-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Bauer, Roger S. McIntyre, Johan Szamosi, Hans Eriksson, Evaluation of adjunct extended-release quetiapine fumarate on sleep disturbance and quality in patients with major depressive disorder and an inadequate response to on-going antidepressant therapy, International Journal of Neuropsychopharmacology, Volume 16, Issue 8, September 2013, Pages 1755–1765, https://doi.org/10.1017/S146114571300031X
Close - Share Icon Share
Abstract
Sleep disturbance is common in depression and is a risk factor for recurrence and suicide. This analysis evaluated the effects of adjunct extended-release quetiapine fumarate (quetiapine XR) on sleep disturbance and quality in patients with major depressive disorder (MDD) and an inadequate response to on-going antidepressant therapy. Pooled data from two 6-wk, randomized, double-blind, placebo-controlled trials were analysed post hoc. Patients received once-daily quetiapine XR [(150 mg/d), n = 309; (300 mg/d), n = 307] or placebo (n = 303) adjunct to on-going antidepressant therapy. Analyses included: change from randomization in Montgomery-Åsberg Depression Rating Scale (MADRS) Item 4 (reduced sleep) score; Hamilton Rating Scale for Depression (HAMD) Items 4 (insomnia-early), 5 (insomnia-middle) and 6 (insomnia-late) scores; HAMD sleep disturbance factor (Items 4+5+6); Pittsburgh Sleep Quality Index (PSQI) global score. Change in MADRS total score was also evaluated in patients stratified by HAMD sleep disturbance factor score (high ⩾4 and low < 4) at randomization. At week 6, adjunct quetiapine XR (150 and 300 mg/d) reduced MADRS Item 4, HAMD Items 4, 5 and 6, HAMD sleep disturbance factor and PSQI global scores from randomization vs. placebo (all p < 0.001). In patients with high sleep disturbance, quetiapine XR (both doses) improved depressive symptoms (MADRS total score) vs. placebo from week 1 onwards (p < 0.01). Adjunct quetiapine XR improved sleep disturbance and quality vs. placebo in patients with MDD and an inadequate response to on-going antidepressant treatment, and was effective against depressive symptoms in patients experiencing high sleep disturbance.
Introduction
Sleep disturbance is a core symptom of major depressive disorder (MDD; APA, 2000), with reported prevalence rates of 50–70% (Carney et al., 2007). Moreover, 40–50% of patients who respond to antidepressant treatment report residual sleep symptoms (Fava et al., 2006; Carney et al., 2007) such as problems with falling asleep, frequent wakening at night, early morning wakening and disturbed sleep duration (either too short or too long) (Mendlewicz, 2009; van Mill et al., 2010).
Alleviation of sleep disturbance is considered to be a key factor in achieving complete remission of MDD (Mendlewicz, 2009; Nierenberg et al., 2010). Moreover, sleep disturbance in patients with MDD is a risk factor for recurrence (Cho et al., 2008), severe suicidal ideation and suicide (Bernert and Joiner, 2007; Wojnar et al., 2009; McCall et al., 2010), and is associated with significantly greater overall treatment costs compared with patients with MDD without sleep disturbance (p < 0.001; Asche et al., 2010). Effective treatment of sleep disturbance is an important consideration when treating patients with MDD. However, there is a paucity of data reporting the efficacy of antidepressants in patients with MDD and a high level of sleep disturbance.
MDD is a severe, chronic and highly prevalent disorder (Kessler et al., 2003) that is associated with significant levels of disability, morbidity and mortality (Lopez and Murray, 1998; WHO, 2008). A variety of pharmacological treatment options are available for MDD including selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). Notwithstanding the availability of numerous pharmacological treatment options, many patients with MDD achieve sub-optimal outcomes and experience residual symptoms (Fava et al., 2007; Iglesias and Alonso, 2009). A number of current antidepressant treatments may cause or exacerbate sleep disturbances (Thase, 2006). For example, treatment with SSRIs, SNRIs, TCAs or MAOIs may be associated with insomnia (Mayers and Baldwin, 2005; Brecht et al., 2008). Additional medications are often used to treat sleep symptoms in patients with MDD (Shelton et al., 2009); thus, a treatment for MDD that also addresses sleep disturbance is desirable.
Extended-release quetiapine fumarate (quetiapine XR) is currently approved in the EU (AstraZeneca, 2012b) and the USA (AstraZeneca, 2012a) and several other countries worldwide as adjunctive treatment for patients with MDD and an inadequate response to previous antidepressants. In addition, once-daily quetiapine XR has demonstrated efficacy as monotherapy in both the acute (Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011) and long-term (Liebowitz et al., 2010) treatment settings and is approved for use as a monotherapy treatment for MDD in a limited number of countries, including Canada and Australia, for patients who are intolerant of, or have had an inadequate response to, alternative antidepressant drugs.
Findings from individual acute studies of adjunct quetiapine XR in patients with MDD and an inadequate response to on-going antidepressant treatment (Bauer et al., 2009; El-Khalili et al., 2010) and a pre-planned analysis of pooled data from these studies (Bauer et al., 2010) demonstrated significant improvements in the primary end-point [reduction in depressive symptoms as assessed by change in Montgomery-Åsberg Depression Rating Scale (MADRS) total score from randomization to week 6] and in key secondary efficacy variables compared with placebo. This post hoc analysis used the pooled data to provide a larger sample size to enable further evaluation of the effects of adjunct quetiapine XR on sleep disturbance and quality and a subgroup analysis investigating the influence of sleep disturbance at baseline on observed antidepressant efficacy.
Method
Study design
Data from two quetiapine XR adjunct therapy studies [D1448C00006; ClinicalTrials.gov study identifier number NCT00326105 (study 6) and D1448C00007; ClinicalTrials.gov study identifier number NCT00351910 (study 7)] were pooled. Details of the methodology employed in these two studies have been reported previously in full (Bauer et al., 2009; El-Khalili et al., 2010). In brief, these were multi-centre, double-blind, randomized, parallel-group, placebo-controlled studies of similar design. Both studies consisted of an enrolment/washout period of ⩽14 d followed by a 6-wk randomized treatment period during which patients received quetiapine XR (150 or 300 mg/d) or placebo as adjunct to on-going antidepressant therapy. Study 6 also included a 2-wk drug-discontinuation/follow-up period. Study 6 was conducted in the USA while study 7 was conducted at centres in Australia, Canada, Europe and South Africa.
The study protocols were approved by Institutional Review Boards or Independent Ethics Committees for each study site. Studies were carried out in accordance with the standards laid down in the Declaration of Helsinki and the International Conference on Harmonisation/Good Clinical Practice guidelines, and all patients provided written informed consent prior to the start of the study.
Patients
Outpatients (aged 18–65 yr) with a Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) or DSM-IV text revision diagnosis of single episode (296.2×) or recurrent (296.3×) MDD, a Hamilton Depression Rating Scale (HAMD; Hamilton, 1960) total score ⩾20 and a HAMD Item 1 (depressed mood) score ⩾2 at enrolment and randomization were eligible for inclusion. In addition, patients were required to have had an inadequate response during their current depressive episode to one of the following antidepressant treatments: amitriptyline; bupropion; citalopram; duloxetine; escitalopram; fluoxetine; paroxetine; sertraline; venlafaxine. Inadequate response was defined as continuing depressive symptoms following ⩾6 wk treatment at an adequate dose (minimum effective dose according to the prescribing information and ⩾1 dose increase as permitted by the prescribing information).
Key exclusion criteria in both studies included: diagnosis of any DSM-IV Axis I disorder other than MDD within 6 months prior to enrolment or any DSM-IV Axis II disorder significantly impacting the patient's current psychiatric status; a current MDD episode lasting >12 months or < 4 wk from enrolment.
Treatment
Patients were randomized using a computer-based system in a 1:1:1 ratio to receive fixed doses of quetiapine XR (150 mg/d), quetiapine XR (300 mg/d) or placebo as adjunct to on-going antidepressant therapy. Quetiapine XR was titrated to a target dose of 50 mg/d on days 1–2, 150 mg/d on days 3–4 and 300 mg/d on day 5. Treatment was administered once daily in the evening. A double-dummy technique was used to ensure blinding of study treatment, with placebo tablets that were identical in appearance, smell and taste to quetiapine XR tablets: quetiapine XR (150 mg; 3 × 50 mg tablets) or quetiapine XR (300 mg; 1 × 300 mg tablet). A combination of tablets was administered to ensure blinding of study treatments.
On-going antidepressant therapy was maintained at the same dose from enrolment through to the end of double-blind treatment.
With the exception of on-going hypnotics to treat insomnia, use of other psychoactive medication was not permitted. Sleep medication could be continued if it had been regular and commenced ⩾28 d before enrolment. The proportion of patients receiving sleep medication was recorded during the studies.
Efficacy assessments
In both studies, the primary efficacy end-point was the change in MADRS total score (Montgomery and Åsberg, 1979) from randomization to week 6. Secondary efficacy end-points including Pittsburgh Sleep Quality Index (PSQI) global score (Buysse et al., 1989) at week 6 have been reported in detail elsewhere (Bauer et al., 2009, 2010; El-Khalili et al., 2010).
Sleep disturbance and quality
Post hoc analyses assessed changes from randomization in the following efficacy variables: MADRS Item 4 (reduced sleep) score at weeks 1, 2, 4 and 6; HAMD Items 4 (insomnia-early), 5 (insomnia-middle) and 6 (insomnia-late); HAMD sleep disturbance factor (Items 4+5+6) scores at week 6.
The change from randomization to weeks 4 and 6 in PSQI individual item scores assessed the following dimensions of sleep: subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbance; use of sleep medication; daytime dysfunction. The change from randomization to week 4 in PSQI global score was also assessed in a post hoc analysis.
Change in MADRS total score was also evaluated in patients stratified by HAMD sleep disturbance factor score (Items 4+5+6: insomnia-early; insomnia-middle; insomnia-late) at randomization. In the pooled studies, mean HAMD sleep disturbance in patients at randomization was 4.3, 4.2 and 4.3 in the quetiapine XR (150 mg/d), (300 mg/d) and placebo groups, respectively (Table 1); therefore, high sleep disturbance was defined as a HAMD sleep disturbance factor score ⩾4 while low sleep disturbance was defined as a HAMD sleep disturbance factor score < 4. These HAMD sleep disturbance severity cut-offs have been reported previously (Fava et al., 2002).
Baseline sleep disturbance scoresa (pooled modified intention-to-treat population)
| Mean (s.d.) . | Placebo +antidepressant (n = 303) . | Quetiapine XR(150 mg/d) +antidepressant (n = 309) . | Quetiapine XR(300 mg/d) +antidepressant (n = 307) . |
|---|---|---|---|
| MADRS Item 4 (reduced sleep) score | 3.4 (1.2) | 3.4 (1.1) | 3.4 (1.3) |
| HAMD Item 4 (insomnia-early) score | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.8) |
| HAMD Item 5 (insomnia-middle) score | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| HAMD Item 6 (insomnia-late) score | 1.2 (0.8) | 1.3 (0.8) | 1.3 (0.8) |
| HAMD sleep disturbance factor (Items 4+5+6) score | 4.2 (1.5) | 4.3 (1.5) | 4.2 (1.5) |
| PSQI global score | 11.8 (3.8) | 12.3 (3.9) | 12.1 (4.0) |
| Mean (s.d.) . | Placebo +antidepressant (n = 303) . | Quetiapine XR(150 mg/d) +antidepressant (n = 309) . | Quetiapine XR(300 mg/d) +antidepressant (n = 307) . |
|---|---|---|---|
| MADRS Item 4 (reduced sleep) score | 3.4 (1.2) | 3.4 (1.1) | 3.4 (1.3) |
| HAMD Item 4 (insomnia-early) score | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.8) |
| HAMD Item 5 (insomnia-middle) score | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| HAMD Item 6 (insomnia-late) score | 1.2 (0.8) | 1.3 (0.8) | 1.3 (0.8) |
| HAMD sleep disturbance factor (Items 4+5+6) score | 4.2 (1.5) | 4.3 (1.5) | 4.2 (1.5) |
| PSQI global score | 11.8 (3.8) | 12.3 (3.9) | 12.1 (4.0) |
XR, Extended-release; MADRS, Montgomery-Åsberg Depression Rating Scale; HAMD, Hamilton Depression Rating Scale; PSQI Pittsburgh Sleep Quality Index.
For patients included in the last assessment (week 6) analysis for each variable.
Baseline sleep disturbance scoresa (pooled modified intention-to-treat population)
| Mean (s.d.) . | Placebo +antidepressant (n = 303) . | Quetiapine XR(150 mg/d) +antidepressant (n = 309) . | Quetiapine XR(300 mg/d) +antidepressant (n = 307) . |
|---|---|---|---|
| MADRS Item 4 (reduced sleep) score | 3.4 (1.2) | 3.4 (1.1) | 3.4 (1.3) |
| HAMD Item 4 (insomnia-early) score | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.8) |
| HAMD Item 5 (insomnia-middle) score | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| HAMD Item 6 (insomnia-late) score | 1.2 (0.8) | 1.3 (0.8) | 1.3 (0.8) |
| HAMD sleep disturbance factor (Items 4+5+6) score | 4.2 (1.5) | 4.3 (1.5) | 4.2 (1.5) |
| PSQI global score | 11.8 (3.8) | 12.3 (3.9) | 12.1 (4.0) |
| Mean (s.d.) . | Placebo +antidepressant (n = 303) . | Quetiapine XR(150 mg/d) +antidepressant (n = 309) . | Quetiapine XR(300 mg/d) +antidepressant (n = 307) . |
|---|---|---|---|
| MADRS Item 4 (reduced sleep) score | 3.4 (1.2) | 3.4 (1.1) | 3.4 (1.3) |
| HAMD Item 4 (insomnia-early) score | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.8) |
| HAMD Item 5 (insomnia-middle) score | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| HAMD Item 6 (insomnia-late) score | 1.2 (0.8) | 1.3 (0.8) | 1.3 (0.8) |
| HAMD sleep disturbance factor (Items 4+5+6) score | 4.2 (1.5) | 4.3 (1.5) | 4.2 (1.5) |
| PSQI global score | 11.8 (3.8) | 12.3 (3.9) | 12.1 (4.0) |
XR, Extended-release; MADRS, Montgomery-Åsberg Depression Rating Scale; HAMD, Hamilton Depression Rating Scale; PSQI Pittsburgh Sleep Quality Index.
For patients included in the last assessment (week 6) analysis for each variable.
Tolerability
Tolerability findings for both studies (individual and pooled data) have been reported in detail previously and showed a profile consistent with acute quetiapine XR use in other indications including schizophrenia and bipolar disorder (Bauer et al., 2009, 2010; El-Khalili et al., 2010); consequently, tolerability data are not presented here.
Statistical analysis
Post hoc efficacy analyses of pooled data from the two adjunct studies were performed on the modified intention-to-treat (MITT) population (all randomized patients who received study medication had a valid MADRS assessment at randomization and ⩾1 valid MADRS assessment post-randomization). Sleep disturbance and sleep quality end-points were included in studies 6 and 7 as they were considered important from a clinical perspective.
For the efficacy assessments, analyses of pooled data from each study were performed using the last observation carried forward (LOCF) approach for missing data, as in the individual studies. Change from randomization was assessed using an analysis of covariance (ANCOVA) model based on the pre-specified analysis for the primary/secondary end-points with randomization score as a covariate, treatment as a fixed effect, centre as a random effect nested within the study but with the addition of study being included as a covariate. Least squares means and associated two-sided 95% confidence intervals were calculated for all pair-wise differences between quetiapine XR and placebo.
The change from randomization in MADRS total score according to high or low sleep disturbance at randomization was analysed using a similar ANCOVA model.
To assess the robustness of the LOCF approach, an analysis using the mixed model repeat measure (MMRM) technique was performed for all sleep disturbance end-points except for those derived from HAMD total score as this was assessed only at randomization and the end of treatment. The MMRM included efficacy variable at baseline as covariate, treatment, visit and treatment × visit as fixed effects and centre as a random effect.
Calculation of effect sizes according to high or low sleep disturbance at randomization used change in MADRS total score from randomization to week 6 for quetiapine XR minus placebo divided by the pooled standard deviation. If a patient had a missing value for any of the sleep disturbance factor variables (HAMD 4, 5 or 6), then that patient was excluded from the subgroup analysis. All statistical analyses were 2-sided with a significance level of 5%. No adjustment was made for multiple comparisons.
Results
Patients
The patient population across the two studies has been described previously in an analysis of pooled data from the two individual studies (Bauer et al., 2010). Briefly, of the 939 patients, 767 (83.5% of MITT population) completed 6 wk treatment. The MITT population comprised 919 patients: 309, 307 and 303 in the quetiapine XR (150 mg/d), (300 mg/d) and placebo groups, respectively (Fig. 1).
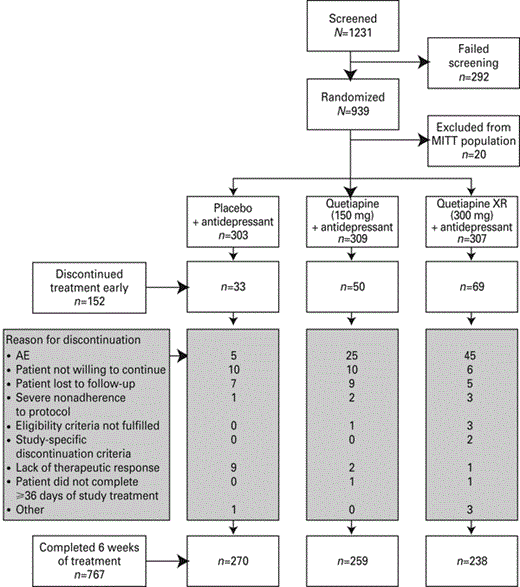
Disposition of patients at each stage of two studies evaluating extended-release quetiapine fumarate (quetiapine XR) as adjunct to on-going antidepressant therapy for the treatment of major depressive disorder. Reprinted from Bauer et al. (2010) with permission from Elsevier. Copyright 2010. AE, Adverse event; MITT, modified intention-to-treat.
Of the MITT population, 226 patients in the quetiapine XR (150 mg/d) group, 215 in the quetiapine XR (300 mg/d) group and 210 in the placebo group experienced high sleep disturbance at randomization. The numbers of patients experiencing low sleep disturbance at randomization in the quetiapine XR (150 mg/d), (300 mg/d) and placebo groups were 83, 92 and 93, respectively.
The three treatment groups were well matched in terms of patient demographics and clinical characteristics at randomization (Bauer et al., 2010). Concomitant antidepressant treatments in the pooled studies included SSRIs, SNRIs, bupropion and amitriptyline. The level of sleep disturbance experienced at randomization was similar in the three treatment groups (Table 1).
At any time during the randomized treatment period, 23.5, 17.3 and 14.9% of patients in the quetiapine XR (150 mg/d), (300 mg/d) and placebo groups, respectively, used sleep medications that had been prescribed prior to study enrolment and permitted according to the study protocols (Bauer et al., 2010). At randomization, 22.1% of patients (68/307) in the quetiapine XR (150 mg/d) and 16.7% of patients (51/306) in the quetiapine XR (300 mg/d) treatment groups, respectively, were using sleep medication. At week 6, the use of sleep medication had reduced to 18.8% of patients (49/260) in the quetiapine XR (150 mg/d) treatment group and 11.4% of patients (27/237) in the quetiapine XR (300 mg/d) treatment group. In the placebo group, 14.5% of patients (44/303) were using sleep medication at randomization compared with 13.1% (35/268) at week 6.
Efficacy assessments
Primary and secondary efficacy outcomes for the pooled population have been reported in detail previously (Bauer et al., 2010) and so are not presented here.
Sleep disturbance
There was a greater reduction in MADRS Item 4 score from baseline with quetiapine XR (150 mg/d) and quetiapine XR (300 mg/d; −1.92 for both; p < 0.001) than with placebo (−0.68) at week 1 and at all subsequent visits including week 6 [quetiapine XR (150 mg/d), −2.27, p< 0.001; quetiapine XR (300 mg/d), −2.23, p < 0.001; placebo −1.30; Fig. 2].

Change from randomization in Montgomery-Åsberg Depression Rating Scale Item 4 (reduced sleep) score over time (last observation carried forward; pooled modified intention-to-treat population). LSM, Least squares means; XR, extended release. *** p < 0.001 vs. placebo + antidepressant.
At week 6, both quetiapine XR doses reduced HAMD Items 4, 5 and 6 scores as well as the HAMD sleep disturbance factor score from randomization compared with placebo (all p < 0.001; Fig. 3).
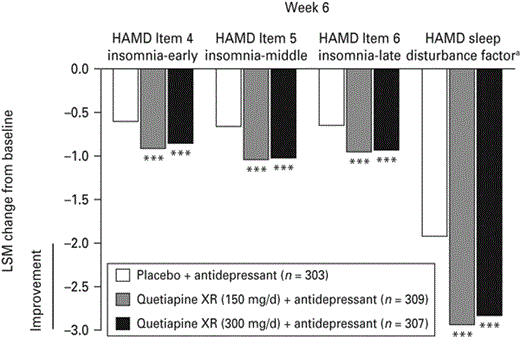
Change from randomization in Hamilton Depression Rating Scale (HAMD) Items 4, 5 and 6 and HAMD sleep disturbance factor scores (last observation carried forward; pooled modified intention-to-treat population). LSM, Least squares means; XR, extended release. a HAMD Items 4+5+6; *** p < 0.001 vs. placebo + antidepressant.
Sleep quality
Greater improvements from randomization in PSQI global score were observed with both doses of quetiapine XR at week 4 (−4.67 for each dose; p< 0.001 each) and week 6 [–4.91 and −4.82 for quetiapine XR (150 mg/d) and (300 mg/d), respectively; p < 0.001 each] vs. placebo (−2.74 and −3.00, respectively; Bauer et al., 2010; Fig. 4). Quetiapine XR (both doses) also improved five out of seven individual PSQI item scores: subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency (p < 0.001 at weeks 4 and 6); sleep disturbance (p < 0.01 at week 4; p < 0.001 at week 6) vs. placebo (Fig. 4). Quetiapine XR did not improve two of the PSQI items, namely use of sleep medication [quetiapine XR (150 mg/d): p = 0.653 at week 4, p = 0.173 at week 6; quetiapine XR (300 mg/d): p = 0.097 at week 4, p = 0.161 at week 6] and daytime dysfunction [quetiapine XR (150 mg/d): p = 0.380 at week 4, p = 0.336 at week 6; quetiapine XR (300 mg/d): p = 0.641 at week 4, p = 0.574 at week 6], to a greater extent than placebo at weeks 4 and 6.
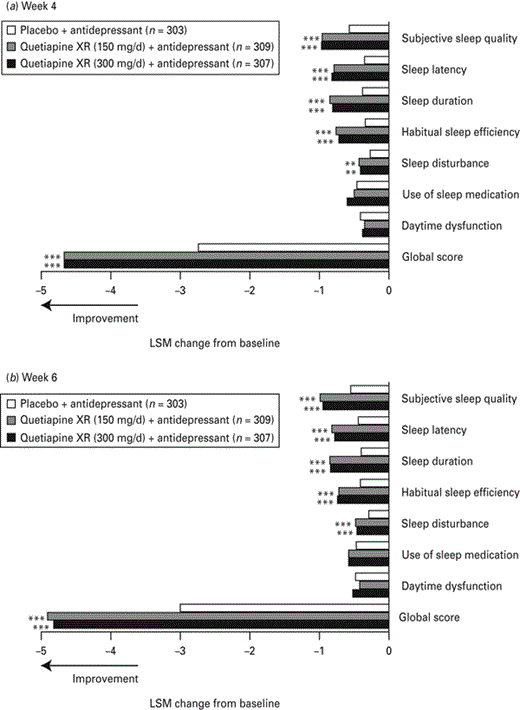
Change from randomization in Pittsburgh Sleep Quality Index global and item scores at (a) week 4 and (b) week 6 (last observation carried forward; pooled modified intention-to-treat population). LSM, Least squares means; XR, extended release. ** p < 0.01, *** p < 0.001 vs. placebo + antidepressant.
Effect of sleep disturbance on changes in depressive symptoms
In patients experiencing high sleep disturbance at randomization, both 150 and 300 mg/d quetiapine XR improved MADRS total score from randomization compared with placebo from week 1 and at all time-points thereafter (Fig. 5a). Effect sizes at week 6 based on change in MADRS total score were 0.32 for quetiapine XR (150 mg/d) and 0.33 for quetiapine XR (300 mg/d).
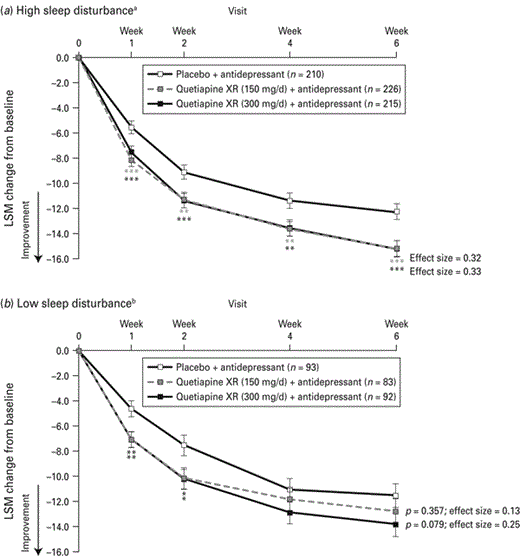
Change from randomization in Montgomery-Åsberg Depression Rating Scale total score over time for patients with (a) high and (b) low sleep disturbance at randomization (last observation carried forward; pooled modified intention-to-treat population). LSM, Least squares means; XR, extended release. a HAMD sleep disturbance factor score ⩾4 at baseline; b HAMD sleep disturbance factor score < 4 at baseline; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. placebo + antidepressant.
In patients with low sleep disturbance at randomization, quetiapine XR (150 and 300 mg/d) improved MADRS total score compared with placebo at weeks 1 and 2 (Fig. 5b). There was no difference between quetiapine and placebo in terms of change in MADRS total score at week 4 [quetiapine XR (150 mg/d) p = 0.535; quetiapine XR (300 mg/d) p = 0.112] or week 6 [quetiapine XR (150 mg/d) p = 0.357; quetiapine XR (300 mg/d) p = 0.079]. Week 6 effect sizes were 0.13 and 0.25, respectively.
MMRM analysis
Results of the MMRM analysis confirmed the findings of the LOCF analysis for sleep disturbance, sleep quality and effect of sleep disturbance on changes in depressive symptoms.
The only difference from the results of the LOCF analysis was in patients with low sleep disturbance at baseline, who achieved greater improvements in MADRS total score with 300 mg/d quetiapine XR at week 6 (p < 0.05) vs. placebo.
Discussion
In this post hoc analysis of pooled data from two acute studies of adjunct quetiapine XR in patients with an inadequate response to on-going antidepressant therapy, approximately two-thirds of patients had high sleep disturbance at randomization and approximately 20% received sleep medication throughout the treatment period. Since sleep disturbance is a common residual symptom in patients with MDD (van Mill et al., 2010), it could be suggested that standard antidepressants are not particularly effective at improving sleep disturbances. Given that the inclusion criteria for the studies reported here stipulated that patients were required to have an inadequate response to antidepressant therapy, it is not surprising that so many patients reported a high level of sleep disturbance at baseline.
Findings from this analysis demonstrated that adjunct quetiapine XR improved a broad range of sleep-related outcome measures. Quetiapine XR (150 and 300 mg/d) led to greater improvements in the duration or depth of sleep compared with an individual's normal sleep pattern (as measured by MADRS Item 4) vs. placebo at the first post-randomization visit (week 1) and this difference persisted at all subsequent visits. Greater improvements in the composite HAMD sleep disturbance factor (sum of the individual items of insomnia-early, insomnia-middle and insomnia-late) were observed with each dose of quetiapine XR vs. placebo. Beyond these clinician-rated measures of sleep disturbance, quetiapine XR (150 and 300 mg/d) also improved patient-reported sleep quality (as measured by PSQI global score at weeks 4 and 6) compared with placebo.
The analysis of patients stratified by sleep disturbance at baseline found that quetiapine XR (150 and 300 mg/d) led to greater improvement in MADRS total score at weeks 1 and 2 compared with placebo irrespective of the baseline level of sleep disturbance. However, from week 4 onwards, for both doses of quetiapine XR, improvement over placebo was only seen in the subgroup of patients with high sleep disturbance at baseline. However, it should be noted that patients were not randomized to treatment based on their level of sleep disturbance at baseline: patient numbers were unbalanced between the sleep disturbance groups (high, n = 651; low n = 268). As reported previously, adverse events of somnolence or sedation were more frequent with both doses of quetiapine XR (150 or 300 mg/d) as adjunctive to antidepressant therapy compared with placebo (Bauer et al., 2010). Studies evaluating the sedative effect of quetiapine XR include a report which evaluated the time-course of sedation for quetiapine XR and immediate-release quetiapine (quetiapine IR) in healthy volunteers (Datto et al., 2009). Quetiapine XR was associated with a reduced intensity of self-reported sedation compared with quetiapine IR. To manage the potential sedative effects during waking hours, prescribing information recommends that quetiapine XR is administered in the evening. It may be expected that the concomitant sedative effect of quetiapine XR may improve sleep in some patients as insomnia is a core symptom of depression. However, the antidepressant effects of quetiapine XR were shown to be independent of either sedation or the observed effects on sleep improvement. Bauer et al., (2010) reported analyses based on pooled data from two studies of quetiapine XR as adjunctive to antidepressant; using two ANCOVA models, treatment effect was greater for quetiapine XR (150 and 300 mg/d) vs. placebo when using MADRS Item 4 (reduced sleep) score as a covariate, and quetiapine XR (150 and 300 mg/d) showed improvements vs. placebo using a modified MADRS total score which omitted Item 4. Furthermore, mean changes in MADRS total score were shown to be irrespective of those who experienced an adverse event of somnolence or sedation (Bauer et al., 2010).
The impact of sleep disturbance on the efficacy of several antidepressants in patients with MDD has been investigated. A randomized double-blind study comparing sertraline, fluoxetine and paroxetine in patients with MDD found no significant differences in efficacy between treatment groups in the overall study population or in subgroups of patients with high or low sleep disturbance at baseline (HAMD sleep disturbance factor ⩾4 and < 4, respectively; Fava et al., 2002). In an analysis of pooled data from three 8-wk randomized, double-blind, placebo-controlled studies of escitalopram in patients with MDD and high levels of sleep disturbance at baseline (MADRS Item 4 score ⩾4), escitalopram significantly reduced MADRS Item 4 scores compared with placebo (p < 0.05; Lader et al., 2005). A meta-analysis of 11 acute (8–9-wk) double-blind, placebo-controlled studies of duloxetine in patients with MDD concluded that it provided negligible benefit to sleep disturbance (Brecht et al., 2008). A 6-wk randomized double-blind comparative study of agomelatine and venlafaxine in patients with MDD found no difference between treatments in terms of antidepressant efficacy; however, agomelatine significantly improved subjective sleep quality vs. venlafaxine, as measured by the Leeds Sleep Evaluation Questionnaire (Lemoine et al., 2007).
There is a paucity of data describing the effects of atypical antipsychotics as adjunct therapy on sleep in patients with MDD. However, our findings for quetiapine XR adjunct therapy are consistent with those from a post hoc analysis of pooled data from studies of adjunct aripiprazole in patients with MDD and an inadequate response to antidepressants, which found significant improvements in the HAMD sleep disturbance factor score and the HAMD Item 4 (early insomnia) score compared with placebo (Nelson et al., 2010). Significant improvements in HAMD Items 5 (insomnia-middle) and 6 (insomnia-late) were not reported for adjunct aripiprazole compared with placebo. Data reported here for quetiapine XR may therefore provide clinicians with important information when assessing patient symptoms at baseline and choosing treatments accordingly.
A key strength of the present analyses is the use of pooled data, which enabled a larger patient population to be evaluated with the potential for reduced sample variation if the study populations are homogenous. However, the smaller sample size in the low sleep disturbance subgroup may have limited any meaningful clinical interpretation of treatment efficacy in this specific group of patients. Based on the previously reported minimum clinically important difference of 1.6–1.9 MADRS units (Duru and Fantino, 2008), our data suggest that patients in the low sleep disturbance subgroup did achieve a clinically meaningful improvement in MADRS total score during treatment with quetiapine XR (300 mg/d) compared with placebo at week 6 (change from baseline of −12.8, −13.9 and −11.6 in the quetiapine XR (150 mg/d), (300 mg/d) and placebo groups, respectively). Moreover, the methodology of these studies imposes limits on the interpretation of our findings given that there was no active comparator to ensure assay sensitivity and that fixed dosing is not representative of clinical practice. Furthermore, the 6-wk duration of these studies precludes any insights into the longer-term efficacy of quetiapine XR adjunct therapy. An additional limitation is that this was a post hoc analysis.
Research suggests that sleep curtailment may result in increased health risk to patients with MDD: sleep disturbance is associated with inflammatory and metabolic changes including detrimental effects on glucose clearance, acute insulin response and insulin sensitivity that increases diabetes risk (Spiegel et al., 2003; Knutson and Van Cauter, 2008; Ruger and Scheer, 2009), although further research is warranted in order to better understand these complex relationships. Future prospective studies could therefore be used to investigate the possibility that improved sleep quality gained from antidepressant therapy may also improve metabolic status.
In summary, in this analysis of pooled data from two randomized placebo-controlled studies, quetiapine XR (150 and 300 mg/d) as adjunct therapy improved sleep disturbance and quality vs. placebo in patients with MDD and an inadequate response to on-going antidepressant treatment, and was effective against depressive symptoms in patients experiencing high levels of sleep disturbance.
Acknowledgements
These studies [D1448C00006 (Pearl) and D1448C00007 (Onyx)] were funded and supported by AstraZeneca. Both studies were registered at ClinicalTrials.gov (D1448C00006 study identifier number NCT00326105; D1448C00007 study identifier number NCT00351910). We thank Varinia Munoz, PhD, from Complete Medical Communications, who provided medical writing support funded by AstraZeneca.
Statement of Interest
Michael Bauer has received grant support or received speaker honoraria from, or has been a consultant for: AstraZeneca; Bristol-Myers Squibb; Otsuka; Deutsche Forschungsgemeinschaft (German Research Foundation); European Commission FP7; NARSAD; Eli Lilly; Lundbeck; NARSAD; Servier; the Stanley Medical Research Institute; Takeda.
Roger McIntyre has received honoraria from, is or has been an advisor/consultant to, or on the speaker bureaus for, or received grant or research support from: AstraZeneca; Bristol-Myers Squibb; CME Outfitters; Eli Lilly; France Foundation; GlaxoSmithKline; Janssen-Ortho; Lundbeck; Merck; NARSAD; Optum Health; Pfizer; Physicians' Postgraduate press; Shire; Stanley Medical Research Institute.
Johan Szamosi and Hans Eriksson are former employees of AstraZeneca.
References



