-
PDF
- Split View
-
Views
-
Cite
Cite
Yuan-Hao Chen, Tung-Tai Kuo, Eagle Yi-Kung Huang, Barry J Hoffer, Jen-Hsin Kao, Yu-Ching Chou, Yung-Hsiao Chiang, Jonathan Miller, Nicotine-Induced Conditional Place Preference Is Affected by Head Injury: Correlation with Dopamine Release in the Nucleus Accumbens Shell, International Journal of Neuropsychopharmacology, Volume 21, Issue 10, October 2018, Pages 949–961, https://doi.org/10.1093/ijnp/pyy055
Close - Share Icon Share
Abstract
Traumatic brain injury is known to impact dopamine-mediated reward pathways, but the underlying mechanisms have not been fully established.
Nicotine-induced conditional place preference was used to study rats exposed to a 6-psi fluid percussion injury with and without prior exposure to nicotine. Preference was quantified as a score defined as (C1 − C2) / (C1 + C2), where C1 is time in the nicotine-paired compartment and C2 is time in the saline-paired compartment. Subsequent fast-scan cyclic voltammetry was used to analyze the impact of nicotine infusion on dopamine release in the shell portion of the nucleus accumbens. To further determine the influence of brain injury on nicotine withdrawal, nicotine infusion was administered to the rats after fluid percussion injury. The effects of fluid percussion injury on conditional place preference after prior exposure to nicotine and abstinence or withdrawal from nicotine were also assessed.
After traumatic brain injury, dopamine release was reduced in the nucleus accumbens shell, and nicotine-induced conditional place preference preference was significantly impaired. Preference scores of control, sham-injured, and fluid percussion injury groups were 0.1627±0.04204, 0.1515±0.03806, and -0.001300±0.04286, respectively. Nicotine-induced conditional place preference was also seen in animals after nicotine pretreatment, with a conditional place preference score of 0.07805±0.02838. Nicotine preexposure substantially increased tonic dopamine release in sham-injured animals, but it did not change phasic release; nicotine exposure after fluid percussion injury enhanced phasic release, though not to the same levels seen in sham-injured rats. Conditioned preference was related not only to phasic dopamine release (r=0.8110) but also to the difference between tonic and phasic dopamine levels (r=0.9521).
Traumatic brain injury suppresses dopamine release from the shell portion of the nucleus accumbens, which in turn significantly alters reward-seeking behavior. These results have important implications for tobacco and drug use after traumatic brain injury.
In this study, a mild concussive traumatic brain injury, elicited by a 6-psi fluid percussion injury (FPI), abolished nicotine-associated conditioned place preference in rats, suggesting that dopamine release in the shell portion of the NAc is affected by such an injury. This could play a crucial role in changes in reward and reinforcing behavior after concussive brain injury. Moreover, the conditioned place preference time seems to correlate with phasic dopamine release and the difference between tonic and phasic dopamine releases. Thus, central dopaminergic transmission dynamics could play a critical role in linking brain injury to nicotine use.
Introduction
Traumatic brain injury (TBI) is often associated with chronic deficits that are not always functionally related to the trauma site (Huang et al., 2014b; Chen et al., 2017a, 2017b). Although widespread processing deficits have been reported, there is evidence that certain physiological networks are uniquely vulnerable to TBI. In particular, alterations in reward processing have been reported, evidenced by increases in tobacco abuse after TBI in both addicts and tobacco-naïve individuals (Ilie et al., 2015a, 2015b). This suggests that mesolimbic-mesocortical dopaminergic reward pathways are affected. We have previously demonstrated that TBI is associated with substantial chronic alterations in nigrostriatal dopamine dynamics, suppressing release and reuptake both ipsilateral and contralateral to the injury (Huang et al., 2014b; Chen et al., 2015). However, the influence of TBI on the mesolimbic-mesocortical dopaminergic pathways is not currently well understood.
Dopamine is a neurotransmitter known to be related to reward (Hoebel, 1985) and is critical for reinforcing effects (Saunders et al., 2013; Vicente et al., 2016) that promote self-administration of drugs of abuse (Di Chiara et al., 2004) as well as compulsions such as eating (Hoebel, 1985; Nestler, 2005). Moreover, dopaminergic neurons in the ventral tegmental area, projecting to the shell of the nucleus accumbens (NAc), play crucial roles in perceptions, which are critical for substance-induced rewards (Benowitz, 2010). Dopaminergic neurons are thought to operate in 2 distinct patterns (Grace, 2000). Tonic firing typically occurs at low frequencies (1–5 Hz) and are related to the basal steady-state concentration of DA in the extracellular compartment. Tonic release can produce a basal input to dopamine receptors, especially to D2 receptors in the mesolimbic system, which includes the NAc (Grace, 1991; Dreyer et al., 2010). In contrast, midbrain dopamine neurons can fire in high-frequency bursts (≥20 Hz), referred to as phasic firing, occurring when animals are presented with motivationally salient stimuli that predict drug or rewarding substance availability. This phasic release produces transient increases in dopamine concentration in the NAc that are sufficient to occupy low-affinity dopamine D1 receptors (Phillips et al., 2003; Dreyer et al., 2010). Nicotine elicits dopamine release in the mesolimbic area, the corpus striatum, and the frontal cortex (Brody, 2006), and lesioning the dopaminergic system blocks self-administration of nicotine in rats (Leikola-Pelho and Jackson, 1992). By stimulating nicotinic cholinergic receptors, nicotine augments the release of many neurotransmitters, including dopamine (Wonnacott, 1997; Dajas-Bailador and Wonnacott, 2004). Previous data have shown that prolonging reuptake in the NAc shell can alter the peak level of dopamine induced by burst stimulation (Chen et al., 2017b). Therefore, we hypothesized that TBI-induced suppression of dopamine release in the NAc affects reward processing. In this study, these mechanisms were evaluated, and this hypothesis was tested using animal behavioral models of nicotine-induced conditioned place preference (CPP) and neurochemical analyses.
Methods
Animals
Adult male Sprague–Dawley rats aged 5 weeks (LASCO Taiwan Co., Ltd.) were used according to procedures that were reviewed and approved by the National Defense Medical Center Animal Care and Use Committee (IACUC protocol no. 16–258). Animals were provided food and water ad libitum and were housed in a 12-h-light/-dark cycle room.
Nicotine-Induced Conditional Place Preference
Nicotine hydrogen tartrate was purchased from Sigma-Aldrich, diluted in 0.9% saline, and administered s.c. at a dose of 0.4 mg/kg (Le Foll and Goldberg, 2005). A solution volume of 0.9% saline was equivalent to the volume of nicotine administered.
Conditioned place preference (CPP) tests were used to determine the rewarding effects of nicotine, employing a test apparatus consisting of 3 chambers separated by removable doors, as described elsewhere (Huang et al., 2003) and calculated as a preference score.
Test time for determining place preference was 15 minutes. During a 16-day conditioning period, rats were given saline on odd days and nicotine on even days. Conditioning time was 40 minutes. After saline or nicotine administration, the rat was placed in the corresponding compartment. Place preference was defined as the proportion of this time that a rat remained in a given compartment, and nicotine-induced reward was defined as the rat spending more time in the nicotine-paired compartment than in the saline-paired compartment (Huang et al., 2003). If place preference exceeded 60 seconds before the 16-day conditioning period ended, the rat was eliminated from the study. The CPP test was applied in 3 experiments as described below (Figure 1).
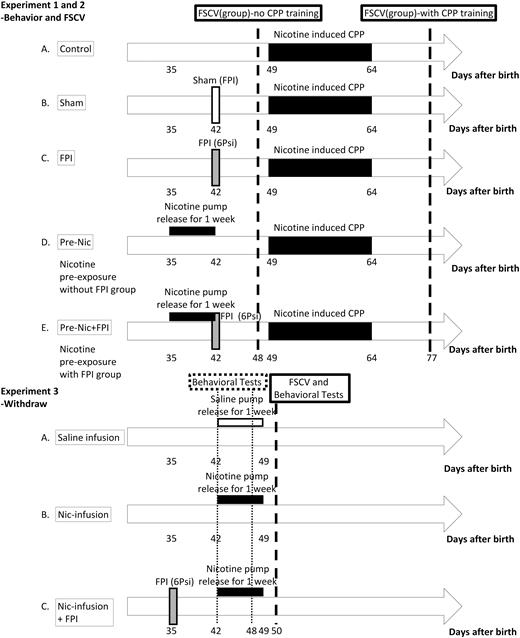
Diagrammatic representation of Experiments 1–3. Experiment 1: The animals are separated into: group (A) conditioned place preference (CPP) group, the rats that only received CPP test; group (B) Sham+CPP, the rats that received a sham operation and then CPP tests; group (C) FPI+CPP group: the rats received 6 Psi fluid percussion injury (FPI) and then CPP tests; group (D) Pre-Nic+CPP group, the rats received 1 week pump implantation releasing nicotine without FPI and then received the CPP tests; group (E) Pre-Nic+FPI+CPP group, the rats received pump implantation releasing nicotine for 1 week and then received 6 Psi-FPI followed by CPP tests. Experiment 2. Electrochemical measurement of dopamine release of using fast scan cyclic voltammetry (FSCV) to determine the dopamine release changes before and after nicotine-induced CPP in each group. Experiment 3. The effect of 6 Psi FPI on nicotine withdrawal signs. The rats were divided into 3 groups: (1) Saline-infusion, rats infused with saline for 7 days; (2) Nic-infusion, rats infused with nicotine (9 mg/kg/d) for 7 days; and (3) Nic-infusion+FPI group, rats infused with nicotine (9 mg/kg/d) for 7 days followed by 6 Psi FPI. (CPP test: nicotine-induced CPP training with a 16-day conditioning period and tests shown in black bar).
Experiment 1: Nicotine-Induced CPP Test
To determine whether fluid percussion injury (FPI) affects nicotine-induced CPP and whether preexposure nicotine effects were similar to patients with tobacco use who suffered from brain injury. The animals were separated into 5 groups: (A) CPP group; the rats that only received nicotine-induced CPP training and tests; (B) Sham+ CPP; the rats that received a sham operation without fluid percussion injury and then received the nicotine-induced CPP training and tests; (C) FPI+ CPP group: the rats that received 6Psi FPI and then received the nicotine-induced CPP training and tests in order to determine if nicotine–induced CPP was affected by FPI; (D) Pre-Nic +CPP group: the rats received 1 week of nicotine exposure by pump implantation without receiving FPI and subsequently received nicotine-induced CPP training and tests to survey if preexposure nicotine would alter nicotine-induced CPP formation; and (E) Pre-Nic+FPI+ CPP group: the rats received nicotine exposure by pump implantation for 1 week and then received 6Psi-FPI, followed by nicotine-induced CPP training and tests to determine if CPP formation could be affected by both FPI and nicotine preexposure. The groupings of animals are shown in Figure 1.
Experiment 2: Electrochemistry Study of Dopamine Release
To determine dopamine release changes before and after nicotine–induced CPP in each group, we performed FSCV in the animals before and after nicotine–induced CPP tests in each of the above groups. Dopamine releases were collected under single pulse stimulation (1 pulse <25 Hz) to mimic tonic release, whereas dopamine release evoked by burst stimulation (10 pulses <25 Hz) was used to mimic phasic release. Subsequently, tonic and phasic release and differences between tonic and phasic release (referred as release probability) data were compared between groups to suggest a hypothesis for dopamine release related to reward systems. Moreover, a linear regression analysis of the relationship between the behavioral test data (preference time score) to tonic, phasic, and differences of DA concentration were performed.
Experiment 3: Abstinence Studies
To determine of the effect of 6 psi FPI on nicotine withdrawal signs in rats, Sprague-Dawley rats were divided into 3 groups: (1) Saline-infusion, rats infused with saline for 7 days; (2) Nic-infusion, rats were infused with nicotine (9 mg/kg/d) for 7 days; and (3) Nic-infusion+FPI, rats were infused with nicotine (9 mg/kg/d) for 7 days followed by 6 Psi FPI. The animals were placed in the observation chamber at 9:00 am. After compensation for a new environment for 30 minutes, the frequency of withdrawal signs was counted for 15 minutes by observers (Malin et al., 1992), modified from the standard checklist of opiate abstinence signs (Malin et al., 1988); the nicotine withdrawal signs included: teeth-chattering/chews, writhes/gasps, shakes/tremors, and ptosis. The presence of these signs would be counted as 1 point/min; the point for miscellaneous less frequent signs (yawns, dyspnea, and seminal ejaculation) were summed (Malin et al., 1992). The behavioral observations were performed each day as follows: at baseline, at the last day of nicotine infusion, and at 16 hours after termination of nicotine infusion. These data are shown in Figure 7.
Surgical Preparation and Fluid Percussion Model
Procedures were similar to methods in our previous papers (Chen et al., 2015, 2017b). Male Sprague-Dawley rats (6 weeks old) weighing 200 to 250 g were anesthetized with Zoletil (50 mg/kg, i.p.; Vibac). Each rat was placed in a stereotaxic frame, and the scalp and temporal muscle were reflected. A 4.8-mm-diameter craniectomy was then performed over the right parietal cortex, 3.8 mm posterior to the bregma and 2.5 mm lateral to the midline, taking care not to penetrate the dura. A cranial Leur adapter of 2.5 mm in inner diameter was placed on the craniectomy site and tightly mounted to the skull using dental acrylic resin. The cranial Leur adapter was filled with saline and attached to the fluid percussion device (Huang et al., 2014a). A fluid percussion device (model HPD-1700; Dragonfly R&D) was used as described elsewhere to produce TBI in rats (Matsushita et al., 2000). Injury was induced by striking the piston of the device with a weighted metal pendulum released from a predetermined angle. Release from 43° produced a 6-psi (0.48 atm) injury.
Infusion Pump Implantation and Drug Protocol
For nicotine or saline infusion, a mini-osmotic pump (ALZET Model 2ML1; DURECT Co.) was implanted in the rat and set to a pumping rate of 10 μL/h. Nicotine was added to 2 mL 0.9% saline to administer 9 mg/kg daily (Cohen and George, 2013). After 7 days, the pump was removed under anesthesia (Cohen and George, 2013).
Open Field Test
The locomotor or behavioral activities of rats were quantified using an infrared (IR) cutoff in a novel environment (Stanford, 2007). Four pairs of IR beams spaced 1–15⁄16 in apart were positioned on 2 sides of a 10-in×18.5-in behavioral chamber (PAS-HC; San Diego Instrument) to measure the frequency of ambulatory activities and total locomotor activity. The former is defined as the number of times a rat crosses 2 or more IR beams within a 15-second detection time interval; the latter is the total number of interruptions detected by a single IR beam.
Brain Slice Preparation for FSCV
Brain slices and carbon fibers (7-μm diameter; GooDfellow Corp.) were prepared as described previously (Chen et al., 2008; Good et al., 2013). After the behavioral experiments, a scaffold was used to decapitate the animals. In Experiments 1 and 2, SD rats were decapitated at 48 days or 77 days after birth. In Experiment 3, SD rats were decapitated at 16 hours in the withdraw period (at 50 days after birth). The animals were decapitated and the brains were removed and placed in a beaker filled with oxygenated (95%O2/5%CO2) ice-cold cutting solution containing (in mM): sucrose 194, NaCl 30, KCl 4.5, MgCl2 1, NaH2PO4 1.2, glucose 10, and NaHCO3 26. A block of brain tissue containing the NAc was glued onto the cutting stage of a vibrating tissue slicer (VT1000, Leica), and the tissue block was immediately submersed in ice-cold, oxygenated cutting solution. Coronal slices 280 μm in thickness containing the NAc were transferred to a holding chamber filled with oxygenated artificial CSF (in mM: NaCl 126, KCl 3, MgCl2 1.5, CaCl2 2.4, NaH2PO4 1.2, glucose 11, NaHCO3 26) at 31 °C for 20 to 30 minutes, after which the holding chamber reached room temperature and was maintained there for the rest of the incubation period. The slices were then transferred to a heated chamber (31–33°C) and perfused with normal artificial CSF (2 mL/min) for FSCV recording (Chen et al., 2017b). Carbon fibers were inserted 100 μm into the NAc under stereoscopic magnification. Fast-scan cyclic voltammetry was performed as described elsewhere (Chen et al., 2015, 2017b). For electrochemical detection, the potential of the carbon fiber was driven from -0.4 to 1.0 V then back to -0.4 V using a triangular waveform (400 V/s, 7-ms duration) applied every 100 ms. A 5-second (50-scan) control period was used to obtain a stable background current that was digitally subtracted from that obtained during the peak of the response following electrical stimulation. Peak oxidation currents were converted to dopamine (DA) concentrations using a calibration performed for each electrode with a 1-μM DA standard solution. All signals used in the statistical analyses matched the expected voltammetric profile for DA (Kawagoe et al., 1993).
To assess the capacity of axon terminals to release DA during stimulation, 2 voltammetric signals were obtained at each recording site using a single pulse (tonic) or 10 pulses (phasic) delivered at 25 Hz under various stimulation intensities (1–10 volts). After tonic and phasic dopamine signals were obtained, values were summed and averaged for each intensity across all sites. Differences in peak DA, obtained immediately after each stimulation, were calculated for each intensity and fit to a linear regression model (y=mx+b; Prism 5.02; GraphPad) where slope m represents the relative change in DA concentration as a function of stimulation intensity (Good et al., 2011; Chen et al., 2017b).
Statistical Analyses
Mean dopamine release (input vs output) was analyzed using 2-way ANOVA followed by a Bonferroni posthoc test for multiple comparisons. A 1-way ANOVA and Bonferroni posthoc test were used to analyze nicotine-induced CPP, locomotor activity, DA concentration under maximum stimulation intensity (5 or 10 volts), slope of the linear regression, and difference in maximum DA concentration. Statistical analyses of abstinence and withdrawal behaviors were performed using paired t tests (baseline vs 16 hours of withdrawal). All data were subjected to repeated-measures ANOVA (pre-test vs post-test), and alpha was set to .05. Posthoc comparisons were performed using an LSD posthoc test. Mixed effects regression analysis for repeated measures was used to evaluate group differences for evoked DA release in the shell of the NAc. All statistical tests were performed using GraphPad Prism 5.02. When P<.05 (2-tailed), significance was identified. The details of statistical analysis for all figures were shown in Supplementary data.
Results
Nicotine-Induced CPP Could Be Induced in Animals after Nicotine Preexposure but Was Impaired after FPI
In this first study we focused on behavioral tests for nicotine-induced CPP assessment to determine whether FPI affected nicotine-induced CPP and if preexposure nicotine here was similar to those patients with tobacco use who suffered from brain injury. We first compared nicotine-induced CPP in control, sham, and FPI groups (Figure 2A). FPI affected nicotine-induced CPP formation; CPP could be induced in the control and sham groups but not in the FPI group (Figure 2A). We expressed the CPP results as a preference score of=(C1 − C2) / (C1 + C2), where C1 is time in the nicotine-paired compartment and C2 is time in the saline-paired compartment. Preference scores were 0.1627±0.04204 (t=4.464, df=16; pair t test, pre-condition vs post-condition, P<.001), 0.1515±0.03806 (t=2.698, df=14; pair t test, pre-condition vs post-condition, P<.05), and -0.001300±0.04286 (t=0.5109, df=13; pair t test, pre-condition vs post-condition, P>.05), respectively. For a behavioral control, locomotion was measured in each group and was not affected by FPI and nicotine pretreatment (Figure 2C–D; 1-way ANOVA [F4,55=0.7741, P=.5468] vs [F4,55=0.3765, P=.8245]).
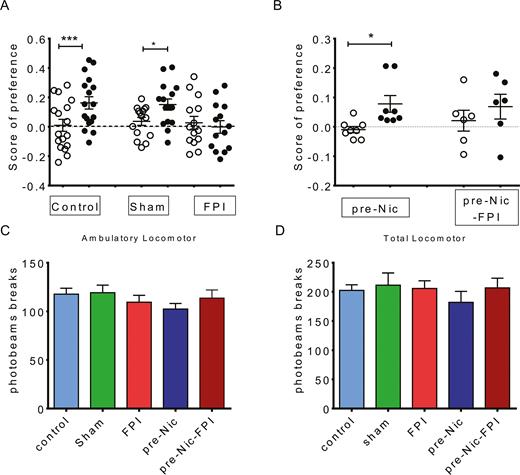
Nicotine-induced conditioned place preference (CPP) was used to determine whether fluid percussion injury (FPI) affected the reward behavior and whether preexposure nicotine had an influence. (A) Nicotine-induced CPP could be induced in control (N=17) and sham (N=15) groups, whereas CPP could not be obtained in animals with FPI (N=14). (B) Nicotine preexposure animals (Pre-Nic, N=8) manifested nicotine-induced CPP. Nicotine-induced CPP could be induced in animals with nicotine preexposure. If animals with nicotine preexposure received FPI (Pre-Nic+6 Psi, N=6), CPP could not be significantly obtained. In each group, white scatter plot: preference before CPP; black scatter plot: preference after CPP. (C) Ambulatory movements of animals and (D) locomotor function were not affected by FPI.
We next analyzed the impact of the history of nicotine preexposure on nicotine-induced CPP to further relate our study to smoking history in humans. Nicotine pretreatment for 1 week (Pre-Nic group) was compared with FPI in nicotine pretreatment groups (Pre-Nic+ FPI) (Figure 2B). The results indicated that nicotine-induced CPP could be induced in animals after nicotine preexposure. Preference scores were 0.07805±0.02838 for the Pre-Nic group, significantly different than those before nicotine conditioning (-0.009641±0.01059; t=2.758, df=5; Pair t test, precondition vs postcondition, P<.05). In the Pre-Nic+FPI group, preference was 0.02094±0.03550 compared to 0.06860±0.04207 (t=1.375, df=5; Pair t test, precondition vs postcondition, P>.05), indicating that CPP was not significantly induced in this group.
Dopaminergic Transmission in the NAc Shell Was Suppressed after FPI and Was Enhanced in Nicotine Preexposed Animals as Well as after CPP Training
To determine if FPI and nicotine preexposure affected dopaminergic transmission in the NAc shell using FSCV, tonic and phasic dopamine releases from the NAc shell were measured using single-pulse and 10-pulse stimulation, respectively, at 25 Hz. Dopamine signals evoked by various stimulation intensities (1~10 Volts) were studied with input/output (I/O) curves to determine dopamine release patterns. First, to analyze the impact of FPI on release of DA in the NAc shell, we compared the I/O curves in control and FPI groups (Figure 3A–B). Tonic and phasic dopamine release were suppressed in the FPI group, similar to our previous report (Chen et al., 2017b). FPI-only animals also had the lowest phasic DA release (Figure 3A–B, solid red squares). Second, we found that the I/O curves could be elevated (left shift) in both control and FPI groups after CPP training (control+CPP: blue open circle vs FPI+CPP: red open square).
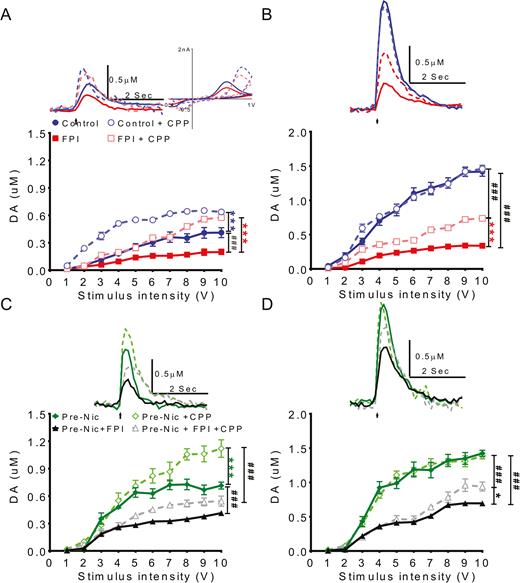
To determine if fluid percussion injury (FPI) and nicotine preexposure affects dopamine transmission in nucleus accumbens (NAc) shell, dopamine release in NAc shell was measured using fast scan cyclic voltammetry (FSCV). Dopamine signals evoked by different stimulation intensities (1~10 V) were plotted as input/output (I/O curves). To evaluate the effect of FPI on dopamine transmission in NAc shell portion during conditioned place preference (CPP) training, (A) tonic and (B) phasic release in control and FPI groups were evaluated. Before CPP training, the tonic and phasic levels of the FPI group were very low. After CPP training, the (A) tonic level increased in both FPI and control animals greater than before CPP (FPI+CPP: white square vs FPI: black square). (B) Phasic release increased after CPP training in the FPI group (FPI+CPP: white square vs FPI: black square), but the increment in the FPI group was less than in the control group (FPI+CPP: white square vs Control+ CPP: white circle). To analyze the effect of FPI on DA transmission in NAc shell of nicotine preexposed animals, the I/O curves of (C) tonic and (D) phasic release in nicotine pretreatment only (Pre-Nic) and FPI with nicotine pretreatment groups were compared. (C) Before CPP training, tonic release in nicotine pretreated animals with FPI (Pre-Nic+FPI) was lower than nicotine pretreated only (Pre-Nic) animals. (Pre-nic+FPI: black solid triangle Pre-Nic: green solid diamond). After CPP training, tonic release in the Pre-Nic+FPI group (gray open triangle) increased but increments were not as high as in the nicotine pretreatment only group (Pre-Nic: green open diamond). (D) The phasic release signal in nicotine pretreatment only animals (Pre-Nic: green open diamond) showed the highest level after CPP training comparing FPI with nicotine pretreatment groups (Pre-Nic+FPI+CPP: gray open triangle). The variation in I/O curves before and after CPP training in FPI animals with nicotine pretreatment showed the phasic release evoked by higher intensities (>7 V) post-CPP training were higher (Pre-Nic+FPI+CPP group: gray open triangle vs Pre-Nic+FPI: black solid triangle).
We then analyzed FPI effects on animals with nicotine pretreatment; the DA release in pre-Nic and Pre-Nic+ FPI groups were compared to determine any FPI effects on DA transmission in animals with nicotine preexposure history (Figure 3C–D). Before CPP training, tonic release in the Pre-Nic group was highest, and this release enhancement in pre-Nic animals was suppressed by FPI. The order of tonic DA release was pre-Nic>pre-Nic+FPI>FPI. After CPP training, tonic release was enhanced in these 2 groups (Figure 3C). Nicotine pretreatment increased phasic release, but this enhancement was suppressed after FPI (Figure 3D, Pre-Nic+FPI: black solid triangle vs Pre-Nic: green solid diamond).
Nicotine-Induced CPP Influence on Dopamine Release: Nicotine Exposure Tends to Increase Dopamine Release
To further determine the effect of nicotine-induced CPP on dopamine release, tonic and phasic releases in brain slice were quantified under maximal stimulation intensities (10 V) in before (pre-CPP state, white bar) and after CPP training (post-CPP state, black bar) animals. For the FPI question, the tonic release in control and FPI groups was compared in Figure 4A. After CPP tests, both control and FPI group tonic release signals were enhanced. Although phasic release before or after CPP training was markedly suppressed in FPI groups compared with control groups, it is interesting that significant enhancement in phasic dopamine release after CPP test could be found in FPI but not in control group (Figure 4B). The enhancement of tonic (Figure 4C) and phasic (Figure 4D) release in the Pre-Nic group was reduced in the Pre-Nic+FPI group. To further determine whether the DA concentrations before CPP and after CPP training would be a factor related to CPP formation, we calculated the tonic and phasic concentration differences before and after CPP training (peri-CPP difference in tonic level = [Tonic]pre-CPP - [Tonic]post-CPP; peri-CPP different in phasic level = [Phasic]pre-CPP - [Phasic]post-CPP) (Figure 4E–F). The peri-CPP difference in tonic release was lowest in Pre-Nic+FPI. However, the peri-CPP difference in phasic was different; these differences were lower in control, Pre-Nic and Pre-Nic+FPI groups, but higher in the FPI only group.
![Nicotine exposure may increase dopamine release. Dopamine concentrations measured with maximal (10 V) stimulation intensities before and after conditioned place preference (CPP) training were analyzed. To determine the fluid percussion injury (FPI) effect, (A) tonic and (B) phasic release values in control and FPI groups were compared. (A)Tonic release was suppressed by FPI. After CPP training, tonic release increased in both control and FPI groups. (B) Either before or after CPP training, phasic release was markedly suppressed in FPI groups compared with control groups. (C) To determine the FPI effect in nicotine preexposed animals, the tonic release data between nicotine pretreated only and FPI with nicotine pretreatment groups were compared. Before CPP training, tonic values in pretreated only animals were higher than in the FPI pretreated animal group. After CPP training, the tonic release increased in both groups, and the tonic release in nicotine pretreatment only animals were again higher than in the nicotine pretreatment with FPI group. (D) For phasic release, analysis between the groups revealed that phasic releases were highest in the nicotine pretreatment only group (Pre-Nic) either before or after CPP training. Phasic release was suppressed in the nicotine pretreatment animals with FPI when compared with nicotine pretreatment only. To further determine whether the DA concentration between before CPP and after CPP training would be a factor related to CPP formation, we calculated the tonic and phasic concentration difference between before and after CPP training (peri-CPP difference in tonic level = [Tonic]pre-CPP - [Tonic]post-CPP; peri-CPP different in phasic level = [Phasic]pre-CPP-[Phasic]post-CPP). (E) The peri-CPP difference in tonic release was lowest in Pre-Nic+ FPI group. (F) The peri-CPP difference in phasic releases was lower in control, Pre-Nic, and Pre-Nic+ FPI groups, but higher in the FPI only group. ##P<.01 and ###<.001, **P<.01 and ***<.001](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijnp/21/10/10.1093_ijnp_pyy055/1/m_pyy05504.jpeg?Expires=1748106016&Signature=1ZpvTCGskhsP5UbTtc6-ApKauGtJEOtmSG~puIwPxeuWFr7Dm3ot~h-GZd48kVEnXJj86k-3TMnTa0XvVAB3iMdn4Eqa47KNHwGANbs759Gb4ABdRUy1AOQyUd0wGea~ZGwBo91GLzI2Ienh7FJakhBVVZp546ZXFOciaysxtXOiv22xdfrJM0lBFP48Aupw74pGQ9J~MXHn~u4zPTUD86zyZ4Nq68WTiT77SWZGJOASlSsIGt-7GJUW8xl6-0fwmNqVNy9-6oN-onRR6m4nc~~k8uJGZsYXL~E4~lEiY6788uwPtGvSrDlZRtOCXBZU8rH-R4TO40YEvK20zb2nJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Nicotine exposure may increase dopamine release. Dopamine concentrations measured with maximal (10 V) stimulation intensities before and after conditioned place preference (CPP) training were analyzed. To determine the fluid percussion injury (FPI) effect, (A) tonic and (B) phasic release values in control and FPI groups were compared. (A)Tonic release was suppressed by FPI. After CPP training, tonic release increased in both control and FPI groups. (B) Either before or after CPP training, phasic release was markedly suppressed in FPI groups compared with control groups. (C) To determine the FPI effect in nicotine preexposed animals, the tonic release data between nicotine pretreated only and FPI with nicotine pretreatment groups were compared. Before CPP training, tonic values in pretreated only animals were higher than in the FPI pretreated animal group. After CPP training, the tonic release increased in both groups, and the tonic release in nicotine pretreatment only animals were again higher than in the nicotine pretreatment with FPI group. (D) For phasic release, analysis between the groups revealed that phasic releases were highest in the nicotine pretreatment only group (Pre-Nic) either before or after CPP training. Phasic release was suppressed in the nicotine pretreatment animals with FPI when compared with nicotine pretreatment only. To further determine whether the DA concentration between before CPP and after CPP training would be a factor related to CPP formation, we calculated the tonic and phasic concentration difference between before and after CPP training (peri-CPP difference in tonic level = [Tonic]pre-CPP - [Tonic]post-CPP; peri-CPP different in phasic level = [Phasic]pre-CPP-[Phasic]post-CPP). (E) The peri-CPP difference in tonic release was lowest in Pre-Nic+ FPI group. (F) The peri-CPP difference in phasic releases was lower in control, Pre-Nic, and Pre-Nic+ FPI groups, but higher in the FPI only group. ##P<.01 and ###<.001, **P<.01 and ***<.001
In summary, comparing DA release at stimulation intensities of 10 V in each group before and after CPP training, we showed that tonic release was elevated by nicotine pretreatment as well as with CPP training itself. Phasic release had the same effect in the FPI groups, suggesting that FPI is associated with significantly diminished DA release compared to noninjury. It also suggests that increasing nicotine exposure time tends to enhance DA release in parallel.
Dopamine Release Capacity Is Reduced after FPI and Nicotine-Induced CPP
To assess the capacity of axon terminals to release DA during phasic stimulation, releasing probability at 25 Hz was assessed. The slopes obtained from the linear regression model of each group were plotted (Figure 5A). Comparing the control and FPI groups, the slopes were suppressed with either tonic or phasic release (Figure 5B). The slope of the FPI group after the CPP test was still lower than that of the control group (Figure 5B). The release probability also decreased under pre-CPP conditions and this became more significant after CPP tests in the nicotine pretreatment group (Figure 5C), which may be the result of the higher tonic release in this group. In FPI with nicotine pretreatment animals (Figure 5, Pre-Nic+FPI), the release probability before CPP was lower than the Pre-Nic group. After CPP, the release probability decreased further.
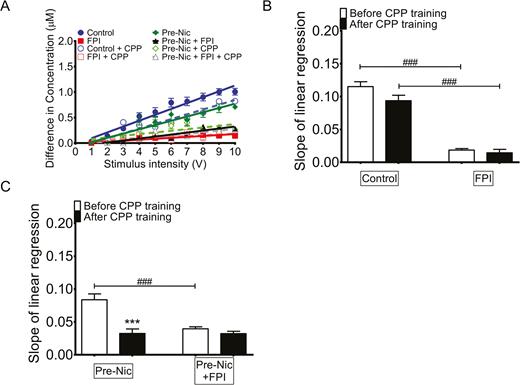
The release capacity of dopaminergic terminals after nicotine-induced conditioned place preference (CPP). (A) To determine whether fluid percussion injury (FPI) may affect the dopamine (DA) transmission in nucleus accumbens (NAc) shell during CPP training, the release probability of control and FPI groups was compared. (B) The slope of linear regression lines representing the release probability of each group was plotted. The release probability after CPP in the control group (blue open circle: control+CPP vs blue solid circle: control) was decreased. In the FPI group, either before (FPI: red solid square) or after CPP (FPI+CPP, red open square), the release probability was markedly suppressed compared with the control group. (C) The FPI effects on DA release probability in NAc shell in nicotine preexposed animals were then measured, and the slope of linear regressions of nicotine pretreated group and nicotine pretreated with FPI groups was compared. The lowest DA release probability was found in the FPI only groups. The DA release probability of the nicotine pretreatment group was high but decreased in FPI with nicotine preexposure history (Pre-Nic+FPI).
The release capacity of dopaminergic terminals in each group, equated to the slope obtained from linear regression, is shown in Figure 5. For all of the groups, the slope decreased after nicotine-induced CPP (black bar vs white bar in each group). After CPP, the largest slope was in the control group (Control+CPP group) followed by the nicotine pretreatment group (Pre-Nic+CPP group), and the FPI with nicotine pretreatment group (Pre-Nic+FPI+CPP group). The lowest slope was in FPI group (FPI+CPP group) (1-way ANOVA [F7, 51=43.77, P<.001]).
Preference for the Conditioned Place Is Related Not only to Phasic Dopamine Release but Also to Release Probability
As previously shown, nicotine-induced CPP was seen in rats that were nicotine pretreated and those that were not pretreated, but it was not significantly found in injured rats (Figure 2A). To further determine the relationship between dopamine release and CPP, after CPP training release probabilities at various stimulation intensities were summed and analyzed using linear regression. The slope of the linear regression line was highest in the CPP group followed by the Control+CPP group. It was lowest in the FPI+CPP group (Figure 5B–C). Linear regression revealed a relationship in tonic release (Figure 6A), phasic release (Figure 6B), and release probability (Figure 6C) concentration differences between pre-CPP and post-CPP states (Figure 6D for tonic and 6E for phasic) at 10-V stimulation intensity. A positive correlation was found between phasic release (Figure 6B, r=0.8110, P=.1890) and release probability (Figure 6C, r=0.9521, P=.0479). Relationships with tonic release were not significant and even negatively correlated with concentration differences between pre-CPP and post-CPP preference scores (Figure 6).
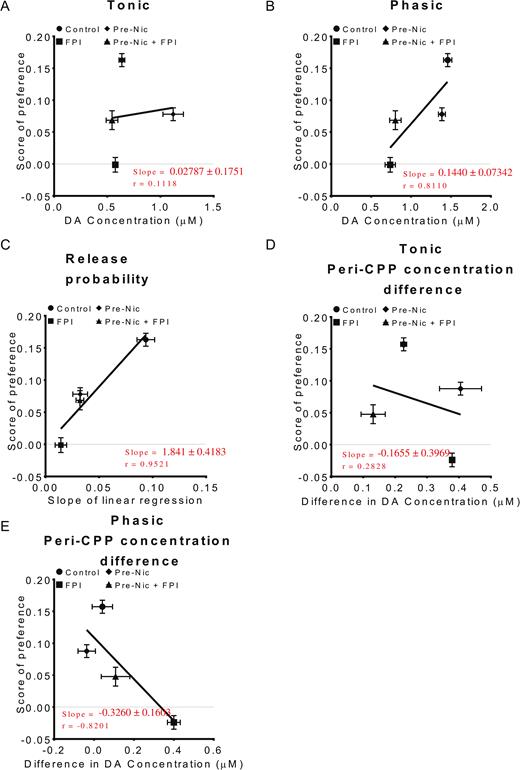
Preference for the conditioned place is correlated with not only dopamine (DA) phasic release but also with differences between tonic and phasic release. Linear regression was used to analyze the relationship between tonic (A, 1p/25 Hz), phasic (B, 10p/25 Hz), and release probability (C) with 10 V stimulation intensity. A positive correlation could be found between phasic release with preference time scores. There was a more significant correlation between release probability and preference time scores (P=.0479). (D–E) Linear regression analysis of peri-CPP concentration differences in (D) tonic releases and (E) phasic releases, which indicated that there were no positive correlations between these peri-CPP concentration differences and conditioned place preference (CPP) preference time scores.
Nicotine Withdrawal Is Not Affected by a FPI
To determine whether TBI affects nicotine withdrawal, rats were exposed to 1 week of nicotine infusion for 7 days after a FPI was induced. Subsequent withdrawal was studied. Both controls and injured rats exhibited withdrawal effects when compared with the saline-infusion group (Figure 7A; 2-way ANOVA [F2,25=27.84, P<.001] saline vs nicotine infusion P<.001). Upon tonic stimulation, DA release was highest among the saline-infusion group followed by the Nic-infusion group. The Nic-infusion+FPI group showed much lower values (Figure 7B; 2-way ANOVA [F3,388=165.7, P<.001]). Upon phasic stimulation, the saline-infusion and Nic-infusion groups showed high values for dopamine release. The Nic-infusion+FPI group had lower values, but injured animals without nicotine treatment had the lowest values (Figure 7C; 2-way ANOVA [F3,500=157.3, P<.001]). Linear regression revealed differences in release probability at various stimulation intensities, reflecting the release capacity of dopaminergic terminals (Figure 7D; 2-way ANOVA [F3, 500=98.76, P<.001]). The slopes of the linear regression lines were small for both groups of injured rats (Figure 7E; 1-way ANOVA [F3,35=506.3, P<.001]).
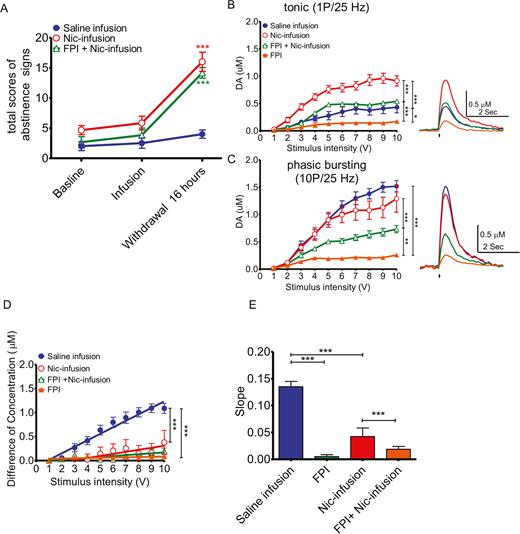
did not affect abstinence/withdrawal. To determine whether traumatic brain injury (TBI) affected withdrawal from nicotine, a 7-day nicotine infusion for 1 week in uninjured or after FPI injury groups, and subsequent withdrawal, was studied. A group of animals with no prior nicotine infusion was also tested (saline infusion). (A) Nicotine infusion in both control (N=6) and FPI (N=6) injured animals showed withdrawal responses after 1 week of nicotine infusion compared to saline-infused control animals (N=6). (B) For tonic release, DA release was highest in the control and saline groups, followed by the control (Saline-infusion; blue solid circle, n=14 slices from 8 rats) and nicotine-treated (Nic-infusion; red open circle, n=6 slices from 3 rats) animals. Both injured groups, with (FPI+Nic-infusion; green open triangle, n=6 slices from 3 rats) and without nicotine pretreatment (FPI; orange solid triangle, n=6 slices from 3 rats) had much lower release values. For phasic release, control animals with and without nicotine had similar high release values. (C) Injured animals with nicotine exposure had lower values, and the injured animals without nicotine treatment had the lowest releases. (D) Linear regression revealed the differences in dopamine concentration between single pulse and 10 pulses at 25 Hz under different stimulation intensities (from 1 to 10 volts), which may reflect the release capacity of dopaminergic synapses. (E) The slopes of the linear regression lines were flat in FPI injured animals with or without nicotine preexposure.
Discussion
In this study, an FPI was found to significantly reduce nicotine-associated CPP in rats, suggesting that dopamine release in the shell portion of NAc is affected by this type of injury. This could play a crucial role in changes in reward or reinforcing behavior. Moreover, preference time seems to correlate with phasic dopamine release and release probability. In addition, nicotine withdrawal was not affected by FPI.
As detailed in the Introduction, addictive drugs can produce physiological changes related to extracellular dopamine fluctuations that are preferentially augmented in the NAc shell rather than its core (Di Chiara and Bassareo, 2007). Therefore, we hypothesized that the reward system is affected by TBI through changes in the dopamine system. Impairment of nicotine-induced CPP, reflecting reward activity, was indeed found here in injured rats.
An electrochemical study of brain slices revealed that nicotine infusions suppressed low-frequency, single-pulse-induced dopamine release but enhanced high-frequency stimulation-induced dopamine release (Cragg, 2006). After nicotine infusion for 1 week, desensitization or inactivation of the nicotine acetylcholine receptors (nAChRs) likely occurs. After 1 week, FSCV data suggest that nAChRs were sensitized with higher tonic dopamine releases (Picciotto et al., 2008) (Figure 3A).
Tonic dopamine release I/O curves before and after CPP training were compared (Figure 4), revealing that increases resulted after training. This was also seen in those animals preexposed to nicotine. The lowest tonic levels were found before CPP training in the CPP and FPI+CPP groups. The I/O curves for phasic dopamine release in each group suggest that release patterns were similar between the CPP group before and after CPP training and the Pre-Nic+CPP group before and after CPP training. The Pre-Nic+FPI+CPP group showed phasic dopamine release both before and after CPP training that was lower than the other groups. The FPI+CPP group showed phasic levels that were higher after CPP training than before training. In fact, the lowest phasic dopamine release levels were in this group before CPP training.
Data from brain slice FSCV assays suggest that nicotine might enhance phasic dopamine release in proportion to pulse or frequency of stimulation (Rice and Cragg, 2004; Cragg, 2006). Because phasic release was low in the FPI+CPP group, incremental increases in DA release after nicotine pretreatment could be seen. Before CPP training, phasic dopamine release in the Pre-Nic+FPI+CPP group was higher than in the FPI+CPP group. After CPP training, phasic dopamine release increased in the latter but remained higher yet in the pretreated rats. There was thus a relationship between phasic dopamine release and nicotine preexposure. In previous studies (Hoebel, 1985; Di Chiara and Bassareo, 2007; Tsai et al., 2009), reward or substance abuse behavior was correlated with phasic dopamine release, and this has now been confirmed and extended by our data.
To assess the release capacity of dopaminergic terminals, the release probability at various stimulation intensities (1–10 V) was analyzed for each group. Multiple linear regression analyses were performed to determine the slopes, which indicated release probabilities. In general, release decreased after CPP training. This phenomenon could be the result of a ceiling effect or the desensitization of nAChRs (Bloem et al., 2014). Among injured groups, the slopes was lower than those found among noninjured groups, whether nicotine pretreated or not.
Nicotine-induced CPP was blunted in nicotine pretreated rats. In these groups, higher baseline tonic levels could have reduced the capacity for phasic dopamine release, or again a ceiling effect may be present (Tizabi et al., 2007). Either possibility could lead to a lower releasing probability, thus resulting in reduced nicotine-induced CPP. On the other hand, lower phasic release after FPI could also lead to a lower releasing probability, again producing reduced CPP. Linear regression analysis of the relationship between CPP parameters and release probability suggests that these are strongly correlated.
Upon examination of the possible impact of TBI on the severity of nicotine withdrawal, no significant differences in withdrawal responses were found between controls and rats with induced FPIs, suggesting that TBI has a minimal effect on the negative reinforcement imparted by withdrawal symptoms, which normally induces continued nicotine use to prevent those symptoms.
In sum, the major conclusions and findings in our study are:
Nicotine-induced CPP could be induced in control animals and animals with a nicotine preexposure history.
Moreover, the change or variation in DA phasic release before and after CPP in each group is found to be one factor that affects the CPP formation.
The factors that are associated with CPP formation in animals were correlated by using linear regression as follows:
a) Dopamine release probability (concentration difference between phasic and tonic release): Pearson’s correlation coefficient r=0.9521.
b) Concentration of phasic release: Pearson’s correlation coefficient is r=0.8110.
c) The concentration difference before and after CPP training: Pearson’s correlation coefficient in tonic concentration difference is r=0.2828 and for phasic concentration difference is r=-0.8201.
Although, the concentration difference between pre- and post-CPP was high in FPI groups, the release probabilities were too low to induce consistent behavior. In humans, reduced capacity for DA release following head injury would lead to a reduction in reward-related DA signaling. Thus, greater amounts of nicotine, or other abused drugs, might be necessary in order to overcome this difference.
Taken together, our findings support the idea that TBI impacts the mesolimbic-mesocortical dopaminergic pathways, thereby reducing nicotine-induced rewards. Therefore, greater stimulation by nicotine might be required to obtain a given level of dopaminergic activity, which in turn might explain increased tobacco abuse among TBI patients. However, in addition to the dopaminergic system, several other neural substrates, including the serotonergic (Wilson et al., 2006; Hein et al., 2007) and noradrenergic systems (Comings and Blum, 2000; Sofuoglu and Sewell, 2009), have been implicated in changes in reward circuitry. Nevertheless, this study demonstrates that TBI has a clear impact on the mesolimbic-mesocortical dopaminergic pathways, reducing dopaminergic release probability in the shell of the NAc, and supporting a connection between reduced rewards of nicotine exposure seen in animal behavioral experiments and clinical findings. In conclusion, an FPI was found to markedly reduce nicotine-associated CPP in rats, and this was accompanied by changes in dopamine release in the shell portion of the NAc. This might play a crucial role in changes in reward and reinforcing behavior. Moreover, CPP time scores correlate with phasic dopamine release and release probability. Finally, nicotine withdrawal is not affected by an FPI.
Statement of Interest
None.
Acknowledgments
This work was supported by the National Science Council of Taiwan (MOST105-2314-B-016-001-MY3), the Tri-Service General Hospital of Taiwan’s Medical Research Project (TSGH-C107-068, TSGH-C107-070, and TSGH-C107-071), and the National Defense Medical Center (MAB-107-022). This project was supported in part by philanthropic support from the George R. and Constance P. Lincoln family.



