-
PDF
- Split View
-
Views
-
Cite
Cite
Madhukar H. Trivedi, Borwin Bandelow, Koen Demyttenaere, George I. Papakosts, Johan Szamosi, Willie Earley, Hans Eriksson, Evaluation of the effects of extended release quetiapine fumarate monotherapy on sleep disturbance in patients with major depressive disorder: a pooled analysis of four randomized acute studies, International Journal of Neuropsychopharmacology, Volume 16, Issue 8, September 2013, Pages 1733–1744, https://doi.org/10.1017/S146114571300028X
Close - Share Icon Share
Abstract
Effects of once-daily extended-release quetiapine fumarate (quetiapine XR) monotherapy on sleep quality and disturbance in patients with major depressive disorder (MDD) were evaluated. Pooled data from four 6- or 8-wk placebo-controlled quetiapine XR (50–300 mg/d) monotherapy studies (D1448C00001; D1448C00002; D1448C00003; D1448C00004) were analysed. Primary efficacy end-point was change from randomization in Montgomery Åsberg Depression Rating Scale (MADRS) score. Post hoc analyses of secondary end-points were conducted for change from randomization in: MADRS item 4 (reduced sleep); Hamilton Rating Scale for Depression (HAMD) items 4 (insomnia-early), 5 (insomnia-middle), 6 (insomnia-late) and sleep disturbance factor (items 4 + 5+6) scores; Pittsburgh Sleep Quality Index (PSQI) global scores. MADRS total score change was also evaluated in patients experiencing high and low baseline sleep disturbance (HAMD sleep disturbance factor scores ⩾4 and < 4, respectively). In total, 1808 patients were randomized to quetiapine XR or placebo across four studies. At last assessment, quetiapine XR reduced MADRS item 4, HAMD items 4, 5 and 6, HAMD sleep disturbance factor score and PSQI global scores from baseline vs. placebo (p < 0.001). For those experiencing high sleep disturbance (n = 865, quetiapine XR; n = 514, placebo), quetiapine XR improved MADRS total score vs. placebo at all visits (p < 0.001). For those with low sleep disturbance (n = 252, quetiapine XR; n = 121, placebo), quetiapine XR improved MADRS total score vs. placebo at weeks 2 (p < 0.001), 4 and 6 (both p < 0.05). In conclusion, quetiapine XR (50–300 mg/d) monotherapy improved symptoms of sleep disturbance vs. placebo in patients with MDD, including those with either high or low baseline sleep disturbance levels.
Introduction
Major depressive disorder (MDD) is a serious and common global health concern; the lifetime prevalence rate of MDD in the community is approximately 16.6% in the USA (Kessler et al., 2005) and 12.8% in Europe (Alonso et al., 2004). It is predicted that by 2030, unipolar depressive disorders will be the second leading cause of disability-adjusted life years after HIV/AIDS (Mathers and Loncar, 2006).
There is a link between depression and sleep, and sleep disturbance is a common symptom of MDD (Nutt et al., 2008) with ‘insomnia (early, middle, late) or hypersomnia nearly every day’ listed as one of its nine symptoms in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV TR; APA, 2000). Furthermore, early morning awakening, at least 2 h before the usual time of awakening, is one of the DSM-IV TR criteria for MDD with melancholic features (APA, 2000), a severe form of MDD. MDD is also associated with impaired sleep efficiency, a reduction in slow-wave sleep and disinhibition of rapid eye movement (REM) sleep (Tsuno et al., 2005): sleep disturbances are common residual symptoms even following antidepressant treatment (Nierenberg et al., 2010; Iovieno et al., 2011; Morehouse et al., 2011). Furthermore, the relief of sleep disturbance symptoms is considered to be an important component of complete remission of MDD (Mendlewicz, 2009). A recent Dutch epidemiological study conducted in patients with MDD and anxiety disorders (n = 2619) confirmed the strong association between MDD and sleep disturbance (van Mill et al., 2010). This epidemiological study also found that insomnia and short sleep duration remain after remission of MDD, again highlighting that insomnia is a common residual symptom.
In patients with MDD, the consequences of sleep disturbance include greater episode severity and higher relapse rates (Franzen and Buysse, 2008), increased severity of suicide ideation (McCall et al., 2010) and increased risk of suicide (McGirr et al., 2007). In addition, direct healthcare costs are greater in patients with MDD who have sleep disturbance (estimated as $4858) than in those with no sleep disturbance (∼$4007; Asche et al., 2010). Thus, the effective treatment of sleep disturbance in patients with MDD is extremely important.
The pharmacological management of patients with MDD who experience sleep disturbance is complicated by the fact that a number of traditional antidepressants may not alleviate sleep disturbance (Mendlewicz, 2009). In the STAR*D study, ∼35% of patients with non-psychotic MDD who received citalopram treatment also used a concomitant psychotropic medication, with insomnia being the most common indication for concomitant medication usage in these patients (Shelton et al., 2009). In addition, some antidepressants may lead to problems with sleep. For example, insomnia is associated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin norepinephrine reuptake inhibitors (SNRIs) venlafaxine and duloxetine, monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs; Mayers and Baldwin, 2005; Brecht et al., 2008), while REM sleep suppression is often observed with MAOIs, SSRIs, venlafaxine, bupropion and most TCAs (Thase, 2000). However, it should be noted that, due to their sedating properties, TCAs (in low doses) and the antidepressant mirtazapine may be used as sleep medication (Becker, 2006).
At the time of writing, extended-release quetiapine fumarate (quetiapine XR) is approved in the USA (AstraZeneca, 2012a), the EU (AstraZeneca, 2012b) and several other countries worldwide as adjunctive treatment for patients with MDD and an inadequate response to previous antidepressants. In addition, quetiapine XR has been approved for use as a monotherapy treatment for MDD in a limited number of countries, including Canada and Australia, for patients who are intolerant of, or have had an inadequate response to, alternative antidepressant drugs.
The pooled analyses reported here evaluated the effects of quetiapine XR monotherapy on sleep quality and sleep disturbance using data from four previously reported studies of acute quetiapine XR monotherapy in patients with MDD (Earley et al., 2008; Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011).
Method
Study design and treatment
For these analyses, data were pooled from four acute monotherapy studies of quetiapine XR in MDD [D1448C00001 (study 1; Weisler et al., 2009); D1448C00002 (study 2; Cutler et al., 2009); D1448C00003 (study 3; Bortnick et al., 2011); D1448C00004 (study 4; Earley et al., 2008)]. The study designs have previously been described in detail. In brief, these were double-blind, randomized, placebo-controlled studies consisting of an enrolment period of up to 28 d and a 6- (studies 1 and 2) or 8-wk (studies 3 and 4) randomized treatment phase followed by a 2-wk post-treatment drug discontinuation phase.
Patients were randomized to receive quetiapine XR (50, 150 or 300 mg/d) or placebo (study 1), quetiapine XR (150 or 300 mg/d), duloxetine (60 mg/d) or placebo (study 2), quetiapine XR (150 or 300 mg/d) or placebo (study 3) or quetiapine XR (150 or 300 mg/d), escitalopram (10 or 20 mg/d) or placebo (study 4). In all four studies, male or female outpatients (aged 18–65 yr) with a DSM-IV (APA, 1994) diagnosis of MDD (single episode or recurrent) were eligible for inclusion. In addition, patients were required to have a HAMD (Hamilton, 1960) total score ⩾22 and a HAMD item 1 (depressed mood) score ⩾2 at enrolment and randomization. Full exclusion criteria have been reported previously (Earley et al., 2008; Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011). In brief, the key exclusion criteria were: DSM-IV Axis I disorder (other than MDD) within 6 months prior to enrolment; DSM-IV Axis II disorder significantly impacting patient's psychiatric status; duration of the current MDD episode > 12 months or < 4 wk from enrolment; history (during the current episode) of an inadequate response to at least 6-wk treatment with two or more classes of antidepressant.
The data from these four studies were pooled to maximize the sample size across the patient subgroups included in these analyses and to enable a best estimate using all available data. In the global quetiapine XR monotherapy MDD clinical trial programme, efficacy was seen for all doses of quetiapine XR from 50 mg/d to 300 mg/d and so it was considered appropriate to combine all quetiapine doses for the purposes of these analyses. Data for duloxetine (study 2) and escitalopram (study 4) were not included in these pooled analyses.
The use of other psychoactive medication was not permitted during these studies, with the exception of ongoing hypnotics for insomnia. The following sleep medications for insomnia were permitted (with usage restricted to bedtime) if treatment had been regular and commenced ⩾28 d before enrolment: lorazepam (or equivalent, maximum 2 mg/d); zolpidem tartrate (10 mg/d); zaleplon (20 mg/d); zopiclone (7.5 mg/d); chloral hydrate (1 g/d). The proportion of patients receiving sleep medication was recorded.
The four studies were performed in accordance with the Declaration of Helsinki and International Conference on Harmonization/Good Clinical Practice guidelines and were approved by the local Institutional Review Board at each centre. Written informed consent was obtained from all patients.
Assessments
Efficacy
The primary end-point in all four studies was change from randomization (baseline) to treatment end [last assessment at week 6 (studies 1 and 2) or 8 (studies 3 and 4)] in Montgomery Åsberg Depression Rating Scale (MADRS; Montgomery and Åsberg, 1979) total score. MADRS total score was assessed at baseline and weeks 1, 2, 4 and 6 in all studies and also at week 8 in studies 3 and 4. Secondary efficacy end-points included MADRS total scores over time and MADRS item; HAMD total and item scores at last assessment (week 6 or 8). In addition, Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) global score was assessed at baseline, week 4 and last assessment (week 6 or 8) to evaluate patients' sleep quality during the previous month by rating the following dimensions of sleep: subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbances; use of sleep medication; daytime dysfunction.
Post hoc sleep disturbance assessments
Post hoc analyses of the pooled data for rating scale sleep items were conducted on changes in MADRS item 4 (reduced sleep) score at weeks 1, 2, 4, 6 and last assessment (week 6 or 8); HAMD items 4 (insomnia-early), 5 (insomnia-middle) and 6 (insomnia-late) and HAMD sleep disturbance factor (items 4 + 5+6) scores at baseline, week 6 and last assessment (week 6 or 8). Also, changes in PSQI global and item scores at week 4 and last assessment (week 6 or 8) were analysed post hoc for the pooled population.
In addition, post hoc analyses were performed for change in MADRS total score in patients experiencing high or low levels of sleep disturbance. In this pooled analysis, the mean baseline HAMD sleep disturbance factor (items 4 + 5+6) score was 4.6 in both treatment groups (Table 1). High and low sleep disturbance at baseline were defined as HAMD sleep disturbance factor scores ⩾4 and < 4, respectively, definitions that have been used previously (Fava et al., 2002). In a pooled data analysis of the effects of adjunct quetiapine XR on sleep disturbance and quality in patients with MDD and an inadequate response to prior antidepressant treatment, the mean baseline HAMD sleep disturbance factor score was approximately 4 with high sleep disturbance being defined by a sleep disturbance factor score ⩾4 and low sleep disturbance by a sleep disturbance factor score < 4 (Bauer et al., 2011). The efficacy of SSRIs on sleep disturbance has also been investigated in a study that defined baseline sleep disturbance as high or low according to HAMD sleep disturbance factor scores ⩾4 and < 4, respectively (Fava et al., 2002).
Demographics, clinical characteristics and disease severity at baseline (pooled MITT population)
| . | Placebo (n = 635) . | Quetiapine XR (all doses combined; n = 1117) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 223 (35.1) | 442 (39.6) |
| Female | 412 (64.9) | 675 (60.4) |
| Age, yr | ||
| Mean (s.d.) | 41.2 (11.6) | 41.2 (11.7) |
| Range | 18–65 | 18–65 |
| Race, n (%) | ||
| White | 425 (66.9) | 786 (70.4) |
| Black | 141 (22.2) | 244 (21.8) |
| Asian | 48 (7.6) | 48 (4.3) |
| Other | 21 (3.3) | 39 (3.5) |
| Weight, kg | ||
| Mean (s.d.) | 82.4 (23.6) | 84.3 (22.2) |
| Range | 36.6–190.0 | 37.0–190.5 |
| DSM-IV diagnosis, n (%) | ||
| 296.2 × MDD, single episode | 99 (15.6) | 164 (14.7) |
| 296.3 × MDD, recurrent | 536 (84.4) | 953 (85.3) |
| Disease severity, mean (s.d.) baseline scores | ||
| MADRS total score | 30.4 (5.3) | 30.6 (5.3) |
| HAMD total score | 25.7 (3.1) | 25.7 (3.2) |
| HAMA total score | 19.2 (5.8) | 19.3 (5.8) |
| CGI-S score | 4.6 (0.7) | 4.6 (0.7) |
| Sleep disturbance assessments, mean (s.d.) baseline scoresa | ||
| MADRS item 4 (reduced sleep) score | 3.8 (1.1) | 3.7 (1.1) |
| HAMD item 4 (insomnia-early) score | 1.6 (0.71) | 1.6 (0.7) |
| HAMD item 5 (insomnia-middle) score | 1.6 (0.6) | 1.6 (0.6) |
| HAMD item 6 (insomnia-late) score | 1.4 (0.7) | 1.4 (0.7) |
| HAMD sleep disturbance factor (items 4 + 5+6) score | 4.6 (1.4) | 4.6 (1.5) |
| PSQI global score | 11.8 (3.92) | 11.7 (3.71) |
| Baseline sleep disturbance level, n (%) | ||
| High (HAMD sleep disturbance factor score ⩾4) | 514 (80.9) | 865 (77.4) |
| Low (HAMD sleep disturbance factor score < 4) | 121 (19.1) | 252 (22.6) |
| . | Placebo (n = 635) . | Quetiapine XR (all doses combined; n = 1117) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 223 (35.1) | 442 (39.6) |
| Female | 412 (64.9) | 675 (60.4) |
| Age, yr | ||
| Mean (s.d.) | 41.2 (11.6) | 41.2 (11.7) |
| Range | 18–65 | 18–65 |
| Race, n (%) | ||
| White | 425 (66.9) | 786 (70.4) |
| Black | 141 (22.2) | 244 (21.8) |
| Asian | 48 (7.6) | 48 (4.3) |
| Other | 21 (3.3) | 39 (3.5) |
| Weight, kg | ||
| Mean (s.d.) | 82.4 (23.6) | 84.3 (22.2) |
| Range | 36.6–190.0 | 37.0–190.5 |
| DSM-IV diagnosis, n (%) | ||
| 296.2 × MDD, single episode | 99 (15.6) | 164 (14.7) |
| 296.3 × MDD, recurrent | 536 (84.4) | 953 (85.3) |
| Disease severity, mean (s.d.) baseline scores | ||
| MADRS total score | 30.4 (5.3) | 30.6 (5.3) |
| HAMD total score | 25.7 (3.1) | 25.7 (3.2) |
| HAMA total score | 19.2 (5.8) | 19.3 (5.8) |
| CGI-S score | 4.6 (0.7) | 4.6 (0.7) |
| Sleep disturbance assessments, mean (s.d.) baseline scoresa | ||
| MADRS item 4 (reduced sleep) score | 3.8 (1.1) | 3.7 (1.1) |
| HAMD item 4 (insomnia-early) score | 1.6 (0.71) | 1.6 (0.7) |
| HAMD item 5 (insomnia-middle) score | 1.6 (0.6) | 1.6 (0.6) |
| HAMD item 6 (insomnia-late) score | 1.4 (0.7) | 1.4 (0.7) |
| HAMD sleep disturbance factor (items 4 + 5+6) score | 4.6 (1.4) | 4.6 (1.5) |
| PSQI global score | 11.8 (3.92) | 11.7 (3.71) |
| Baseline sleep disturbance level, n (%) | ||
| High (HAMD sleep disturbance factor score ⩾4) | 514 (80.9) | 865 (77.4) |
| Low (HAMD sleep disturbance factor score < 4) | 121 (19.1) | 252 (22.6) |
CGI-S, Clinical Global Impressions-Severity of Illness; HAMA, Hamilton Rating Scale for Anxiety; HAMD, Hamilton Rating Scale for Depression; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; MITT, modified intent-to-treat; PSQI, Pittsburgh Sleep Quality Index; XR, extended release.
For patients included in the last assessment (week 6 or week 8) analysis for each variable.
Demographics, clinical characteristics and disease severity at baseline (pooled MITT population)
| . | Placebo (n = 635) . | Quetiapine XR (all doses combined; n = 1117) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 223 (35.1) | 442 (39.6) |
| Female | 412 (64.9) | 675 (60.4) |
| Age, yr | ||
| Mean (s.d.) | 41.2 (11.6) | 41.2 (11.7) |
| Range | 18–65 | 18–65 |
| Race, n (%) | ||
| White | 425 (66.9) | 786 (70.4) |
| Black | 141 (22.2) | 244 (21.8) |
| Asian | 48 (7.6) | 48 (4.3) |
| Other | 21 (3.3) | 39 (3.5) |
| Weight, kg | ||
| Mean (s.d.) | 82.4 (23.6) | 84.3 (22.2) |
| Range | 36.6–190.0 | 37.0–190.5 |
| DSM-IV diagnosis, n (%) | ||
| 296.2 × MDD, single episode | 99 (15.6) | 164 (14.7) |
| 296.3 × MDD, recurrent | 536 (84.4) | 953 (85.3) |
| Disease severity, mean (s.d.) baseline scores | ||
| MADRS total score | 30.4 (5.3) | 30.6 (5.3) |
| HAMD total score | 25.7 (3.1) | 25.7 (3.2) |
| HAMA total score | 19.2 (5.8) | 19.3 (5.8) |
| CGI-S score | 4.6 (0.7) | 4.6 (0.7) |
| Sleep disturbance assessments, mean (s.d.) baseline scoresa | ||
| MADRS item 4 (reduced sleep) score | 3.8 (1.1) | 3.7 (1.1) |
| HAMD item 4 (insomnia-early) score | 1.6 (0.71) | 1.6 (0.7) |
| HAMD item 5 (insomnia-middle) score | 1.6 (0.6) | 1.6 (0.6) |
| HAMD item 6 (insomnia-late) score | 1.4 (0.7) | 1.4 (0.7) |
| HAMD sleep disturbance factor (items 4 + 5+6) score | 4.6 (1.4) | 4.6 (1.5) |
| PSQI global score | 11.8 (3.92) | 11.7 (3.71) |
| Baseline sleep disturbance level, n (%) | ||
| High (HAMD sleep disturbance factor score ⩾4) | 514 (80.9) | 865 (77.4) |
| Low (HAMD sleep disturbance factor score < 4) | 121 (19.1) | 252 (22.6) |
| . | Placebo (n = 635) . | Quetiapine XR (all doses combined; n = 1117) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 223 (35.1) | 442 (39.6) |
| Female | 412 (64.9) | 675 (60.4) |
| Age, yr | ||
| Mean (s.d.) | 41.2 (11.6) | 41.2 (11.7) |
| Range | 18–65 | 18–65 |
| Race, n (%) | ||
| White | 425 (66.9) | 786 (70.4) |
| Black | 141 (22.2) | 244 (21.8) |
| Asian | 48 (7.6) | 48 (4.3) |
| Other | 21 (3.3) | 39 (3.5) |
| Weight, kg | ||
| Mean (s.d.) | 82.4 (23.6) | 84.3 (22.2) |
| Range | 36.6–190.0 | 37.0–190.5 |
| DSM-IV diagnosis, n (%) | ||
| 296.2 × MDD, single episode | 99 (15.6) | 164 (14.7) |
| 296.3 × MDD, recurrent | 536 (84.4) | 953 (85.3) |
| Disease severity, mean (s.d.) baseline scores | ||
| MADRS total score | 30.4 (5.3) | 30.6 (5.3) |
| HAMD total score | 25.7 (3.1) | 25.7 (3.2) |
| HAMA total score | 19.2 (5.8) | 19.3 (5.8) |
| CGI-S score | 4.6 (0.7) | 4.6 (0.7) |
| Sleep disturbance assessments, mean (s.d.) baseline scoresa | ||
| MADRS item 4 (reduced sleep) score | 3.8 (1.1) | 3.7 (1.1) |
| HAMD item 4 (insomnia-early) score | 1.6 (0.71) | 1.6 (0.7) |
| HAMD item 5 (insomnia-middle) score | 1.6 (0.6) | 1.6 (0.6) |
| HAMD item 6 (insomnia-late) score | 1.4 (0.7) | 1.4 (0.7) |
| HAMD sleep disturbance factor (items 4 + 5+6) score | 4.6 (1.4) | 4.6 (1.5) |
| PSQI global score | 11.8 (3.92) | 11.7 (3.71) |
| Baseline sleep disturbance level, n (%) | ||
| High (HAMD sleep disturbance factor score ⩾4) | 514 (80.9) | 865 (77.4) |
| Low (HAMD sleep disturbance factor score < 4) | 121 (19.1) | 252 (22.6) |
CGI-S, Clinical Global Impressions-Severity of Illness; HAMA, Hamilton Rating Scale for Anxiety; HAMD, Hamilton Rating Scale for Depression; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; MITT, modified intent-to-treat; PSQI, Pittsburgh Sleep Quality Index; XR, extended release.
For patients included in the last assessment (week 6 or week 8) analysis for each variable.
Tolerability
Tolerability assessments in the four studies, including adverse event (AE) reporting, have been described previously (Earley et al., 2008; Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011) and so are not presented in detail here.
Statistical analysis
All analyses were performed using last observation carried forward (LOCF) data (for imputation of missing data) for the pooled modified intent-to-treat (MITT) population. For inclusion in the MITT, randomized patients must have taken at least one dose of study medication and have both a valid MADRS total score at randomization and at least one valid post-randomization assessment of MADRS total score.
For all efficacy outcomes, an analysis of covariance (ANCOVA) model was used to analyse change from randomization; least squares means (LSM) estimates and two-sided 95% confidence intervals (CI) were provided. The ANCOVA model included score at randomization as a covariate, treatment and study as fixed effects and centre nested within study as a random effect. A similar ANCOVA model was used to analyse change from randomization in MADRS total score according to high or low sleep disturbance at baseline. Effect sizes for quetiapine XR in patients with high or low sleep disturbance status at baseline were calculated using change in MADRS total score from randomization to final assessment for quetiapine XR (all doses combined) minus placebo divided by the pooled standard deviation of the change in MADRS total score (ANCOVA; LOCF; MITT population).
LSM differences and 95% CIs for quetiapine XR vs. placebo in terms of change in PSQI global score were determined using data from the individual monotherapy studies and the current pooled analysis. The difference between treatment groups was not classed as statistically significant if the 95% CIs for the comparison between quetiapine XR and placebo included 1.
Post hoc assessments of baseline variables on outcome
To evaluate the effect of HAMD sleep disturbance factor score at baseline on efficacy outcomes at the last assessment, a post hoc analysis was conducted using an ANCOVA model (with MADRS total score at randomization as covariate, treatment and baseline HAMD sleep disturbance factor score as fixed effects and centre as a random effect) to analyse change in MADRS total score from randomization in patients with high and low baseline sleep disturbance.
In addition, descriptive statistics were provided to compare baseline disease severity [MADRS, HAMD, Hamilton Rating Scale for Anxiety (HAMA), Clinical Global Impressions-Severity of Illness (CGI-S) total and PSQI global scores] according to the level of sleep disturbance at baseline (high or low).
Results
Individual studies
The results for the primary and key secondary efficacy variables for the four individual studies have been reported previously (Earley et al., 2008; Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011). Three of the studies were positive in favour of quetiapine XR (Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011). Study 4 was a failed study: neither quetiapine XR nor escitalopram demonstrated a statistically significant separation from placebo (Earley et al., 2008). Study 4 was included in this pooled analysis in order that the entire dataset from the quetiapine XR acute monotherapy clinical development programme in adults could be analysed.
Pooled data analyses
Patients
In total, 1808 patients were randomized to receive quetiapine XR or placebo across the four studies. For the pooled analyses, the MITT population comprised 1752 patients (n = 1117, quetiapine XR; n = 635, placebo). Overall, 1291 patients completed the randomized treatment period (6 or 8 wk), with 312 and 149 patients discontinuing early in the quetiapine XR and placebo groups, respectively; in both treatment groups the main reasons for discontinuation were AE, patient not willing to continue and patient lost to follow-up.
The two treatment groups were well matched in terms of demographics, clinical characteristics and disease severity at baseline (Table 1). In the pooled MITT population, 865 patients in the quetiapine XR group and 514 in the placebo group experienced high sleep disturbance at baseline; 252 and 121 patients in the quetiapine XR and placebo groups, respectively, experienced low sleep disturbance at baseline.
In the pooled MITT population, 3.6% of quetiapine XR-treated patients and 6.9% of placebo-treated patients used concomitant sleep medication at baseline; the level of sleep medication usage remained similarly low throughout the study, with 3.2 and 4.9% of quetiapine XR-treated and placebo-treated patients, respectively, using sleep medication at treatment end.
Sleep disturbance assessments: efficacy outcomes
MADRS item 4 (reduced sleep), HAMD items 4, 5 and 6 and HAMD sleep disturbance factor (sum of items 4 + 5+6) scores at baseline are provided in Table 1; the level of sleep disturbance experienced at baseline was similar in the two treatment groups.
A reduction in MADRS item 4 score from baseline was seen with quetiapine XR (all doses combined) compared with placebo at week 1 (−2.28 and −1.04, respectively; p < 0.001) and all subsequent visits including last assessment (−2.53 and −1.69, respectively; p < 0.001; Fig. 1).
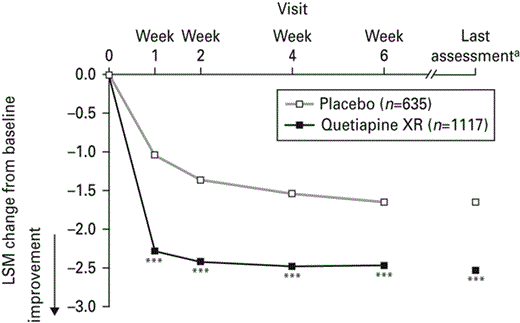
Least squares means (LSM) change from baseline in Montgomery Åsberg Depression Rating Scale item 4 score over time (last observation carried forward; pooled modified intent-to-treat population). XR, Extended release. a Pooled scores for week 6 of studies 1 and 2 and for week 8 of studies 3 and 4; *** p < 0.001 vs. placebo.
Similarly, at last assessment (week 6 or 8), HAMD items 4, 5 and 6 and HAMD sleep disturbance factor scores were all reduced from baseline with quetiapine XR (all doses combined) compared with placebo (p < 0.001; Fig. 2).
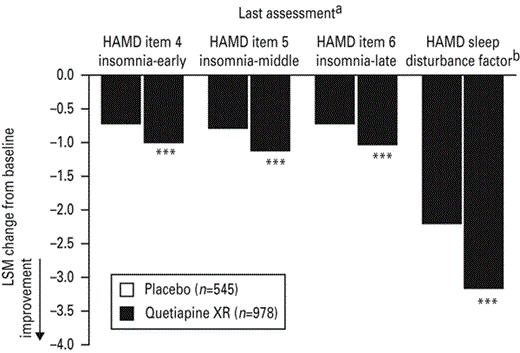
Least squares means (LSM) change from baseline in Hamilton Rating Scale for depression (HAMD) items 4, 5 and 6 and HAMD sleep disturbance factor scores at last assessment (last observation carried forward; pooled modified intent-to-treat population). XR, Extended release. a Pooled scores for week 6 of studies 1 and 2 and for week 8 of studies 3 and 4; b HAMD items 4 + 5+6; *** p < 0.001 vs. placebo.
Reductions from baseline in PSQI global scores (indicating an improvement in sleep quality) were seen at week 4 with quetiapine XR (all doses combined; −4.20; p < 0.001) compared with placebo (−2.58; Fig. 3a). In addition, improvements were seen in five out of seven individual PSQI item scores [subjective sleep quality, sleep latency, sleep duration and sleep disturbances (p < 0.001 each) and habitual sleep efficiency (p < 0.01)] at week 4 for quetiapine XR compared with placebo. There was also a reduction in PSQI global scores from baseline at last assessment with quetiapine XR (all doses combined; −4.63; p < 0.001) compared with placebo (−3.36; Fig. 3b). Furthermore, a reduction was seen with quetiapine XR in the same five individual PSQI items at last assessment as at week 4 [subjective sleep quality, sleep latency, sleep duration and sleep disturbance (p < 0.001 each) and habitual sleep efficiency (p < 0.01) vs. placebo]. The daytime dysfunction item was improved in both treatment groups at last assessment; however, the improvement was greater with placebo (p < 0.05).
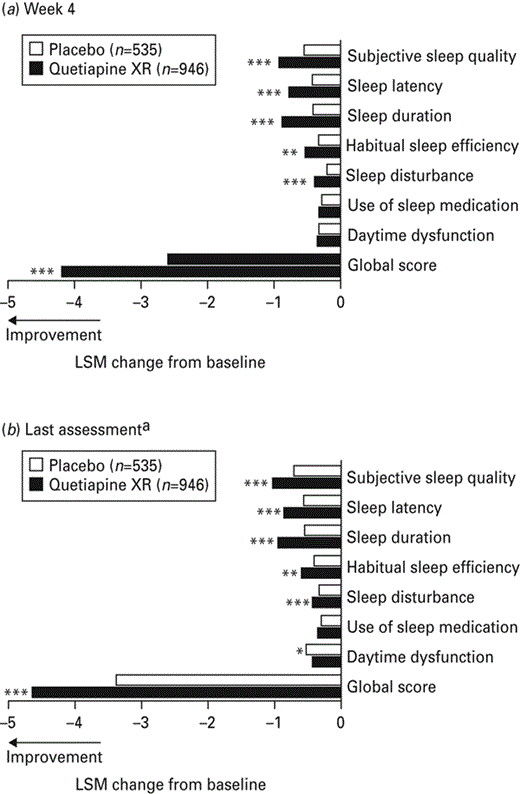
Least squares means (LSM) change from baseline in Pittsburgh Sleep Quality Index global and individual item scores at (a) week 4 and (b) last assessment (last observation carried forward; pooled modified intent-to-treat population). XR, Extended release. a Pooled scores for week 6 of studies 1 and 2 and for week 8 of studies 3 and 4. * p < 0.05; ** p < 0.01; *** p < 0.001 vs. placebo.
Efficacy outcome in patients with MDD and high or low levels of sleep disturbance
There was an improvement in MADRS total score from randomization with quetiapine XR compared with placebo at week 1 (−8.75 and −6.71, respectively; p < 0.001) and all subsequent time points including last assessment [−16.29 (n = 865) and −12.99 (n = 514), respectively; p < 0.001] in the subgroup of patients experiencing high sleep disturbance (Fig. 4a).
![Least squares means (LSM; ±s.e.) change from baseline in Montgomery Åsberg Depression Rating Scale total score over time for patients with (a) high sleep disturbance [baseline Hamilton Rating Scale for depression (HAMD) sleep disturbance factor score (sum of items 4 + 5+6) ⩾ 4] and (b) low sleep disturbance [baseline HAMD sleep disturbance factor score (sum of items 4 + 5+6) < 4; last observation carried forward; pooled modified intent-to-treat population]. XR, Extended release. a Pooled scores for week 6 of studies 1 and 2 and for week 8 of studies 3 and 4. * p < 0.05; *** p < 0.001 vs. placebo.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijnp/16/8/10.1017_S146114571300028X/1/m_s146114571300028x_fig4.gif?Expires=1750189005&Signature=qLxTZ7A4WZLKCJFKXf6d7apCdSjt5jLSgZu0b4kVAKxBOn4mwlbS~ETaDUk7-q0xR-81xvvYKCG4NgsMSicDAZDg7zPVWXAKx~MIe00VqW7fVP9esjjZSrnY5ppYW7csTAkmEUwJXCK~ETEUmGmqCHaKa4Cc6sk7cvhkoZKhxxa4nOYktCwWVMrbSaFWhMkbcYkm3uANydeyOJAw8UUV0a3Ue4Whhub~48yVYmFbRorUoUM7rUWbcFcPhVpkV32pyGn3YiZkGc-KLMmNLzpVrKjZs3ajK8UtkFxfgpXk3dKL-q8d6ImYOLCH3SjFBEm8d-dmFNv~JDsTV~cKnOAi5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Least squares means (LSM; ±s.e.) change from baseline in Montgomery Åsberg Depression Rating Scale total score over time for patients with (a) high sleep disturbance [baseline Hamilton Rating Scale for depression (HAMD) sleep disturbance factor score (sum of items 4 + 5+6) ⩾ 4] and (b) low sleep disturbance [baseline HAMD sleep disturbance factor score (sum of items 4 + 5+6) < 4; last observation carried forward; pooled modified intent-to-treat population]. XR, Extended release. a Pooled scores for week 6 of studies 1 and 2 and for week 8 of studies 3 and 4. * p < 0.05; *** p < 0.001 vs. placebo.
In the subgroup of patients with low sleep disturbance, quetiapine XR also improved MADRS total score vs. placebo at weeks 2 (−10.94 and −7.83, respectively; p < 0.001), 4 (−12.31 and −10.04, respectively; p < 0.05) and 6 (−13.85 and −11.78, respectively; p < 0.05; Fig. 4b). At last assessment (pooled scores for week 6 for studies 1 and 2 and week 8 for studies 3 and 4) there was no difference between quetiapine XR (n = 252) and placebo (n = 121) in terms of improvements in MADRS total score in patients with low sleep disturbance (−13.94 and −11.94, respectively; p = 0.052).
The effect sizes (using change in MADRS total score from baseline to last assessment for quetiapine XR vs. placebo) were 0.33 for patients with high sleep disturbance at baseline and 0.23 for patients with low sleep disturbance at baseline. The effect size (quetiapine XR vs. placebo) for the pooled population was 0.30.
Effect of baseline variables on outcome
There was no difference in MADRS total score change at last assessment for patients with high sleep disturbance at baseline compared with patients with low sleep disturbance at baseline (p = 0.6189).
The assessment of baseline disease severity showed that patients with high sleep disturbance had higher MADRS, HAMD, HAMA total and PSQI global scores than patients with low sleep disturbance (Table 2).
Baseline scores for disease severity variables according to high or low sleep disturbance levels at baseline (pooled MITT population)
| Baseline score, mean (s.d.) . | High sleep disturbance at baselinea . | Low sleep disturbance at baselineb . | ||
|---|---|---|---|---|
| Placebo (n = 514) . | Quetiapine XR (all doses combined; n = 865) . | Placebo (n = 121) . | Quetiapine XR (all doses combined; n = 252) . | |
| MADRS total score | 30.7 (5.3) | 31.1 (5.4) | 29.2 (5.0) | 29.1 (4.6) |
| HAMD total score | 26.1 (3.2) | 26.3 (3.3) | 24.3 (2.2) | 23.7 (1.9) |
| HAMA total score | 19.4 (6.0) | 19.8 (6.0) | 18.3 (5.0) | 17.8 (5.1) |
| CGI-S total score | 4.6 (0.7) | 4.6 (0.7) | 4.4 (0.6) | 4.4 (0.5) |
| PSQI global score | 12.6 (3.5) | 12.5 (3.3) | 8.6 (3.7) | 8.7 (3.5) |
| Baseline score, mean (s.d.) . | High sleep disturbance at baselinea . | Low sleep disturbance at baselineb . | ||
|---|---|---|---|---|
| Placebo (n = 514) . | Quetiapine XR (all doses combined; n = 865) . | Placebo (n = 121) . | Quetiapine XR (all doses combined; n = 252) . | |
| MADRS total score | 30.7 (5.3) | 31.1 (5.4) | 29.2 (5.0) | 29.1 (4.6) |
| HAMD total score | 26.1 (3.2) | 26.3 (3.3) | 24.3 (2.2) | 23.7 (1.9) |
| HAMA total score | 19.4 (6.0) | 19.8 (6.0) | 18.3 (5.0) | 17.8 (5.1) |
| CGI-S total score | 4.6 (0.7) | 4.6 (0.7) | 4.4 (0.6) | 4.4 (0.5) |
| PSQI global score | 12.6 (3.5) | 12.5 (3.3) | 8.6 (3.7) | 8.7 (3.5) |
CGI-S, Clinical Global Impressions-Severity of Illness; HAMA, Hamilton Rating Scale for Anxiety; HAMD, Hamilton Rating Scale for Depression; MADRS, Montgomery Åsberg Depression Rating Scale; MITT, modified intent-to-treat; PSQI, Pittsburgh Sleep Quality Index; XR, extended release.
HAMD sleep disturbance factor (items 4 + 5+6) score ⩾4.
HAMD sleep disturbance factor (items 4 + 5+6) score < 4.
Baseline scores for disease severity variables according to high or low sleep disturbance levels at baseline (pooled MITT population)
| Baseline score, mean (s.d.) . | High sleep disturbance at baselinea . | Low sleep disturbance at baselineb . | ||
|---|---|---|---|---|
| Placebo (n = 514) . | Quetiapine XR (all doses combined; n = 865) . | Placebo (n = 121) . | Quetiapine XR (all doses combined; n = 252) . | |
| MADRS total score | 30.7 (5.3) | 31.1 (5.4) | 29.2 (5.0) | 29.1 (4.6) |
| HAMD total score | 26.1 (3.2) | 26.3 (3.3) | 24.3 (2.2) | 23.7 (1.9) |
| HAMA total score | 19.4 (6.0) | 19.8 (6.0) | 18.3 (5.0) | 17.8 (5.1) |
| CGI-S total score | 4.6 (0.7) | 4.6 (0.7) | 4.4 (0.6) | 4.4 (0.5) |
| PSQI global score | 12.6 (3.5) | 12.5 (3.3) | 8.6 (3.7) | 8.7 (3.5) |
| Baseline score, mean (s.d.) . | High sleep disturbance at baselinea . | Low sleep disturbance at baselineb . | ||
|---|---|---|---|---|
| Placebo (n = 514) . | Quetiapine XR (all doses combined; n = 865) . | Placebo (n = 121) . | Quetiapine XR (all doses combined; n = 252) . | |
| MADRS total score | 30.7 (5.3) | 31.1 (5.4) | 29.2 (5.0) | 29.1 (4.6) |
| HAMD total score | 26.1 (3.2) | 26.3 (3.3) | 24.3 (2.2) | 23.7 (1.9) |
| HAMA total score | 19.4 (6.0) | 19.8 (6.0) | 18.3 (5.0) | 17.8 (5.1) |
| CGI-S total score | 4.6 (0.7) | 4.6 (0.7) | 4.4 (0.6) | 4.4 (0.5) |
| PSQI global score | 12.6 (3.5) | 12.5 (3.3) | 8.6 (3.7) | 8.7 (3.5) |
CGI-S, Clinical Global Impressions-Severity of Illness; HAMA, Hamilton Rating Scale for Anxiety; HAMD, Hamilton Rating Scale for Depression; MADRS, Montgomery Åsberg Depression Rating Scale; MITT, modified intent-to-treat; PSQI, Pittsburgh Sleep Quality Index; XR, extended release.
HAMD sleep disturbance factor (items 4 + 5+6) score ⩾4.
HAMD sleep disturbance factor (items 4 + 5+6) score < 4.
Tolerability
Full results for the tolerability assessments in the four studies, including AE reporting, have been described previously (Earley et al., 2008; Cutler et al., 2009; Weisler et al., 2009; Bortnick et al., 2011) and so are not presented in detail here.
Of interest is that AEs potentially related to somnolence occurred at an incidence of 29.2 and 4.5% (sedation); 24.9 and 6.9% (somnolence); 1.4 and 0.5% (sluggishness) and 1.6 and 0.9% (lethargy), in the quetiapine XR (all doses) and placebo groups, respectively (unpublished observations). The majority of AEs associated with somnolence were of mild or moderate intensity and the proportion of these AEs leading to withdrawal from the study was 15.4 and 6.0% in the quetiapine XR (all doses) and placebo groups, respectively. Of the quetiapine XR groups, the incidence of AEs leading to discontinuation was lowest in the 50 mg/d group. Sedation (6.1%) and somnolence (2.4%) were the most common AEs leading to discontinuation in quetiapine XR patients.
Discussion
These pooled analyses of four acute (6- or 8-wk) studies demonstrate that quetiapine XR monotherapy improved patients' symptoms of sleep disturbance and sleep quality over the short term. Quetiapine XR monotherapy improved sleep disturbances (assessed by MADRS item 4 score) compared with placebo from week 1 onwards. The effect size for quetiapine XR compared with placebo was larger for patients with high baseline sleep disturbance than for patients with low baseline sleep disturbance, which may be considered as small-to-medium and small effect sizes, respectively (Cohen, 1988). In addition, with the exception of study 4 that had a lower effect size of 0.14, as expected these effect sizes were of a similar magnitude to those calculated for quetiapine XR 50, 150 or 300 mg/d in the individual studies (0.25–0.42; J. Szamosi et al., unpublished observations; Cutler et al., 2009; Bortnick et al., 2011). Furthermore, improvements in HAMD sleep items and sleep disturbance factor scores confirmed the positive effect on sleep restoration of short-term therapy with quetiapine XR monotherapy compared with placebo. In addition, overall sleep quality was improved by quetiapine XR monotherapy compared with placebo, with its beneficial effects being seen on the PSQI items of subjective sleep quality, sleep latency, sleep disturbance and sleep duration.
In these pooled analyses, the PSQI scores at baseline demonstrate that patients in this pooled population were experiencing poor sleep quality at study entry (mean PSQI global scores of ∼12 in both treatment groups): a PSQI global score > 5 has been suggested as indicating significant sleep disturbance (Buysse et al., 1989). The PSQI results indicate that quetiapine XR improved sleep disturbance, with a positive effect on several aspects of sleep quality. The PSQI assesses sleep quality during the previous 4 wk and, since it was assessed in these studies at baseline, week 4 and last assessment, it was not possible to establish whether the improvement in sleep quality with quetiapine XR occurred earlier in treatment. There was a difference between quetiapine XR and placebo on the PSQI item daytime dysfunction in favour of placebo at last assessment. Possible explanations for this finding could be either an effect of daytime sedation experienced by patients receiving quetiapine XR or a symptom of poor sleep quality during the previous night.
In the present pooled analyses, quetiapine XR was effective against depressive symptoms (as assessed by MADRS total score) in all patients regardless of whether they were experiencing high or low baseline sleep disturbance, although its effect appeared to be greatest in those patients with high sleep disturbance. The post hoc analysis to determine whether baseline levels of sleep disturbance affect efficacy outcomes found no relationship between outcome at last assessment and baseline sleep disturbance levels (high or low); thus, quetiapine XR has an antidepressant effect in this patient population irrespective of baseline sleep disturbance.
It is important for clinicians to be aware of any effect that antidepressant therapy may have on sleep disturbance. Although some standard antidepressants may be associated with sleep disturbances when evaluated through polysomnography (Thase, 2006), there is a paucity of clinical studies reporting the efficacy of traditional antidepressant agents in patients with MDD and high levels of sleep disturbance. A study by Fava et al. (2002) found no differences in efficacy between sertraline, fluoxetine and paroxetine in patients with MDD with either high or low sleep disturbance levels. Furthermore, in the three treatment groups, mean HAMD sleep disturbance factor scores at baseline were ∼3 and had reduced at treatment end to ∼1.6 (data estimated from published figure); this is slightly higher than the mean score reported here for quetiapine XR at treatment end (1.4). Also, fewer patients had high levels of sleep disturbance and baseline HAMD sleep disturbance factor scores were lower (indicating a reduced level of sleep disturbance) in the Fava et al. (2002) study than in the current analyses.
Three 8-wk studies demonstrated that escitalopram was more effective than placebo at reducing MADRS item 4 scores in patients with MDD and high levels of sleep disturbance (MADRS item 4 score ⩾4) at baseline (Lader et al., 2005). A 6-wk study comparing agomelatine with venlafaxine in patients with MDD (n = 332) found that, while the two treatments have similar efficacy against depressive symptoms, agomelatine was significantly better at improving symptoms of sleep disturbance (assessed by Leeds Sleep Evaluation Questionnaire and HAMD sleep disturbance factor scores; Lemoine et al., 2007). HAMD sleep disturbance factor scores at baseline (4.6 for both treatment groups) in the study by Lemoine et al. (2007) were the same as those in the present analyses, suggesting that patients in the two studies were experiencing a similar level of sleep disturbance at baseline. In addition, an indirect comparison shows the HAMD sleep disturbance factor score at treatment end seen here with quetiapine XR (1.4) to be similar to that for agomelatine (1.4), but lower than that for venlafaxine (1.8; Lemoine et al., 2007).
More recently, a meta-analysis of 11 short-term studies in patients with MDD found duloxetine to be associated with only a minor benefit, not considered to be clinically relevant, in terms of sleep disturbance (rated using HAMD sleep items and sleep disturbance factor scores; Brecht et al., 2008). It is of note that two of the studies included in the current pooled analyses had an active treatment arm, neither of which demonstrated significant change from baseline in PSQI global score at treatment end vs. placebo: study 2 (Cutler et al., 2009; duloxetine LSM change −3.24 vs. −2.95) and study 4 (Earley et al., 2008; escitalopram LSM change −3.32 vs. −3.37). In clinical practice, the addition of a sedative/hypnotic medication is common in patients with MDD who experience sleep disturbances not adequately relieved by treatment with an SSRI or SNRI (APA, 2010).
Patients with MDD and sleep disturbance tend to have more severe depressive symptoms (Sunderajan et al., 2010). The comparison of baseline disease severity levels in patients with high or low levels of baseline sleep disturbance reported here support this finding (with the exception of CGI-S total score), since baseline rating scale scores were higher in patients with high baseline sleep disturbance. In addition, in a community-based study, volunteers with insomnia experienced more severe depression and were approximately 10 times more likely to have clinically relevant levels of depression than subjects without insomnia (Taylor et al., 2005). Therefore, it is of note that in the present analysis quetiapine XR reduced the depressive symptoms experienced by patients with high sleep disturbance.
In the present pooled analysis, there was a higher incidence of AEs potentially related to somnolence in the quetiapine XR treatment groups than in the placebo group, with an apparent dose-effect. Sedation and somnolence were the most common AEs leading to discontinuation in quetiapine XR patients; however, the majority of AEs associated with somnolence were classified by the investigator as mild or moderate. Similarly, controlled US clinical studies of mirtazapine showed that somnolence occurred in 54% of patients treated with mirtazapine compared with 18% who received placebo (Merck, 2012). In these studies, somnolence resulted in discontinuation for 10.4% of patients treated with mirtazapine compared with 2.2% for placebo.
The main strength of the current analyses lies in the pooling of data from four similar studies to provide a larger patient sample than that of the individual studies alone and give an adequate population size for subgroup analyses to be conducted by high or low sleep disturbance level at baseline. Data pooling is a common practice that allows data to be evaluated from a greater number of patients, which reduces sample variation. In addition, the use of different assessment tools provided a more complete picture of the effect of study treatment on sleep disturbance. MADRS item 4 assesses any reductions in the duration or depth of sleep during the whole night compared with the patient's normal sleep pattern. In contrast, HAMD sleep items evaluate three periods of sleep: going to sleep (insomnia-early); during the night (insomnia-middle); early morning (insomnia-late). Limitations of our analyses include the different durations of the studies (two 6-wk and two 8-wk studies) and the smaller sample of patients with low sleep disturbance, which may have limited statistical power. Also, the effects of treatment on daytime sleepiness/dysfunction were only evaluated through the single PSQI item of daytime dysfunction. That concomitant sleep medication was permitted (although usage was restricted) in patients already receiving this type of medication can be viewed as both a strength as it reflects the real-life clinical situation and as a limitation since a small number of patients with high sleep disturbance levels may not have been classified as such due to the effects of concomitant sleep medication. Another limitation is that this report does not include assessments, such as polysomnography, that directly measure sleep quality.
In summary, these pooled analyses of four acute studies showed that quetiapine XR monotherapy (50–300 mg/d) effectively improved symptoms of sleep disturbance compared with placebo, with improvements seen as early as week 1, and was effective against depressive symptoms in patients experiencing either high or low baseline sleep disturbance levels. Further evaluation of the effect on symptoms of sleep disturbance with quetiapine XR as adjunct therapy in patients with MDD is warranted.
Acknowledgements
These studies were supported by AstraZeneca Pharmaceuticals and were registered at ClinicalTrials.gov (D1448C00001 study identifier number NCT00320268; D1448C00002 study identifier number NCT00321490; D1448C00003 study identifier number NCT00326144; D1448C00004 study identifier number NCT00351169). We thank Jocelyn Woodcock, MPhil, from Complete Medical Communications, who provided medical writing support funded by AstraZeneca.
Statement of Interest
Madhukar H. Trivedi is or has been an advisor/consultant to, or on the Speakers' Bureaus within the past 3 yr and anticipates receiving fees in the near future for: Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Rexahn Pharmaceuticals, Sepracor, SHIRE Development, Sierra, SK Life and Science, Takeda, Tal Medical/Puretech Venture, Transcept, VantagePoint and Wyeth-Ayerst Laboratories. In addition, he has received research support from: Corcept Therapeutics, Inc., Cyberonics, Inc., Merck, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), Solvay Pharmaceuticals, Inc., Targacept and Valient.
Borwin Bandelow has received consulting fees and honoraria within the past 3 yr from AstraZeneca, Bristol-Myers Squibb, Essex, Lilly, GlaxoSmithKline, Janssen-Cilag, Jazz Pharmaceuticals, Lundbeck, Ono Pharma, Pfizer, Roche, Servier, Wyeth and Xian-Janssen.
Koen Demyttenaere has received honoraria from, or has been an advisor/consultant to, or on the Speakers' Bureaus for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, Lundbeck, Otsuka, Servier, Takeda and Wyeth.
George Papakostas has received honoraria from or is/has been a consultant to, or on the Speakers' Bureaus for: Abbott Laboratories, AstraZeneca PLC, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Lundbeck, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Takeda Pharmaceutical Company Ltd, Theracos, Inc., Titan Pharmaceuticals and Wyeth, Inc. In addition, he has received research support from AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories) and Sunovion Pharmaceuticals.
Johan Szamosi, Willie Earley and Hans Eriksson were employees of AstraZeneca at the time this analysis was conceived, conducted and completed.
References



