacturing practice
- cyclic phosphodiesterase, type 2
- central nucleus of amygdala



