-
PDF
- Split View
-
Views
-
Cite
Cite
Ching-Hua Lin, Chun-Jen Huang, Cheng-Chung Chen, ECT Has Greater Efficacy Than Fluoxetine in Alleviating the Burden of Illness for Patients with Major Depressive Disorder: A Taiwanese Pooled Analysis, International Journal of Neuropsychopharmacology, Volume 21, Issue 1, January 2018, Pages 63–72, https://doi.org/10.1093/ijnp/pyx114
Close - Share Icon Share
Abstract
The burden of major depressive disorder includes suffering due to symptom severity, functional impairment, and quality of life deficits. The aim of this study was to compare the differences between electroconvulsive therapy and pharmacotherapy in reducing such burdens.
This was a pooled analysis study including 2 open-label trials for major depressive disorder inpatients receiving either standard bitemporal and modified electroconvulsive therapy with a maximum of 12 sessions or 20 mg/d of fluoxetine for 6 weeks. Symptom severity, functioning, and quality of life were assessed using the 17-item Hamilton Rating Scale for Depression, the Modified Work and Social Adjustment Scale, and SF-36. Side effects following treatment, including subjective memory impairment, nausea/vomiting, and headache, were recorded. The differences between these 2 groups in 17-item Hamilton Rating Scale for Depression, Modified Work and Social Adjustment Scale, quality of life, side effects, and time to response (at least a 50% reduction of 17-item Hamilton Rating Scale for Depression) and remission (17-item Hamilton Rating Scale for Depression ≤7) following treatment were analyzed.
Electroconvulsive therapy (n=116) showed a significantly greater reduction in 17-item Hamilton Rating Scale for Depression, Modified Work and Social Adjustment Scale, and quality of life deficits and had significantly shorter time to response/remission than fluoxetine (n=126). However, the electroconvulsive therapy group was more likely to experience subjective memory impairment and headache.
Compared with fluoxetine, electroconvulsive therapy was more effective in alleviating the burden of major depressive disorder and had a substantially increased speed of response/remission in the acute phase. Increased education and information about electroconvulsive therapy for clinicians, patients, and their families and the general public is warranted.
The results of research conducted in an Asian country revealed that ECT was more effective in reducing the burden of acute phase depression than fluoxetine. Patients with treatment-resistant depression were excluded from the fluoxetine 20 mg group. ECT had a substantially increased speed of symptomatic response and remission compared with fluoxetine, although the ECT group had a higher rate of treatment-resistant depression (86.2%) and experienced more subjective memory impairment and headache, while other side effects were not systematically evaluated in the pooled analysis.
Introduction
Major depressive disorder (MDD) is a common mental disorder. The burden of MDD includes suffering due to symptom severity, functional impairment, and quality of life (QOL) deficits. Such burdens may lead to increased suffering, with negative consequences for families as well as for society (Ishak et al., 2013). MDD is predicted to become the second-leading contributor to the global burden of disease by the year 2020 (Mathers et al., 2008). The overall goals of treatment of MDD should focus on achieving symptom resolution and episode remission, in addition to alleviating functional impairments and QOL deficits (Gelenberg et al., 2010).
In clinical practice, pharmacological treatment and electroconvulsive therapy (ECT) are used to treat MDD patients. ECT is significantly more effective than pharmacotherapy in treating severe and treatment-resistant depression (UK ECT Review Group, 2003; Kellner et al., 2012). However, traditional initial assessment and outcome measurement of mental disorders has been focused on symptom severity. It is generally assumed that changes in depressive symptoms equal changes in functioning and QOL. However, numerous studies support that symptoms, functioning, and QOL are dissociable domains (McCall et al., 2001, 2004; Angermeyer et al., 2002; Mathew et al., 2007; Lam et al., 2011). The American Psychiatric Association practice guideline for the treatment of patients with MDD (Gelenberg et al., 2010) emphasizes the importance of adding functioning and QOL measures to adequately capture the full burden of depression. Functioning refers to an individual’s actual involvement and participation in health and life activities, whereas QOL reflects the patient’s satisfaction with such activities and psychological well-being (WHO, 1998; IsHak et al., 2011).
There are several reasons to replicate and extend the outcomes of ECT from traditional symptom severity to functioning and QOL for depressed patients. First, few studies have simultaneously explored the differences in alleviating depressive symptoms, improving functioning, and reducing QOL deficits between ECT and pharmacotherapy. Second, ECT use in Western countries is much more common than in Asian countries. Psychiatrists in Asia seem reluctant to prescribe ECT and patients may hesitate to receive it. Almost all the studies about ECT outcome for depressed patients are from Western countries. Consequently, data for comparison are limited (Chanpattana et al., 2010). Third, ECT is an “orphan treatment,” as there is no marketing supporting it (Kotzalidis et al., 2015).
The aim of this study was to compare existing data from 2 previously published open-label studies in regard to depressive symptoms, functioning, QOL, and tolerability. The subjects from the first study (Lin et al., 2016) were 130 MDD patients who received ECT with a maximum of 12 treatments. Subjects from the second study (Lin et al., 2011) were 131 MDD patients without a history of treatment-resistant depression who were prescribed 20 mg/d fluoxetine as monotherapy for a period of up to 6 weeks. We hoped to ascertain the differences between the ECT and fluoxetine groups in efficacy in relieving the burden of MDD and speed of response/remission. We hypothesized that ECT-treated patients would yield greater improvements in reducing symptom severity, functional impairments, and QOL deficits than the fluoxetine-treated patients, but would be less tolerated.
Methods
Subjects
The data used in this pooled analysis were drawn from 2 open-label trials for depressed inpatients receiving ECT (ClinicalTrials.gov identifier: NCT02032576; duration: from Jan. 2008 to Oct. 2013) (Lin et al., 2016) or fluoxetine (NCT01075529; from May 2007 to Feb. 2010) (Lin et al., 2011). Two trials were conducted at the Psychosomatic Ward of the Kai-Syuan Psychiatric Hospital, Kaohsiung, Taiwan, and were approved by the hospital’s institutional review board. Patients who satisfied the DSM-IV criteria for MDD as confirmed using the Structured Clinical Interview for DSM-IV Axis I (APA, 1994), were aged ≥18 years, had a baseline 17-item Hamilton Depression Rating Scale (HAMD-17) (Hamilton, 1960)≥18, and no diagnosis of schizophrenia or other psychotic disorders, bipolar disorders, or organic mental disorders were included in both trials. Formal psychotherapy was not permitted during the study period.
Procedure
For the ECT group, MDD inpatients were enrolled if they met the indications of ECT (i.e., need for a rapid and definitive response, high suicide risk, severe psychomotor retardation, and treatment-resistant depression) (Waite and Easton, 2013) and had no serious medical conditions restricting the use of ECT. The practice of ECT was in accord with the American Psychiatric Association Task Force on ECT (APA, 2001). Psychotropic agents, including antidepressants, antipsychotics, and mood stabilizers, were discontinued for 3 days before initiating ECT if emergency ECT was not required. Patients remained medication free during the ECT course, except for anxiolytic or sedative-hypnotic medications as needed for insomnia or severe anxiety. Standard bitemporal and modified ECT was performed. Modified ECT refers to the administration of anesthesia, muscle relaxant, and seizure-inducing electrical stimulus, in that order. For our ECT protocol, anesthesia was induced by thiopental or thiamylal, both at doses of 1.5 to2.0 mg/kg i.v. Neuromuscular blockade was induced by succinylcholine at a dosage of 0.5to1.0 mg/kg i.v. ECT was conducted using the Thymatron System IV machine with brief-pulse and constant current (pulse width, 0.5 ms; frequency, 60 Hz; current, 0.9 A). Seizure duration was at least 20 seoncds as measured by electromyogram and 25 seconds measured by electroencephalography. Treatment was given 3 times a week before Aug. 2009 and later 2 times a week, with a maximum of 12 treatments. The number of ECT treatments was determined by the treating psychiatrist, depending on whether remission (HAMD-17≤7) had been reached, if patients could not tolerate the side effects, or if patients decided to discontinue ECT (APA, 2001).
For the fluoxetine group, physically healthy inpatients who required acute treatment of MDD were enrolled. Patients with treatment-resistant depression or substance dependence/abuse were excluded. Treatment-resistant depression was defined as a lack of response to 2 or more adequate trials of different classes of antidepressants (Souery et al., 1999). An adequate trial was 4 to 6 weeks of treatment with an antidepressant at a dosage considered therapeutic. After a washout period of at least 72 hours, patients received open-label fluoxetine treatment at a fixed dose of 20 mg/d for 6 weeks. No other psychotropic agents were administered during the treatment period, except for anxiolytic or sedative-hypnotic medications as needed for insomnia or severe anxiety. Judgment of treatment adherence was based on the nursing staff’s observations. Both sets of subjects, receiving either ECT or fluoxetine, would complete their trials within 6 weeks.
Outcome Measures
Symptom severity was assessed by independent board-certified psychiatrists using HAMD-17. Higher HAMD-17 scores (ranging from 0 to 52) indicate more severe depression. The Work and Social Adjustment Scale (WSAS) (Mundt et al., 2002) is a 5-item self-rating scale designed to measure functional impairment. Each item is scored from 0 (not affected at all) to 8 (severely affected). Item 1 assesses work ability, but it may be difficult for patients to demonstrate a high level of work functioning while in the hospital when their jobs are outside of the hospital, or for those patients who have retired. Therefore, Item 1 has been omitted. We renamed the Work and Social Adjustment Scale, without Item 1, the Modified Work and Social Adjustment Scale (MWSAS) (ranging from 0 to 32) to assess functioning. The Medical Outcomes Study Short-Form 36 (SF-36) (Brazier et al., 1992), a self-rating scale with 2 primary-factor analytic components, the physical component summary (PCS) and the mental component summary (MCS), was used to measure QOL. Lower PCS and MCS scores reflect worse QOL.
For the ECT group, symptom severity and functioning were assessed using HAMD-17 and MWSAS before ECT, after every 3 ECT treatments, and after the final ECT treatment. QOL was assessed before ECT. A study by Daly et al. (J. J. Daly et al., 2001) found that an average of 6 ECT treatments is needed to reach initial response. Therefore, if patients received at least 6 ECT treatments, QOL was reassessed after the final ECT. To prevent post-ECT confusion from influencing the assessment, all measures were conducted 1 to 2 days after treatment.
For the fluoxetine group, symptom severity and functioning were assessed using HAMD-17 and MWSAS at baseline, and again at weeks 1, 2, 3, 4, and 6. QOL as determined by SF-36 was assessed at baseline and reassessed if patients completed the 6-week fluoxetine treatment.
Side Effects
For the ECT group, side effects not present before ECT, including subjective memory impairment, nausea/vomiting, and headache, either first observed by the psychiatrist at each visit or first reported spontaneously by the patient indicated side effects “cases.”
For the fluoxetine group, side effects were assessed using the Utvalg for Kliniske Undersogelser Side Effect Rating Scale (UKU) (Lingjaerde et al., 1987) and by the registration of side effects at baseline and at weeks 1, 2, 3, 4, and 6. UKU, a clinician-rating scale with 48 items, contains a Likert scale of 0 to 3 for degree of severity. A score of 1, 2, or 3 on any UKU item that first occurred or worsened during treatment indicated a “case” (E. J. Daly et al., 2011). To compare the ECT group with the fluoxetine group, only subjective memory impairment (Item 1.4), nausea/vomiting (Item 3.4), and headache (Item 4.17) were selected.
Statistical Analysis
Analysis was on a modified intent-to-treat basis for subjects reporting at least one post-baseline assessment. Data were analyzed using the SPSS version 17.0 for Windows (SPSS Inc.). Statistical significance was defined as P < .05. Pearson χ2 test or Fisher’s exact test was used to compare categorical variables, and independent t test was used for continuous variables.
In the first step, 2 groups at baseline were compared in terms of sex, age, age at onset, baseline HAMD-17, baseline MWSAS, baseline PCS, baseline MCS, anxiolytic/sedative-hypnotic medications used during the trial period, employment in the 6 months before the trial, and side effects following treatment. Age at onset was defined as the age at which the first major depressive episode occurred. Employment was defined as working for pay in the 6 months before the trial (Lerner et al., 2004).
In the second step, the median pre-post differences in the HAMD-17 and MWSAS scores in both groups were calculated. The generalized estimating equations (GEE) method with the first-order autoregressive working correlation structure (AR 1) (Liang and Zeger, 1986) was applied to examine the differences in HAMD-17 and MWSAS scores between the 2 groups during the course of acute treatment, after adjusting for sex, age, age at onset, and baseline severity (baseline HAMD-17 or MWSAS). Analyses of group differences in PCS and MCS were performed by ANCOVA, with treatment (ECT vs fluoxetine) as a fixed factor and sex, age, age at onset, and the baseline value (baseline PCS or MCS) as covariates.
In the last step, the treating psychiatrists, patients, and their families need to know the onset of a meaningful benefit of treatment, such as symptomatic response or remission. Response was defined as an at least 50% reduction of the baseline HAMD-17 score. Kaplan-Meier survival analysis was used to determine the differences in speed of response/remission between 2 groups.
In contrast to the fluoxetine trial, which is typically of fixed duration and involves fixed time points for assessment (i.e., weeks 0, 1, 2, 3, 4, and 6), ECT is administered over a brief period (i.e., 2 or 3 times weekly) with considerable variability in the time points of clinical assessment (i.e., before ECT, after every 3 ECT treatments, and after the final ECT treatment). We therefore used the exact number of days since the baseline assessment as the time variable in both the GEE and Kaplan-Meier survival analysis. This method has been used previously (Schoeyen et al., 2015).
Results
Subjects
A flow chart of the participant selection process is shown in Figure 1. In the ECT group, 130 patients treated with ECT (n=113 due to treatment-resistant depression, n=15 due to high suicide risk, and n=2 due to severe psychomotor retardation) participated in the study. One-hundred sixteen patients receiving at least 3 ECT treatments (n=100 due to treatment-resistant depression, n=14 due to high suicide risk, and n=2 due to severe psychomotor retardation) were included in the analysis. Twelve of the 116 discontinued ECT prematurely; the remainder (n=104) completed the course of ECT. In the fluoxetine group, 131 patients were enrolled. One hundred twenty-six who had at least one post-baseline assessment at week 1 entered the analysis. Fourteen of the 126 discontinued the fluoxetine trial prematurely; the remainder (n=112) completed the 6-week trial. In the ECT group, the mean number of treatments was 8.9 ± 2.5. One hundred (86.2%) were diagnosed as having treatment-resistant depression, and 103 received ECT twice weekly. Table 1 reveals that the clinical variables at baseline did not significantly differ between the treatment groups. However, the ECT group had significantly lower employment rates than the fluoxetine group. In the ECT group, 105 patients completed the SF-36 assessment before ECT, and 95 of 105 once again after ECT. In the fluoxetine group, 119 patients completed the SF-36 assessment at baseline, and 106 of 119 once again after the 6-week fluoxetine trial.
Baseline Clinical Characteristics and Side Effects Comparing Patients Receiving ECTa and Those Receiving Fluoxetine
| . | ECT . | Fluoxetine . | P . | ||
|---|---|---|---|---|---|
| Variables | n | n | |||
| Sex, female, n (%) | 116 | 82 (70.7) | 126 | 96 (76.2) | .332a |
| Age, mean (SD), year | 116 | 46.9 (12.3) | 126 | 45.3 (11.0) | .286b |
| Age at onset, mean (SD), year | 116 | 38.1 (12.8) | 126 | 38.9 (11. 8) | .636b |
| Baseline HAMD-17 score, mean (SD) | 116 | 30.9 (7.0) | 126 | 31.3 (6.5) | .602b |
| Baseline MWSAS score, mean (SD) | 116 | 23.5 (7.3) | 126 | 23. 8 (7.9) | .747b |
| Baseline SF-36 PCS, mean (SD), | 105 | 42.4 (9.1) | 119 | 40.1 (10.6) | .088b |
| Baseline SF-36 MCS, mean (SD), | 105 | 20.5 (7.7) | 119 | 21.5 (9.0) | .396b |
| Employment in the 6 months before the trial, n (%) | 116 | 19 (16.4) | 126 | 46 (36.5) | <.001a |
| Anxiolytic/sedative-hypnotic medication used, n (%) | 116 | 105 (90.5) | 126 | 113 (89.7) | .828a |
| Side effects following treatment | 116 | 48 (41.4) | 126 | 21 (16.7) | <.001a |
| Subjective memory impairment, n (%) | 116 | 24 (20.7) | 126 | 20 (15.9) | 0.332a |
| Nausea/vomiting n (%) | 116 | 70 (60.3) | 126 | 21 (16.7) | <.001a |
| Headache n (%) | |||||
| . | ECT . | Fluoxetine . | P . | ||
|---|---|---|---|---|---|
| Variables | n | n | |||
| Sex, female, n (%) | 116 | 82 (70.7) | 126 | 96 (76.2) | .332a |
| Age, mean (SD), year | 116 | 46.9 (12.3) | 126 | 45.3 (11.0) | .286b |
| Age at onset, mean (SD), year | 116 | 38.1 (12.8) | 126 | 38.9 (11. 8) | .636b |
| Baseline HAMD-17 score, mean (SD) | 116 | 30.9 (7.0) | 126 | 31.3 (6.5) | .602b |
| Baseline MWSAS score, mean (SD) | 116 | 23.5 (7.3) | 126 | 23. 8 (7.9) | .747b |
| Baseline SF-36 PCS, mean (SD), | 105 | 42.4 (9.1) | 119 | 40.1 (10.6) | .088b |
| Baseline SF-36 MCS, mean (SD), | 105 | 20.5 (7.7) | 119 | 21.5 (9.0) | .396b |
| Employment in the 6 months before the trial, n (%) | 116 | 19 (16.4) | 126 | 46 (36.5) | <.001a |
| Anxiolytic/sedative-hypnotic medication used, n (%) | 116 | 105 (90.5) | 126 | 113 (89.7) | .828a |
| Side effects following treatment | 116 | 48 (41.4) | 126 | 21 (16.7) | <.001a |
| Subjective memory impairment, n (%) | 116 | 24 (20.7) | 126 | 20 (15.9) | 0.332a |
| Nausea/vomiting n (%) | 116 | 70 (60.3) | 126 | 21 (16.7) | <.001a |
| Headache n (%) | |||||
Abbreviations: ECT, electroconvulsive therapy; HAMD-17, 17-item Hamilton Rating Scale for Depression; MCS, mental component summary, lower scores of PCS reflect worse quality of life; MWSAS, Modified Work and Social Adjustment Scale = Work and Social Adjustment Scale (WSAS) without Item 1; PCS, physical component summary, lower scores of PCS reflect worse quality of life;
SF-36, Medical Outcomes Study Short-Form 36.
Bold, statistically significant.
aPearson’s χ2 test
bIndependent t test.
Baseline Clinical Characteristics and Side Effects Comparing Patients Receiving ECTa and Those Receiving Fluoxetine
| . | ECT . | Fluoxetine . | P . | ||
|---|---|---|---|---|---|
| Variables | n | n | |||
| Sex, female, n (%) | 116 | 82 (70.7) | 126 | 96 (76.2) | .332a |
| Age, mean (SD), year | 116 | 46.9 (12.3) | 126 | 45.3 (11.0) | .286b |
| Age at onset, mean (SD), year | 116 | 38.1 (12.8) | 126 | 38.9 (11. 8) | .636b |
| Baseline HAMD-17 score, mean (SD) | 116 | 30.9 (7.0) | 126 | 31.3 (6.5) | .602b |
| Baseline MWSAS score, mean (SD) | 116 | 23.5 (7.3) | 126 | 23. 8 (7.9) | .747b |
| Baseline SF-36 PCS, mean (SD), | 105 | 42.4 (9.1) | 119 | 40.1 (10.6) | .088b |
| Baseline SF-36 MCS, mean (SD), | 105 | 20.5 (7.7) | 119 | 21.5 (9.0) | .396b |
| Employment in the 6 months before the trial, n (%) | 116 | 19 (16.4) | 126 | 46 (36.5) | <.001a |
| Anxiolytic/sedative-hypnotic medication used, n (%) | 116 | 105 (90.5) | 126 | 113 (89.7) | .828a |
| Side effects following treatment | 116 | 48 (41.4) | 126 | 21 (16.7) | <.001a |
| Subjective memory impairment, n (%) | 116 | 24 (20.7) | 126 | 20 (15.9) | 0.332a |
| Nausea/vomiting n (%) | 116 | 70 (60.3) | 126 | 21 (16.7) | <.001a |
| Headache n (%) | |||||
| . | ECT . | Fluoxetine . | P . | ||
|---|---|---|---|---|---|
| Variables | n | n | |||
| Sex, female, n (%) | 116 | 82 (70.7) | 126 | 96 (76.2) | .332a |
| Age, mean (SD), year | 116 | 46.9 (12.3) | 126 | 45.3 (11.0) | .286b |
| Age at onset, mean (SD), year | 116 | 38.1 (12.8) | 126 | 38.9 (11. 8) | .636b |
| Baseline HAMD-17 score, mean (SD) | 116 | 30.9 (7.0) | 126 | 31.3 (6.5) | .602b |
| Baseline MWSAS score, mean (SD) | 116 | 23.5 (7.3) | 126 | 23. 8 (7.9) | .747b |
| Baseline SF-36 PCS, mean (SD), | 105 | 42.4 (9.1) | 119 | 40.1 (10.6) | .088b |
| Baseline SF-36 MCS, mean (SD), | 105 | 20.5 (7.7) | 119 | 21.5 (9.0) | .396b |
| Employment in the 6 months before the trial, n (%) | 116 | 19 (16.4) | 126 | 46 (36.5) | <.001a |
| Anxiolytic/sedative-hypnotic medication used, n (%) | 116 | 105 (90.5) | 126 | 113 (89.7) | .828a |
| Side effects following treatment | 116 | 48 (41.4) | 126 | 21 (16.7) | <.001a |
| Subjective memory impairment, n (%) | 116 | 24 (20.7) | 126 | 20 (15.9) | 0.332a |
| Nausea/vomiting n (%) | 116 | 70 (60.3) | 126 | 21 (16.7) | <.001a |
| Headache n (%) | |||||
Abbreviations: ECT, electroconvulsive therapy; HAMD-17, 17-item Hamilton Rating Scale for Depression; MCS, mental component summary, lower scores of PCS reflect worse quality of life; MWSAS, Modified Work and Social Adjustment Scale = Work and Social Adjustment Scale (WSAS) without Item 1; PCS, physical component summary, lower scores of PCS reflect worse quality of life;
SF-36, Medical Outcomes Study Short-Form 36.
Bold, statistically significant.
aPearson’s χ2 test
bIndependent t test.
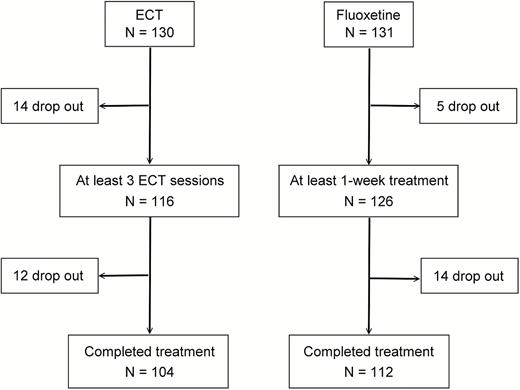
Study design for patients with major depressive disorder treated with electroconvulsive therapy or fluoxetine.
Premature Discontinuation
The clinical baseline characteristics of dropout patients did not differ significantly between the ECT group (n=26) and the fluoxetine group (n=19) with respect to sex (P=.487), age (P=.944), age at onset (P=.869), baseline HAMD-17 (P=.135), and baseline MWSAS (P=.169) (data not shown in the table). There were no significantly different dropout rates between the ECT group (20.0%=26/130) and the fluoxetine group (14.5%=19/131) (χ2=1.31, df =1, P=.240). Thirteen (13/26=50%) patients did not complete the ECT course due to side effects: complaints of pain (n=6), confusion or memory problems (n=2), hypoxia after ECT (n=3), and a high frequency of ventricular premature contraction, lasting for a long duration, which occurred after ECT (n=2). Nineteen patients did not complete the fluoxetine trial due to lack of efficacy (n=3), premature discharge (n=14), and withdrawal of consent (n=2). In contrast to the patients treated with ECT, none of fluoxetine-treated patients dropped out due to side effects (Fisher’s exact test, P<.001).
Efficacy and Side Effects
The ECT group had greater median pre-post differences than the fluoxetine group, regardless of rating by HAMD-17 (23.0 vs 16.0) or MWSAS (12.0 vs 4.0) (data not shown in the table). After adjusting for sex, age, age at onset, and baseline severity (baseline HAMD-17 or MWSAS) using GEE, the ECT group had significantly decreased posttreatment HAMD-17 and MWSAS scores compared with the fluoxetine group during the course of acute treatment, on average by 4.29 and 3.59 points, respectively (Table 2). Figure 2 shows the HAMD-17 scores between the ECT group and the fluoxetine group during the course of acute treatment. Similarly, score gains in PCS and MCS for the ECT group were significantly greater than those for the fluoxetine group, as analyzed by ANCOVA, with sex, age, age at onset, and baseline values (baseline PCS or MCS) as covariates (Table 3). However, the ECT group experienced significantly higher rates of subjective memory impairment and headache than did the fluoxetine group following treatment (Table 1).
Effects of Sex, Treatment (ECT vs Fluoxetine), Treatment Duration, Age, Age at Onset, and Baseline Severity (Baseline HAMD-17 or MWSAS) over Time on the HAMD-17 Score or MWSAS Score Using the Generalized Estimating Equations
| . | HAMD-17 . | MWSAS . | ||||
|---|---|---|---|---|---|---|
| Variable . | Estimate . | SE . | P . | Estimate . | SE . | P . |
| Male vs female | -0.30 | 0.55 | .583 | -0.97 | 0.68 | .150 |
| ECT vs fluoxetine | -4.29 | 0.53 | <.001 | -3.59 | 0.62 | <.001 |
| Treatment duration (1-day increments) | -0.52 | 0.02 | <.001 | -0.25 | 0.02 | <.001 |
| Age (1-year increments) | 0.04 | 0.04 | .249 | -0.01 | 0.04 | .771 |
| Age at onset (1-year increments) | -0.05 | 0.04 | .193 | -0.03 | 0.04 | .513 |
| Baseline HAMD-17 or MWSAS (1-point increments) | 0.59 | 0.04 | <.001 | 0.74 | 0.04 | <.001 |
| . | HAMD-17 . | MWSAS . | ||||
|---|---|---|---|---|---|---|
| Variable . | Estimate . | SE . | P . | Estimate . | SE . | P . |
| Male vs female | -0.30 | 0.55 | .583 | -0.97 | 0.68 | .150 |
| ECT vs fluoxetine | -4.29 | 0.53 | <.001 | -3.59 | 0.62 | <.001 |
| Treatment duration (1-day increments) | -0.52 | 0.02 | <.001 | -0.25 | 0.02 | <.001 |
| Age (1-year increments) | 0.04 | 0.04 | .249 | -0.01 | 0.04 | .771 |
| Age at onset (1-year increments) | -0.05 | 0.04 | .193 | -0.03 | 0.04 | .513 |
| Baseline HAMD-17 or MWSAS (1-point increments) | 0.59 | 0.04 | <.001 | 0.74 | 0.04 | <.001 |
Bold, statistically significant.
Effects of Sex, Treatment (ECT vs Fluoxetine), Treatment Duration, Age, Age at Onset, and Baseline Severity (Baseline HAMD-17 or MWSAS) over Time on the HAMD-17 Score or MWSAS Score Using the Generalized Estimating Equations
| . | HAMD-17 . | MWSAS . | ||||
|---|---|---|---|---|---|---|
| Variable . | Estimate . | SE . | P . | Estimate . | SE . | P . |
| Male vs female | -0.30 | 0.55 | .583 | -0.97 | 0.68 | .150 |
| ECT vs fluoxetine | -4.29 | 0.53 | <.001 | -3.59 | 0.62 | <.001 |
| Treatment duration (1-day increments) | -0.52 | 0.02 | <.001 | -0.25 | 0.02 | <.001 |
| Age (1-year increments) | 0.04 | 0.04 | .249 | -0.01 | 0.04 | .771 |
| Age at onset (1-year increments) | -0.05 | 0.04 | .193 | -0.03 | 0.04 | .513 |
| Baseline HAMD-17 or MWSAS (1-point increments) | 0.59 | 0.04 | <.001 | 0.74 | 0.04 | <.001 |
| . | HAMD-17 . | MWSAS . | ||||
|---|---|---|---|---|---|---|
| Variable . | Estimate . | SE . | P . | Estimate . | SE . | P . |
| Male vs female | -0.30 | 0.55 | .583 | -0.97 | 0.68 | .150 |
| ECT vs fluoxetine | -4.29 | 0.53 | <.001 | -3.59 | 0.62 | <.001 |
| Treatment duration (1-day increments) | -0.52 | 0.02 | <.001 | -0.25 | 0.02 | <.001 |
| Age (1-year increments) | 0.04 | 0.04 | .249 | -0.01 | 0.04 | .771 |
| Age at onset (1-year increments) | -0.05 | 0.04 | .193 | -0.03 | 0.04 | .513 |
| Baseline HAMD-17 or MWSAS (1-point increments) | 0.59 | 0.04 | <.001 | 0.74 | 0.04 | <.001 |
Bold, statistically significant.
Changes of PCS and MCS before and after Treatment for Patients Receiving ECT and Fluoxetine
| . | ECT . | Fluoxetine . | Pa . | ||
|---|---|---|---|---|---|
| Variables . | n . | . | n . | . | . |
| SF-36 PCS change, mean (SD) | 95 | 6.1 (9.4) | 106 | 1.3 (9.3) | <.001 |
| SF-36 MCS change, mean (SD) | 95 | 12.0 (11.2) | 106 | 6.4 (11.6) | .013 |
| . | ECT . | Fluoxetine . | Pa . | ||
|---|---|---|---|---|---|
| Variables . | n . | . | n . | . | . |
| SF-36 PCS change, mean (SD) | 95 | 6.1 (9.4) | 106 | 1.3 (9.3) | <.001 |
| SF-36 MCS change, mean (SD) | 95 | 12.0 (11.2) | 106 | 6.4 (11.6) | .013 |
aP values were determined by ANCOVA, with sex, age, age at onset, and baseline value as a covariate.
Bold, statistically significant.
Changes of PCS and MCS before and after Treatment for Patients Receiving ECT and Fluoxetine
| . | ECT . | Fluoxetine . | Pa . | ||
|---|---|---|---|---|---|
| Variables . | n . | . | n . | . | . |
| SF-36 PCS change, mean (SD) | 95 | 6.1 (9.4) | 106 | 1.3 (9.3) | <.001 |
| SF-36 MCS change, mean (SD) | 95 | 12.0 (11.2) | 106 | 6.4 (11.6) | .013 |
| . | ECT . | Fluoxetine . | Pa . | ||
|---|---|---|---|---|---|
| Variables . | n . | . | n . | . | . |
| SF-36 PCS change, mean (SD) | 95 | 6.1 (9.4) | 106 | 1.3 (9.3) | <.001 |
| SF-36 MCS change, mean (SD) | 95 | 12.0 (11.2) | 106 | 6.4 (11.6) | .013 |
aP values were determined by ANCOVA, with sex, age, age at onset, and baseline value as a covariate.
Bold, statistically significant.
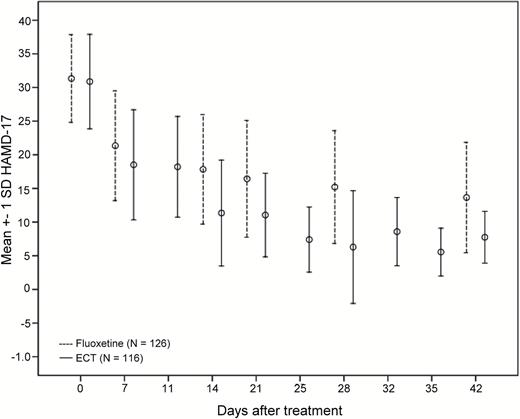
Error bars showing the 17-item Hamilton Rating Scale for Depression (HAMD-17) scores between the electroconvulsive therapy (ECT) group and the fluoxetine group during the course of acute treatment.
Remission and Response
Among the patients who completed treatment, the response rate of 92.3% (96/104) in the ECT group was significantly higher than the 58.9% (66/112) in the fluoxetine group (χ2=32.04, df=1, P<.001). The ECT group (71.2%=74/104) also had a significantly higher remission rate than the fluoxetine group (27.7%=31/112) (χ2=40.80, df=1, P<.001). Patients treated with ECT (mean time±SE=19.3±0.9 days) also had a significantly shorter time to response than those treated with fluoxetine (mean time±SE=24.3±1.3 days) (log rank=13.48, df=1, P <.001). Time to remission for ECT-treated patients (mean time±SE=29.9±1.0 days) was also significantly shorter than for fluoxetine-treated patients (mean time±SE=35.2 ± 1.0 days) (log rank=36.48, df=1, P<.001). Figure 3 compares the time to response between the 2 groups, and Figure 4 compares the time to remission between the 2 groups.
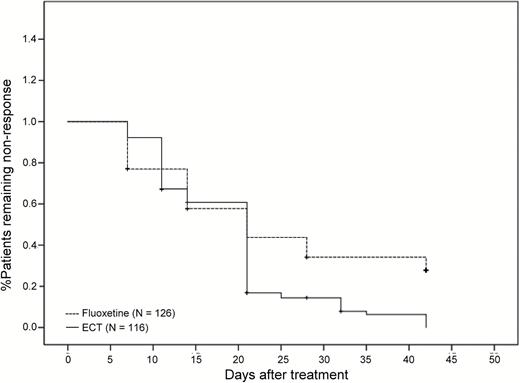
Time to response (log rank=13.48, df=1, P<.001) for patients with major depressive disorder receiving ECT or fluoxetine.
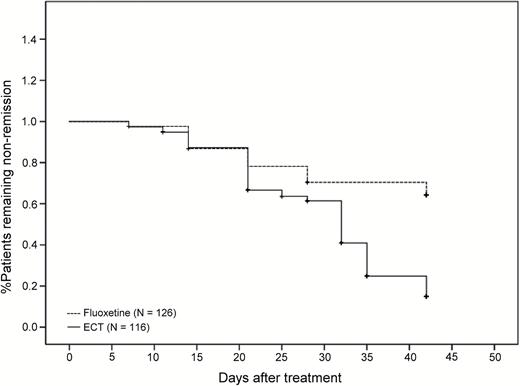
Time to remission (log rank=36.48, df=1, P<.001) for patients with major depressive disorder receiving ECT or fluoxetine.
Discussion
This pooled analysis study revealed 3 main findings: (1) ECT alleviated the burden of MDD more than fluoxetine in the acute phase (Tables 2 and 3); (2) compared to fluoxetine-treated patients, ECT-treated patients had a more rapid onset of response/remission. Our results may be comparable with the findings by a randomized study that showed ECT is superior to paroxetine in treatment-resistant depression in terms of both degree and speed of response (Folkerts et al., 1997). (3) The ECT group had a dropout rate comparable with the fluoxetine group. One-half of the dropout patients in the ECT group discontinued treatment due to side effects, whereas none of the patients in the fluoxetine group dropped out of the trial because of side effects. Patients treated with ECT were likely to experience subjective memory impairment and headache more often than fluoxetine following treatment (Table 1). This indicates that fluoxetine is better tolerated than ECT. Even though unpleasant side effects are widely believed to impair the patient’s QOL (Wisniewski et al., 2007), ECT still improved QOL more than fluoxetine (Table 3).
The patients in the ECT or fluoxetine groups represented a relatively severely ill population who had not responded adequately to outpatient treatment and needed hospitalization (Gelenberg et al., 2010). Previous studies have found that patients with severe depression are associated with significant functional impairment and poor QOL (Coryell et al., 1993; Thase, 2000; Trivedi et al., 2006). Both groups had comparable symptom severity (Table 1), even though 86% of the patients in the ECT group were treatment-resistant. It is reasonable that there were no statistically significant differences between these 2 groups with respect to functional impairment and QOL deficit at baseline (Table 1). Compared with the fluoxetine group, the ECT group showed significantly lower employment rates in the 6 months before the trial (Table 1). There was a distinct possibility that a high percentage of the patients in the ECT group were treatment-resistant, because patients with treatment-resistant depression have been reported to experience lower employment rates than those without (Souery et al., 2007). This may be associated with longer durations of current episodes and longer stays in hospital after several ineffective treatment options (Souery et al., 2007; Zaninotto et al., 2013).
Although the ECT can be superior to antidepressant drugs in reducing the burden of depression for patients, it is generally applied as the treatment of last resort. There is still a majority of MDD patients with an indication for ECT who have not received ECT. One possible reason is that side effects may compromise adherence to ECT, even though most side effects are transient (Fink, 2014). Psychiatrists must help patients reduce their fear and manage such side effects. For example, prophylaxis with antiemetic agents and analgesics to relieve nausea/vomiting and headache might be considered before undergoing ECT (Payne and Prudic, 2009). Some strategies recommended to decrease memory impairment include setting the ECT machine with brief pulse waveform, unilateral nondominant stimulus electrode placement, and ultra-brief pulse stimuli. Also, decreasing stimulus intensity and decreasing the frequency and number of treatments may prove beneficial (Payne and Prudic, 2009; Mankad, 2010). On the other hand, more professional education about ECT and related mental health laws for psychiatrists may increase the numbers of ECT prescriptions or referrals (Finch et al., 1999; Dauenhauer et al., 2011).
The ECT group was treated with bi-temporal ECT. Unilateral electrode placement might have reduced the observed incidence of subjective memory impairment (Waite and Easton, 2013). Therefore, whether the present findings can be extrapolated to those treated with right unilateral ECT requires additional study. However, one study (Prudic et al., 1996) has concluded that ECT outcome appears to be independent of electrode placement.
Treatment with a fixed dose of 20 mg/d of fluoxetine treatment might be considered insufficient to demonstrate response, as in clinical practice individuals vary in their dosage for optimal treatment response. However, earlier fixed-dose studies (Schweizer et al., 1990; Stokes, 1993) have demonstrated that 20 mg of fluoxetine daily is the optimal dose for most patients. A meta-analysis study by Beasley et al. (Beasley et al., 2000) also found that fluoxetine therapy at 20 mg daily is a critical factor for adequate therapy and has good treatment tolerance. Berney (Berney, 2005) concluded that a flat dose-response curve is a class phenomenon for selective serotonin reuptake inhibitors, regardless of whether patients have mild or moderate-to-severe depression. Therefore, the poor outcome of the fluoxetine group did not appear to be due to the 20 mg/d of fluoxetine. Additionally, because plasma levels were not analyzed in the fluoxetine group, the 20 mg/d of fluoxetine prescribed might be criticized as to whether the therapeutic plasma levels could be reached. However, previous studies reported no evidence of a relationship between fluoxetine plasma concentrations and clinical response (Kelly et al., 1989; Norman et al., 1993; Amsterdam et al., 1997).
Several strengths of this study should be addressed. First, to our knowledge, this is the first study to focus on symptom severity, functioning, QOL, side effects, and the speed of symptomatic response/remission when simultaneously comparing the results of ECT and antidepressant medication in depressed patients. Symptom severity reflects only a portion of the burden of major depressive disorder (Cohen et al., 2013). Both functioning and QOL assessed by self-rating scales (i.e., MWSAS and SF-36) may reflect patients’ perspectives on ECT. Second, the GEE offers advantages over standard regression techniques in that it permits examination of the relationships between variables at all time points, adjusts for the within-subject dependence effect, and allows for the inclusion of subjects with missing data (Liang and Zeger, 1986; Twisk, 2003; Madhoo and Levine, 2015). Third, survival analysis may display the greatest sensitivity in detecting treatment group differences in speed of symptomatic response/remission (Nobler et al., 1997). Fourth, our results could be generalized to a clinical setting (Nierenberg et al., 1995).
The present study was also subject to certain limitations. First, this pooled analysis study was nonrandomized and unblinded, which could contribute to major bias. The pooled analysis study came from 2 short-term and open-label studies. We did not know how long such outcome differences between 2 groups could last. Additionally, it was difficult to estimate the degree to which clinical improvements were due to treatments, placebo effect, or other psychiatric interventions. For example, depressed patients in hospital do obtain relief from milieu approaches (Rasmussen, 2009). Patients and clinicians both know the treatments and both may anticipate the outcomes. These anticipations may contribute to a placebo effect. However, it is unlikely the clinical response was solely attributable to placebo effects for the following reasons: first, the response rates of the ECT (92.3%) and the fluoxetine groups (58.9%) were too high to be accountable by the typical placebo effect, i.e., around 30 %, as estimated from past clinical placebo-controlled antidepressant trials (Walsh et al., 2002); second, it has been demonstrated that patients with severe depression and with treatment-resistant depression present a lower placebo response (Khan et al., 2002; Brunoni et al., 2009). However, both trials were open-label. The placebo effect might not be comparable in the 2 groups and must not be underestimated.
Second, the 6-week treatment with fluoxetine might also be criticized as being of too short a duration for the stability of the result to be verified, as longer treatment durations may lead to further improvement in depressive symptoms and functioning (Kocsis et al., 2002; Rush et al., 2006). Therefore, prolonged fluoxetine treatment may yield greater improvement, thereby lessening the difference between fluoxetine and ECT. However, the 6-week period was relatively long and sufficient for inpatient trials to detect initial responses to antidepressant medication. For the ECT group, 6 to 12 treatments are necessary for most patients in usual clinical practice (APA, 2001; Charles H. Kellner, 2012), but a patient with poor response after 12 treatments is not likely to have a favorable response even after receiving more ECT treatments (Waite and Easton, 2013). However, the rate and quality of response to ECT are highly individualized. Some patients may need as many as 20 treatments to obtain maximal improvement (Mankad, 2010).
Third, the side effects of ECT trial were determined by clinical observation rather than UKU, which was developed to assess the side effects in a system way (Lingjaerde et al., 1987). Therefore, the intensity of ECT side effects was not reported, and other common side effects which occurred with fluoxetine were not compared. In addition, there was no objective measurement of memory impairment and other adverse cognitive effects following treatments.
Fourth, the SF-36 is designed to assess the QOL over the previous 4 weeks (Brazier et al., 1992). We did not measure the post-ECT QOL for patients who did not complete at least 6 ECT or the entire fluoxetine trial.
Fifth, the wash-out period of each trial was relatively short due to ethical concerns. It would be inhumane to leave patients with severe depression untreated for too long, even though all inpatients were under close surveillance. Consequently, the carry over effects of medication used before the trials may confound the outcome during the early treatment period.
Sixth, the side effects of anxiolytics/sedative-hypnotic medication may be mistaken as being due to the ECT or fluoxetine. The anxiolytic/sedative-hypnotic medication may also lower the HAMD-17 score during the trial period, often resulting in improvement. Thus, some initial improvement may be due to the sedating effects of anxiolytics/sedative-hypnotic medication rather than the ECT or fluoxetine (Roose and Nobler, 2001; Smith et al., 2002). However, no significant difference existed in the rates of anxiolytics/sedative-hypnotic medication used between the ECT and fluoxetine groups (Table 1). The impact on outcome or tolerability of each group should therefore be comparable.
In conclusion, the current results revealed that bi-temporal ECT was more effective in reducing the burden of acute phase depression than fluoxetine. Bi-temporal ECT had a substantially increased speed of symptomatic response and remission compared to fluoxetine, although the ECT group had a higher rate of treatment-resistant depression (86.2%) and experienced more unpleasant side effects. Increased education and information about ECT for clinicians, patients and their family, and the general public is indispensable.
Funding
This study was supported by the Kaohsiung Municipal Kai-Syuan Psychiatric Hospital (KSPH-2007-16, KSPH-2008-12) and the Ministry of Science and Technology, Taiwan (MOST-103-2314-B-280-001-MY3).
Statement of interest
None.
Acknowledgments
We would like to thank all the participants for this study.
References



