-
PDF
- Split View
-
Views
-
Cite
Cite
Ling-Zhi Xu, De-Feng Xu, Ying Han, Li-Jing Liu, Cheng-Yu Sun, Jia-Hui Deng, Ruo-Xi Zhang, Ming Yuan, Su-Zhen Zhang, Zhi-Meng Li, Yi Xu, Jin-Sheng Li, Su-Hua Xie, Su-Xia Li, Hong-Yan Zhang, Lin Lu, BDNF-GSK-3β-β-Catenin Pathway in the mPFC Is Involved in Antidepressant-Like Effects of Morinda officinalis Oligosaccharides in Rats, International Journal of Neuropsychopharmacology, Volume 20, Issue 1, 1 January 2017, Pages 83–93, https://doi.org/10.1093/ijnp/pyw088
Close - Share Icon Share
Abstract
Morinda officinalis oligosaccharides have been reported to exert neuroprotective and antidepressant-like effects in the forced swim test in mice. However, the mechanisms that underlie the antidepressant-like effects of Morinda officinalis oligosaccharides are unclear.
Chronic unpredictable stress and forced swim test were used to explore the antidepressant-like effects of Morinda officinalis oligosaccharides and resilience to stress in rats. The phosphoinositide-3 kinase inhibitor LY294002 was microinjected in the medial prefrontal cortex to explore the role of glycogen synthase kinase-3β in the antidepressant-like effects of Morinda officinalis oligosaccharides. The expression of brain-derived neurotrophic factor, phosphorylated-Ser9-glycogen synthase kinase 3β, β-catenin, and synaptic proteins was determined in the medial prefrontal cortex and the orbitofrontal cortex by western blot.
We found that Morinda officinalis oligosaccharides effectively ameliorated chronic unpredictable stress-induced depression-like behaviors in the sucrose preference test and forced swim test. The Morinda officinalis oligosaccharides also significantly rescued chronic unpredictable stress-induced abnormalities in the brain-derived neurotrophic factor-glycogen synthase kinase-3β-β-catenin pathway and synaptic protein deficits in the medial prefrontal cortex but not orbitofrontal cortex. The activation of glycogen synthase kinase-3β by the phosphoinositide-3 kinase inhibitor LY294002 abolished the antidepressant-like effects of Morinda officinalis oligosaccharides in the forced swim test. Naïve rats that were treated with Morinda officinalis oligosaccharides exhibited resilience to chronic unpredictable stress, accompanied by increases in the expression of brain-derived neurotrophic factor, phosphorylated-Ser9-glycogen synthase kinase-3β, and β-catenin in the medial prefrontal cortex.
Our findings indicate that the brain-derived neurotrophic factor-glycogen synthase kinase-3β-β-catenin pathway in the medial prefrontal cortex may underlie the antidepressant-like effect of Morinda officinalis oligosaccharides and resilience to stress.
Depression is one of the most prevalent and debilitating disorders worldwide that causes high social and economic burden. Currently available antidepressants have undesirable side effects, and only one-half of patients experience improvement with these therapies, highlighting the pressing need to develop novel antidepressants with limited side effects. In the present study, we tested the antidepressant effects of Morinda officinalis oligosaccharides in 2 models of depression, chronic unpredictable stress and the forced swim test. We found that Morinda officinalis oligosaccharides treatment produced antidepressant-like effects and promoted resilience to stress, accompanied by increased expression of brain-derived neurotrophic factor, phosphorylated-Ser9-glycogen synthase kinase-3β, β-catenin, and synaptic proteins in the medial prefrontal cortex. Glycogen synthase kinase-3β activation by LY294002 abolished the antidepressant-like effects of Morinda officinalis oligosaccharides in the forced swim test. Altogether, our findings suggest that the brain-derived neurotrophic factor-glycogen synthase kinase-3β-3β-β-catenin pathway in the medial prefrontal cortex may mediate the antidepressant-like effect of Morinda officinalis oligosaccharides and resilience to stress.
Introduction
Major depressive disorder is one of the most common neuropsychiatric syndromes, with a high lifetime prevalence that places significant burdens on the economy and society worldwide (Kessler et al., 2005; Belmaker and Agam, 2008). However, only ~50% of depressed patients respond to current antidepressants, and 70% of them fail to achieve full remission (Gartlehner et al., 2007, 2011). The currently available antidepressants also have undesirable side effects, such as sexual dysfunction (Perlis et al., 2009; Bishop et al., 2012). The development of novel antidepressants with high efficacy and limited side effects is necessary.
Morinda officinalis is an herb that is widely used as a Yang-tonic agent to invigorate renal function and improve sexual performance (Song et al., 2015). Morinda officinalis oligosaccharides (MOs) have been detected as the active ingredient (Zhou et al., 2014; Wu et al., 2015). Several studies have reported the neuroprotective effects of MOs in vitro and in vivo, and MOs have been shown to reduce immobility time in the forced swim test (FST) in rodents (Zhang et al., 2002; Li et al., 2003, 2004; Chen et al., 2013, 2014). However, the molecular mechanisms that underlie the antidepressant-like effects of MOs and resilience to stress are still unclear.
Glycogen synthase kinase-3β (GSK-3β) is a ubiquitous serine/threonine protein kinase that is constitutively active under basal conditions. The phosphorylation of GSK-3β at the Ser9 residue inhibits the activity of GSK-3β in response to upstream signals (Li and Jope, 2010). Akt is the major downstream target of phosphoinositide-3 kinase (PI3K), which regulates cell growth and migration. Once activated, Akt leads to GSK-3β inactivation through phosphorylation at the Ser9 residue (Cross et al., 1995). Growing evidence indicates that GSK-3β is an important target of the therapeutic actions of drugs that are used to treat mood disorders, and the inhibition of GSK-3β activity is required for the rapid antidepressant-like effects of ketamine (Li and Jope, 2010; Beurel et al., 2011; Duman and Aghajanian, 2012; Liu et al., 2013). Phosphorylated GSK-3β decreased in the medial prefrontal cortex (mPFC) and hippocampus in rats that were subjected to chronic unpredictable stress (CUS) (Zhu et al., 2013). GSK-3β activation reduces the stability of β-catenin and leads to its degradation through ubiquitination, whereas GSK-3β inhibition stabilizes β-catenin and increases its nuclear translocation to regulate gene expression, synaptic plasticity, and neurogenesis (Li and Jope, 2010; Garza et al., 2012), which in turn exerts antidepressant effects (Beurel et al., 2011; Duman and Aghajanian, 2012; Zhu et al., 2013). β-Catenin has also been implicated in neuropsychiatric disorders, including depression (Madsen et al., 2003), and mediates resilience to stress (Wilkinson et al., 2011; Dias et al., 2014). Accumulating evidence indicates that brain-derived neurotrophic factor (BDNF) plays a critical role in the pathophysiology and treatment of depression and is involved in the therapeutic response to antidepressants (Shimizu et al., 2003; Duman and Monteggia, 2006; Frodl et al., 2007; Groves, 2007; Licinio et al., 2009; Autry et al., 2011; Duman and Aghajanian, 2014; Hosang et al., 2014; Molendijk et al., 2014). BDNF binds to tyrosine kinase receptor B to activate PI3K and Akt, thus inhibiting GSK-3β activity (Li and Jope, 2010). We found that MOs increased BDNF levels in the hippocampus in our previous study (Xu et al., 2015).
In the present study, we tested the antidepressant-like effects of MOs in a model of CUS and the FST in adult male rats and determined whether the BDNF-GSK-3β-β-catenin pathway in the mPFC is involved in this process.
Methods
Animals and Drugs
Male Sprague-Dawley rats were purchased from the Center of Laboratory Animal Science, Peking University Health Science Center. The rats weighed 240 to 260 g upon arrival. They were group-housed under a constant temperature (23 ± 2ºC) and a 12-h-light/12-h-dark cycle (8:00 am lights on, 8:00 pm lights off). Food and water were available ad libitum in the home cages. The rats were acclimated to handling and allowed to adapt for a minimum of 7 days before starting the experiment. All of the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011) and were approved by the Peking University Animal Use Committee (LA2012/21). All of the behavioral tests and drug administration were performed during the dark phase of the light/dark cycle.
The MOs were dissolved in distilled H2O (Beijing Tongrentang Ltd. Co., Beijing, China). The doses of MOs (12.5, 25, and 50 mg/kg) were based on our previous study (Xu et al., 2015). Fluoxetine (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled H2O, as described previously (Xu et al., 2015). LY294002 (10 nM/0.5 μL; Sigma-Aldrich) was dissolved in phosphate-buffered saline (pH 7.4) that contained 50% dimethylsulfoxide as described previously (Zhu et al., 2013). The MOs (12.5, 25, and 50 mg/kg, i.g.) and fluoxetine (10 mg/kg, i.g.) were administered once per day for 14 days from the beginning of week 4 until the end of the CUS protocol. For the experiments that assessed resilience to stress, MOs (50 mg/kg) were administered i.g. once per day for 14 days before the CUS protocol.
Animal Treatments and Behavioral Measurements
Locomotor Activity
Locomotor activity was measured with an automated video tracking system (DigBehv-LM4, Shanghai Jiliang Software Technology, Shanghai, China) that consisted of 8 identical clear Plexiglas chambers (40 cm × 40 cm×65 cm). The video files were analyzed using DigBehv analysis software. Locomotor activity is expressed as the total distance traveled (in centimeters) during a predetermined period of time (5 minutes) as described previously (Suo et al., 2013).
FST
Each rat was individually placed in a 25-cm diameter × 65-cm high plastic cylinder that was filled with 23 to 25°C water to a depth of 45cm for 15minutes. The rats were tested 24 hours later for 5 minutes. Immobility was defined as the minimum movement required to passively keep the animal’s head above the water without other motions. The results are expressed as the amount of time (in seconds) that the animals spent immobile during the 5-minute test (Shi et al., 2012; Suo et al., 2013; Zhu et al., 2013).
Sucrose Preference Test
We measured sucrose preference as previously described (Shi et al., 2012; Suo et al., 2013; Zhu et al., 2013). The rats were trained to adapt to a 1% sucrose solution (w/v) for 48 hours at the beginning of the experiment, during which 2 bottles of 1% sucrose solution were placed in each cage. After adaptation, the rats were deprived of water and food for 4 hours, followed by the sucrose preference test (SPT), in which the rats were housed in individual cages for 1 hour and had free access to 2 identical bottles that contained 1% sucrose or tap water. The position of the 2 bottles was counterbalanced across the left and right sides of the cages at 30 minutes during the test. After 1 hour, sucrose and water consumption (in grams) was measured, and sucrose preference (percent) was calculated as the ratio of sucrose consumption to sucrose + water consumption (sucrose consumption / [sucrose consumption + water consumption] × 100%).
CUS
In the CUS procedure, the animals were exposed to a variable sequence of mild and unpredictable stressors, with 2 stressors per day for 35 days. The stressors included food or water deprivation for 24 hours, crowding for 12 hours, forced cold swim for 5 minutes, immobilization for 1 hour, cage rotation for 1 hour, tilted cages (45°) for 24 hours, cold stress for 1 hour, light/dark cycle reversal for 36 hours, and soiled bedding for 24 hours. Control rats were handled daily without any stress in the housing room. The stress protocol was adapted from previous studies (Shi et al., 2012; Suo et al., 2013; Zhu et al., 2013).
Surgery and Drug Infusion
Male rats, weighing 280 to 300 g, were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Stainless-steel guide cannulas (22 gauge) were bilaterally implanted in the mPFC (anterior/posterior, +3.2 mm; medial/lateral, ±2.0 mm; dorsal/ventral, -2.8 mm) (He et al., 2011; Suo et al., 2013; Xue et al., 2015). The cannulas were placed at a 16º angle toward the midline to avoid penetration of the lateral ventricle. The cannulas were anchored to the skull with screws and dental cement. After 1 week of recovery, drugs and vehicle were infused bilaterally in the mPFC using a 1-mm projection cannula injector 30 minutes before testing at a flow rate of 0.25 μL/min. The volume of the infusion was 0.5 μL for each side. The number of animals per group is indicated after excluding animals that had incorrect cannula placements, determined by histology.
Histology
The animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with 0.01 M phosphate-buffered saline, followed by 4% paraformaldehyde in 0.2 M phosphate buffer. The brain was extracted, postfixed overnight at 4°C, and cryoprotected in 30% sucrose in 0.2 M phosphate buffer. The cannula placements were confirmed in 40-μm-thick sections using Nissl staining under light microscopy. Rats with misplaced cannulas were excluded from the statistical analysis (Han et al., 2016; Xue et al., 2015). The locations of the cannula tips are shown in Figure 3B.
Tissue Sample Preparation
The procedure was based on our previous studies (Lu et al., 2005; Luo et al., 2015; Zhang et al., 2016). The brains were extracted, and bilateral tissue punches (16 gauge) of the mPFC were obtained and homogenized with an electrical disperser (WiggenHauser, Sdn Bhd) after being lysed with RIPA lysis buffer with protease inhibitor and a phosphatase inhibitor mixture (Applygen Technology, Beijing, China) for 30 minutes. The homogenate was then subjected to 10000 × g centrifugation at 4°C for 20 minutes. All of the above procedures were performed at 0 to 4°C. The protein concentrations of all of the samples were determined using the BCA assay kit (Applygen Technology). Dilution of the samples with RIPA lysis buffer was used to normalize the protein concentration.
To purify crude synaptoneurosomes, the mPFC from adult rats was dissected and homogenized in a solution that contained 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM ethylenediaminetetraacetic acid, 1× protease inhibitor cocktail, 5 mM NaF, and 1 mM sodium vanadate. The homogenate was centrifuged at 2800 rotations per minute for 10 minutes at 4°C. The pellet (nuclear fraction) contained nuclei and large cellular debris. The supernatant was then centrifuged at 12000 rotations per minute for 10 minutes. After centrifugation, the supernatant (cytosolic fraction) was removed, and the pellet (crude synaptosomal fraction) was resuspended and sonicated in protein lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 2 mM ethylenediaminetetraacetic acid, 1 mM NaVO3, 5 mM NaF, and 1× protease inhibitor cocktail). The protein concentration was determined using a BCA protein assay kit (Li et al., 2011).
Western Blot
The western-blot assays were based on previous studies (Lu et al., 2005; Wang et al., 2010; Xue et al., 2012). The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide/0.27% N,N’-methylenebisacrylamide resolving gel) for approximately 40 minutes at 80 V in stacking gel and approximately 1 hour at 120 V in resolving gel. Proteins were electrophoretically transferred to polyvinylidene fluoride transfer membranes (Millipore, Bedford, MA) at 250 mA for 1 to 3 hours. Membranes were washed with TBST (Tris-buffered saline plus 0.05% Tween-20, pH 7.4) and then Incubated in blocking buffer (5% bovine serum albumin in TBST) overnight at 4°C. The next day, the membranes were incubated for 45 minutes at room temperature on a shaker with anti-BDNF antibody (1:2000; Abcam, Cambridge, UK; catalog no. ab108319), anti-GSK-3β antibody (1:2000; Abcam; catalog no. ab32391), anti-phospho-S9 GSK-3β antibody (1:2000; Abcam; catalog no. ab75814), anti-β-catenin antibody (1:5000; Abcam; catalog no. ab32572), anti-synapsin I antibody (1:1000; Cell Signaling Technology, Danvers, MA; catalog no. 6710), anti-postsynaptic density 95 (PSD95) antibody (1:1000; Abcam; catalog no. ab76115), anti-GluR1 antibody (1:2000; Abcam; catalog no. ab109450), anti-GADPH antibody (1:2000, Cell Signaling Technology, Danvers, MA; catalog no. 8884), or β-actin (1:2000; Sigma; catalog no. A5316) in TBST plus 5% bovine serum albumin and 0.05% sodium azide. After four 5-minute washes in TBST buffer, the blots were incubated for 45 to 55 minutes at room temperature on a shaker with horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG for β-actin and goat anti-rabbit IgG for the others; Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. sc-130657/sc-47778) diluted 1:5000 in blocking buffer. The blots then underwent four 5-minute washes with TBST, and immunostaining was visualized using the EZ-ECL chemiluminescence detection kit. The immunoblots were quantified with the Gel Doct EZ system (Bio-Rad). Band intensities for BDNF, total GSK-3β (t-GSK-3β), and β-catenin were normalized to β-actin. p-Ser9-GSK-3β was normalized to t-GSK-3β. Synapsin I, GluR1, and PSD95 were normalized to GADPH. The band intensities were analyzed using Quantity One 4.4.0 software (Bio-Rad).
Statistical Analysis
The data are expressed as mean ± SEM. Independent-sample t tests were used to analyze the behavioral and molecular data in 2 groups. One- or 2-way ANOVA was used with appropriate between- and within-subjects factors for the different experiments (see Results). Significant main effects and interactions (P<.05) in the factorial ANOVAs were further analyzed using Tukey’s posthoc test or paired comparisons as appropriate. Values of P<.05 were considered statistically significant.
Results
MOs Rescued CUS-Induced Depression-Like Behaviors and the Abnormality of BDNF-GSK-3β-β-catenin Pathway in the mPFC
To explore the antidepressant-like effects of MOs, adult male rats underwent 5-week CUS and were administered MOs (12.5, 25, and 50 mg/kg) and fluoxetine (10 mg/kg) i.g. once per day from day 22 until the end of the CUS protocol, for a total of 14 days. The SPT and FST were then conducted on days 36 and 37 (Figure 1A). The data were analyzed using 1-way ANOVA, with group as the between-subjects factor, followed by Tukey’s posthoc test. The analysis revealed significant main effects of group in the SPT (F7,64 = 6.447, P<.01; Figure 1B) and FST (F7,64 = 11.096, P<.01; Figure 1D). The CUS-induced decrease in sucrose preference and increase in immobility time were reversed by MOs at 50 mg/kg (P<.01) and fluoxetine at 10 mg/kg (P<.05). Total fluid consumption was not altered by CUS or drug administration (Figure 1C). MOs and fluoxetine treatment had no significant effects on sucrose preference or immobility time in nonstressed rats, which may be attributable to a “ceiling effect” or “floor effect.”
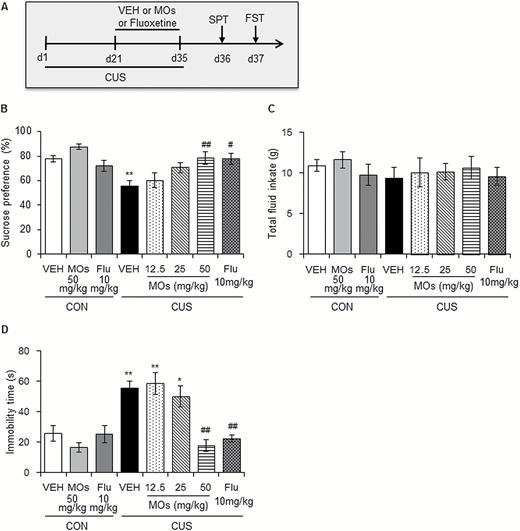
Morinda officinalis oligosaccharide (MOs) treatment produced antidepressant-like effects in rats. (A) Timeline of chronic unpredictable stress (CUS) exposure and the behavioral tests (n = 8–9/group). Rats underwent 35 days of CUS, and MOs (12.5, 25, and 50 mg/kg) and fluoxetine (10 mg/kg) were administered i.g. during the last 14 days. (B-C) Sucrose preference and total fluid intake in the sucrose preference test (SPT). (D) Immobility time in the forced swim test (FST).
To explore the molecular mechanisms that underlie the antidepressant-like effects of MOs, the rats underwent 5-week CUS exposure and were administered MOs (50 mg/kg, i.g.) and fluoxetine (10 mg/kg) i.g. for 2 weeks. Brain areas were then collected after the CUS procedure (Figure 2A). The data were analyzed by 1-way ANOVA, with group as the between-subjects factor, followed by Tukey’s posthoc test. The analysis revealed a significant main effect of group on the expression of β-catenin (F3,28 = 16.553, P<.01), p-Ser9-GSK-3β (F3,28 = 26.748, P<.01), and BDNF (F3,28 = 16.515, P<.01) but not t-GSK-3β (P>.05; Figure 2B) in the mPFC. The MOs and fluoxetine treatment effectively rescued the CUS-induced decreases in β-catenin, p-Ser9-GSK-3β, and BDNF levels in the mPFC.
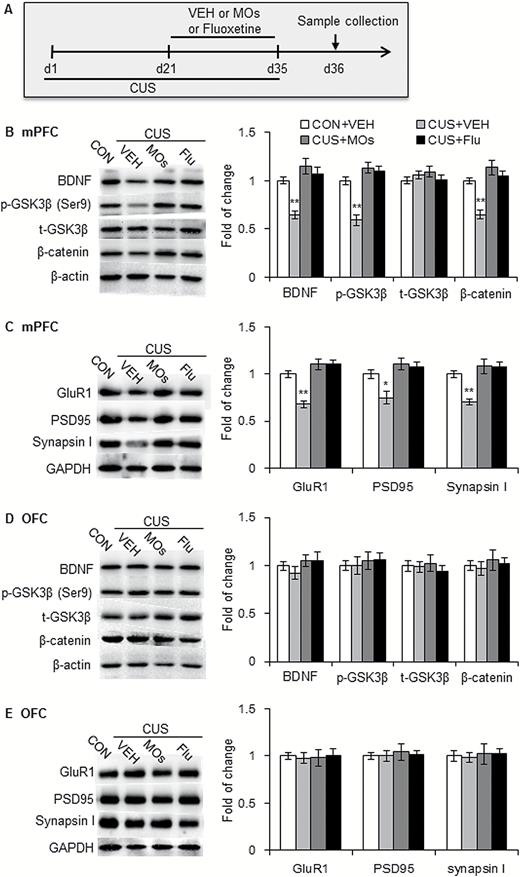
Morinda officinalis oligosaccharides (MOs) rescued abnormalities in the brain-derived neurotrophic factor (BDNF)-glycogen synthase kinase-3β (GSK-3β) -3β-β-catenin pathway and synaptic protein deficits that were induced by chronic unpredictable stress (CUS) in the medial prefrontal cortex (mPFC). (A) Timeline of CUS exposure and sample collection (n = 8/group). (B, D) Representative Western blots and levels of BDNF, p-Ser9-GSK-3β, t-GSK-3β, and β-catenin in the mPFC (B) and orbitofrontal cortex (OFC) (D) in rats. (C, E) Representative Western blots and levels of synaptic proteins in the mPFC (C) and OFC (E) in rats. The data are expressed as mean ± SEM. *P<.05, **P<.001, compared with control (CON) group. SPT, sucrose preference test; VEH, vehicle.
Previous studies demonstrated the role of the GSK-3β-β-catenin pathway in neurogenesis and synaptic plasticity. We explored the effects of MOs on CUS-induced synaptic deficits in the mPFC. Male rats underwent 5-week CUS exposure and 2-week administration of MOs (50 mg/kg) and fluoxetine (10 mg/kg), followed by the determination of synaptic proteins in the mPFC (Figure 2A). The data were analyzed by 1-way ANOVA, with group as the between-subjects factor, followed by Tukey’s posthoc test. The analysis revealed a significant main effect of group on the expression of PSD95 (F3,28 = 8.976, P<.01), synapsin I (F3,28 = 13.333, P<.01), and GluR1 (F3,28 = 25.550, P<.01) (Figure 2C). The CUS-induced deficits in synaptic proteins (PSD95, synapsin I, and GluR1) were effectively reversed by MOs and fluoxetine treatment.
Additionally, CUS and drug administration had no effect on the expression of BDNF, p-Ser9-GSK-3β, β-catenin, GluR1, PSD95, or synapsin I in the orbitofrontal cortex (OFC; all P>.05) (Figure 2D-E). These results indicate that MOs and fluoxetine treatment significantly rescued CUS-induced abnormalities in the BDNF-GSK-3β-β-catenin pathway and synaptic protein deficits in the mPFC but not OFC.
Activation of GSK-3β Reversed the Antidepressant-Like Action of MOs in the FST
We found that MOs rescued CUS-induced depression-like behavior and the activation of GSK-3β in the mPFC. To further verify that GSK-3β is involved in the antidepressant-like effects of MOs, 1 day after 15-minute adaptation, we microinjected LY294002 (10 nM/side), a PI3K inhibitor, in the mPFC in naive rats, followed by MOs administration (50 mg/kg) and then the locomotor activity test and FST (Figure 3A). The behavioral data were analyzed by 2-way ANOVA, with LY294002 and MOs as between-subjects factors, followed by paired-comparison posthoc tests. The analysis revealed significant main effects of LY294002 (F1,31 = 9.340, P<.01) and MOs (F1,31 = 12.313, P<.01) on immobility time in the FST and a significant LY294002 × MOs interaction (F1,31 = 17.322, P<.01) (Figure 3D). The MOs significantly decreased immobility time in the FST, and the microinjection of LY294002 in the mPFC abolished the effect of MOs (P<.01). There were no significant main effects of LY294002 (P>.05) or MOs (P>.05) on locomotor activity and no LY294002 × MOs interaction (P>.05) (Figure 3C).
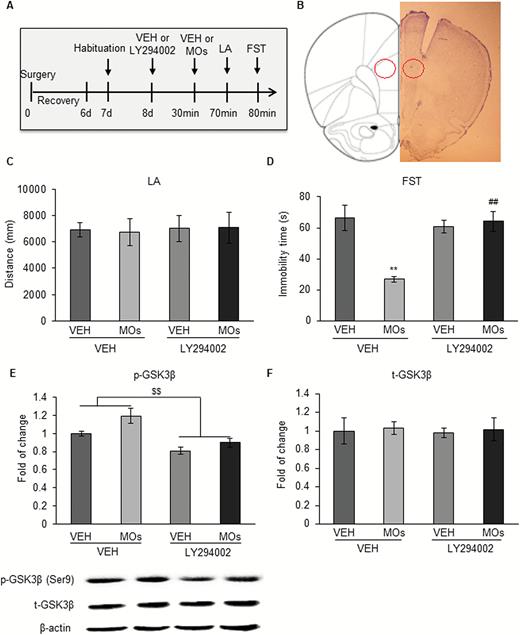
Activation of glycogen synthase kinase-3β (GSK-3β) reversed the antidepressant-like effect of Morinda officinalis oligosaccharides (MOs) in the forced swim test (FST). (A) Timeline of the experimental procedure. Rats were bilaterally implanted with cannulas in the prelimbic cortex and microinjected with the phosphoinositide-3 kinase (PI3K) inhibitor LY294002 before MOs administration, followed by the behavioral tests (n = 8–9/group). (B) Microinjection sites in the medial prefrontal cortex (mPFC). (C) Locomotor activity test. (D) Forced swim test (FST). (E) Expression of p-Ser9-GSK-3β. (F) Expression of t-GSK-3β. The data are expressed as mean ± SEM. **P<.01, compared with vehicle (VEH)+VEH group; ##P<.01, compared with VEH+MOs group; $$P<.01, main effect of LY294002 on phosphorylated GSK-3β levels. CON, control; LA, locomotor activity test.
The expression of t-GSK-3β and p-Ser9-GSK-3β was analyzed using 2-way ANOVA, with LY294002 and MOs as between-subjects factors, followed by paired-comparison posthoc tests. The analysis revealed significant main effects of LY294002 (F1,27 = 28.406, P<.01) and MOs (F1,27 = 7.540, P<.01) on p-Ser9-GSK-3β, with no LY294002 × MOs interaction (F1,27 = 0.991, P>.05) (Figure 3E). The microinjection of LY294002 in the mPFC reversed the activation of GSK-3β. There were no significant main effects of LY294002 or MOs on t-GSK-3β and no LY294002 × MOs interaction (P>.05) (Figure 3F).
MOs Enhanced the Resilience to Stress
Previous studies demonstrated that BDNF and β-catenin are related to the vulnerability to stress. We explored the effects of MOs on the susceptibility to stress in male rats. Fourteen-day MOs administration (50 mg/kg) was performed before the 5 weeks of CUS, and the SPT was performed at baseline, before CUS, and at weeks 1, 2, 3, 4, and 5 of the CUS protocol (Figure 4B). Sucrose preference was analyzed by repeated-measures ANOVA, with group as the between-subjects factor and time as the within-subjects factor, followed by Tukey’s posthoc test. There was a significant main effect of group on sucrose preference (F3,30 = 12.509, P<.01), with no group × time interaction (F18,180 = 1.255, P>.05). A significant decrease in sucrose preference was found in rats pretreated with vehicle since the 3rd weekend of the CUS (P<.05) but not in rats pretreated with MOs (P>.05) (Figure 4C). No significant changes in total fluid consumption were observed after CUS or MOs treatment (all P>.05) (Figure 4D).
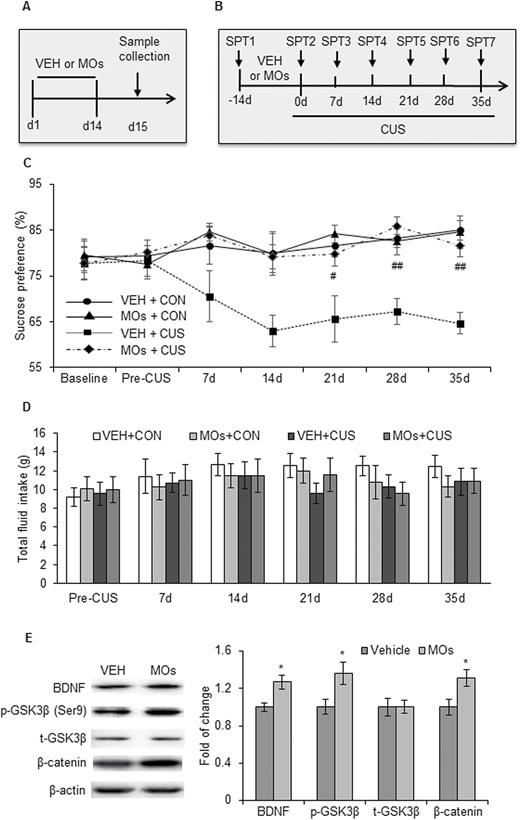
Morinda officinalis oligosaccharides (MOs) enhanced the resilience to stress. (A) Timeline of MOs administration and sample collection (n = 8–9/group). (B) Timeline of sucrose preference test (SPT) and MOs administration (n = 8–9/group). (C-D) Sucrose preference and total fluid intake in the SPT. (E) Representative Western blots and levels of β-catenin, p-Ser9-glycogen synthase kinase-3β (GSK-3β), t-GSK-3β, and brain-derived neurotrophic factor (BDNF) in the medial prefrontal cortex (mPFC). The data are expressed as mean ± SEM. *P<.05, compared with vehicle (VEH) group; #P<.05, ##P<.01, compared with VEH+chronic unpredictable stress (CUS) group. CON, control.
The mPFC was collected from rats that received 14-day MOs administration (50 mg/kg) but were not subjected to 5 weeks of CUS to detect the expression of β-catenin, GSK-3β, and BDNF (Figure 4A). The data were analyzed using independent-sample t tests, with drug as the between-subjects factor. The analysis revealed that MOs significantly increased the expression of β-catenin (t14 = -2.534, P<.05), p-Ser9-GSK-3β (t14 = -2.374, P<.05), and BDNF (t14 = -3.019, P<.05) but not t-GSK3β (t14 = -0.086, P>.05) in the mPFC (Figure 4E). These results indicate that MOs enhanced resilience to stress in rats, and this effect might result from MO-induced activation of the BDNF-GSK-3β-β-catenin pathway in the mPFC.
Discussion
The present study showed that MOs treatment produced antidepressant-like effects and reversed CUS-induced behavioral deficits in rats. The administration of MOs also rescued the decreases in β-catenin, p-Ser9-GSK-3β, and BDNF levels and synaptic protein deficits that were induced by CUS in the mPFC but not OFC. GSK-3β activation by LY294002 prevented the antidepressant-like effects of MOs, indicating that the behavioral response to MOs might be mediated by GSK-3β signaling in the mPFC. Moreover, we found that MOs treatment promoted resilience to CUS. We propose a model that depicts the involvement of BDNF–GSK-3β-β-catenin pathway in the antidepressant-like effect of MOs and resilience to stress (Figure 5).
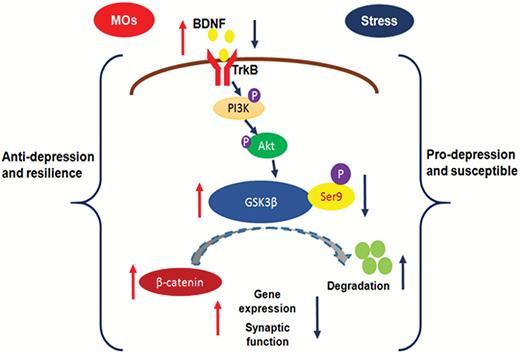
Schematic illustration of the involvement of the brain-derived neurotrophic factor (BDNF)-glycogen synthase kinase-3β (GSK-3β)-β-catenin pathway in the antidepressant-like effects of Morinda officinalis oligosaccharides (MOs) and resilience to stress. Chronic stress decreases the expression of BDNF, p-Ser9-GSK-3β, and β-catenin. GSK-3β is regulated by BDNF through phosphoinositide-3 kinase (PI3K)/AKT signaling. An increase in GSK3β activity reduces the stability of β-catenin and leads to β-catenin degradation through ubiquitination. A decrease in GSK3β activity increases β-catenin and promotes gene expression and synaptic function. The MOs rescue the stress-induced changes in the BDNF-GSK-3β-β-catenin pathway and promote gene expression and synaptic function. These molecular changes may underlie the antidepressant-like effects of MOs and resilience to stress.
GSK-3β has been identified as a protein kinase that modulates many aspects of neuronal function, such as gene expression, neurogenesis, neurodevelopment, synaptic maintenance, and plasticity (Peineau et al., 2008; Wexler et al., 2008; Liu et al., 2013), and has been implicated in the pathophysiology of dopamine-associated behaviors and diseases (Xu et al., 2009, 2011; Wu et al., 2011). Several lines of evidence indicate that GSK-3β is involved in mood disorders and an important target of therapeutic antidepressants (Li and Jope, 2010; Duman and Aghajanian, 2014). Postmortem studies reported an increase in GSK-3β activity or decrease in its expression in major depressive disorder, depressed suicide, and bipolar depression patients (Karege et al., 2007; Li and Jope, 2010; Pandey et al., 2015). Animal studies reported that chronic stress decreased the phosphorylation of GSK-3β in the mPFC and hippocampus (Zhu et al., 2013). Chronic administration of traditional antidepressants, including fluoxetine and venlafaxine, significantly increased p-Ser9-GSK-3β levels in the hippocampus (Okamoto et al., 2010). GSK-3β inhibition is also required for the rapid antidepressant effects of ketamine (Li and Jope, 2010; Beurel et al., 2011; Liu et al., 2013). In the present study, we found that MOs treatment increased p-Ser9-GSK-3β in the mPFC, and GSK-3β activation by LY294002 prevented the antidepressant-like effects of MOs, indicating that the behavioral response to MOs might be mediated by GSK-3β signaling in the mPFC. Indeed, LY294002 is a PI3K inhibitor. Because no specific GSK3β activator has yet been developed, we used LY294002 to activate GSK3β indirectly, based on previous studies (Zhu et al., 2013; Li et al., 2014). The western-blot results confirmed that LY294002 administration decreased p-Ser9-GSK3β levels (i.e., increased its activity). However, LY294002 administration also raises the possibility that it may have affected other kinases that regulated by PI3K/Akt signaling, such as GSK-3α. Less evidence supports the involvement of GSK3α in the actions of antidepressants, although one study showed that GSK3α knockout mice exhibited a decrease in depressive-like behavior (Kaidanovich-Beilin et al., 2009). Further investigations are needed to test the effects of MOs on GSK3α activity.
β-Catenin is a major transcriptional modulator that is inhibited by GSK-3β activation and activated by GSK-3β inhibition, which produces antidepressant effects and mediates behavioral resilience (Madsen et al., 2003; Kaidanovich-Beilin et al., 2004; Wilkinson et al., 2011; Karege et al., 2012; Dias et al., 2014). GSK-3β activation results in β-catenin phosphorylation and proteasomal degradation. A decrease in GSK-3β activity increases the stability of β-catenin and enhances its biological function (Garza et al., 2012). The loss of BDNF is involved in the pathophysiology of depression (Shimizu et al., 2003; Duman and Monteggia, 2006; Frodl et al., 2007; Groves, 2007; Licinio et al., 2009; Autry et al., 2011; Duman and Aghajanian, 2014; Hosang et al., 2014; Molendijk et al., 2014), and the restoration of BDNF underlies the therapeutic efficacy of antidepressant treatment (Krishnan and Nestler, 2008; Autry et al., 2011; Monteggia et al., 2013; Duman and Aghajanian, 2014). BDNF binds to tyrosine kinase receptor B to activate PI3K and Akt, which subsequently phosphorylates the N-terminal serine of GSK-3β and thus inhibits GSK-3β activity (Li and Jope, 2010). GSK-3β inhibition has also been shown to enhance the protective effects of BDNF in a model of Alzheimer’s disease (Liu et al., 2015).
Consistent with a previous study (Zhang et al., 2002), we found that MOs at 50 mg/kg produced antidepressant-like effects and prevented CUS-induced depressive-like behavior. Accumulating evidence shows that CUS reduces the expression of BDNF, p-Ser9-GSK-3β, and β-catenin in the mPFC and hippocampus (Kaidanovich-Beilin et al., 2004; Gould et al., 2007; Peineau et al., 2008; Li and Jope, 2010; Zhu et al., 2013; Dias et al., 2014; Molendijk et al., 2014), and this lower expression was rescued by MOs treatment. Based on these findings, we speculate that the antidepressant-like effects of MOs might be mediated by the BDNF-GSK-3β-β-catenin pathway in the mPFC. This possibility is also supported by evidence that demonstrates the involvement of the BDNF-GSK-3β-β-catenin pathway in the pathophysiology and treatment of depression (Karege et al., 2002; Shimizu et al., 2003; Groves, 2007; Beurel et al., 2011; Karege et al., 2012; Zhu et al., 2013; Molendijk et al., 2014). GSK-3β activation in the mPFC did not affect locomotor activity or t-GSK3β in the mPFC.
The CUS-induced decrease in synaptic protein levels is consistent with previous studies showed that chronic stress caused neuronal atrophy and synaptic loss in limbic brain regions, including the mPFC (Radley et al., 2008; Li et al., 2011; Duman and Aghajanian, 2012). Treatment with MOs rescued the CUS-induced synaptic protein deficits in the mPFC. Previous studies reported the involvement of the GSK-3β-β-catenin pathway in the regulation of synaptic function and transmission (Peineau et al., 2008; Li and Jope, 2010; Wei et al., 2010; Duman and Aghajanian, 2012, 2014; Ochs et al., 2015). The BDNF-GSK-3β-β-catenin pathway plays a critical role in the resilience to stress (Bergstrom et al., 2008; Wilkinson et al., 2011; Van den Hove et al., 2013; Dias et al., 2014). We also found that MOs increased the resilience to stress in rats, accompanied by a significant increase in the expression of BDNF, p-Ser9-GSK-3β, and β-catenin in the mPFC. These results suggest that the BDNF-GSK-3β-β-catenin pathway might mediate the resilience to stress that is afforded by MOs in rats.
In summary, the present findings indicate that MOs, active ingredients of traditional Chinese medicine, produce antidepressant-like effects and resilience to stress, which may be mediated by the BDNF-GSK-3β-β-catenin pathway in the mPFC.
Statement of Interest
None.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81171251) and National New Drug Innovation Program (no. 2014ZH09301307-014).
References
Author notes
L.-Z.X., D.-F.X., and Y.H. contributed equally to the article



