-
PDF
- Split View
-
Views
-
Cite
Cite
Caroline D. Rae, Vincent H.-C. Lee, Roger J. Ordidge, Angelo Alonzo, Colleen Loo, Anodal transcranial direct current stimulation increases brain intracellular pH and modulates bioenergetics, International Journal of Neuropsychopharmacology, Volume 16, Issue 8, September 2013, Pages 1695–1706, https://doi.org/10.1017/S1461145713000084
Close - Share Icon Share
Abstract
Transcranial direct current stimulation is an emerging treatment for brain disorders but its mode of action is not well understood. We applied 10 min 1 mA anodal transcranial direct current stimulation (tDCS) inside the bore of a 3 T MRI scanner to the left dorsolateral prefrontal cortex of 13 healthy volunteers (aged 19–28 yr) in a blinded, sham-controlled, cross-over design. Brain bioenergetics were measured from the left temporo-frontal region using 31P magnetic resonance spectroscopy before, during and for 20 min following tDCS. Brain pH rose during tDCS and remained elevated afterwards. Phosphomonoesters were significantly decreased while inorganic phosphate (Pi) also fell. Partial-least squares discriminant analysis of the data revealed two significantly different subject groups: one where phosphocreatine (PCr), ATP and Pi fell along with a larger increase in pH and one where PCr and ATP increased along with a smaller increase in pH and a slower and more sustained decrease in Pi. Group membership was predicted by baseline pH and ATP. We interpreted the effects of tDCS as driving two biochemical processes: cellular consumption of ATP causing hydrolysis of PCr via the creatine kinase reaction driving the increase in pH; synthesis of ATP and PCr by mitochondria with concomitant drop in Pi and phosphomonoester levels.
Introduction
Transcranial direct current stimulation (tDCS) is a safe method for modulating cortical excitability and is of current interest as a possible treatment for psychiatric conditions as well as neurological disorders (Stagg and Nitsche, 2011). Electrodes are applied to the scalp and low currents applied via cathodal and anodal electrodes. Depending on the site of application, a range of effects have been demonstrated, including motor, somatosensory, cognitive, visual and affective (Utz et al., 2010).
Application of currents alters blood flow, with widespread increases in the vicinity of the anode and decreased blood flow near the cathode, with effects lasting for at least 50 min (10 min tDCS, 1 mA; Lang et al., 2005). Increased oxygen delivery in the vicinity of the anode has been shown using near-infrared spectroscopy, with effects lasting for several minutes after stimulation had ceased (Merzagora et al., 2010). tDCS alters cortical excitability, with sustained increases of up to 150% lasting for up to 90 min, provided the period of tDCS application exceeded 9 min in duration. These effects may be mediated by NMDA receptors, dopaminergic activity and/or by the serotonin system (Stagg and Nitsche, 2011).
Current thinking on tDCS is that it acts by altering the polarity of membranes without causing action potentials, although polarity is dependent on the orientation of axons and dendrites in the induced field (Zaghi et al., 2010). Application of electric currents to brain cortical tissue increases metabolism (McIlwain, 1953), possibly through membrane polarization causing subsequent activation of NMDA receptors (Nitsche et al., 2003; Fritsch et al., 2010) with bursts of lactate production noted following stimulation indicative of increased metabolism of glucose (McIlwain, 1955). Periods of anodal stimulation exceeding 5 min result in prolonged (up to 5 h with direct cortical stimulation in rats) increases in excitability (Creutzfeldt et al., 1962; Bindman et al., 1964). Electric pulses have a larger proportionate effect on metabolism in white matter than in grey (Kurokawa, 1960) due to the larger spread of the pulse through this medium. Early work where ‘electrical pulses’ were applied to tissue slices showed breakdown of phosphocreatine (PCr) and increased inorganic phosphate (Pi), which recovered to baseline levels following cessation of the pulses (Heald, 1954). This effect was independent of, and occurred on a faster time-scale to, changes induced by K+ depolarization.
In humans, application of tDCS has shown mixed outcomes. Rango et al. (2008), who stimulated the right motor cortex (1.5 mA for 15 min), reported increases in myoinositol 30 min after tDCS but no effect on any other metabolite measured. Stagg et al. (2009) stimulated the left sensorimotor cortex (1 mA for 10 min) and reported reduced GABA with no significant change in glutamate/glutamine 15–20 min post stimulus, while a 30 min 2 mA stimulus increased glu/gln 30 min post stimulus (Clark et al., 2011). Anodal tDCS applied to the primary motor cortex for 20 min at 1 mA resulted in decreased bioenergetic ratios [adenosine triphosphate (ATP)/Pi and PCr/Pi] 90 min after commencement of tDCS, which correlated with cerebral glucose uptake rates (Binkofski et al., 2011).
Brain bioenergetics are known to be sensitive to brain workload (Kato et al., 1996; Rango et al., 1997) and are known to be altered in some psychiatric disorders, e.g. (Volz et al., 1998). We wished to determine whether brain bioenergetics changed during tDCS and to delineate what these changes, if any, were.
Method
Participants
Altogether, 13 healthy subjects (age range 19–28 yr, median 22 yr; five males) were recruited with informed consent from the student population of the University of New South Wales (NSW, Australia). Subjects were considered ineligible if there was any current or past psychiatric illness, general systemic or neurological illness (epilepsy, seizures or head trauma). Also excluded were those taking or recently taking psychotropic medications, including benzodiazepines, those with a history of alcohol abuse [2001 Australian National Health and Medical Research Council (NHMRC) guidelines], recreational drug users, excessive caffeine consumption (NSW Health guidelines), magnetic resonance imaging (MRI) contraindications or those with any possibility of being pregnant. Potential subjects were screened for the above using a 22-item questionnaire, adapted from the Transcranial Magnetic Stimulation Adult Safety Screen (Keel et al., 2001), including additional questions about past and present neurological and psychiatric disorder, drug use and general medical illness. A positive response on any item was further assessed by a psychiatrist (C.L.). This study was conducted within the parameters for human research as specified by the Australian NHMRC and approved by the University of NSW Human Research Ethics Committee.
Procedure
Study design followed a double-blind, cross-over design with subjects receiving either 10 min of active or sham tDCS (see below), where the subject and the researcher analysing the results were blind to treatment condition. Four subjects received active treatment prior to sham and nine received sham treatment first. Subjects were studied twice at similar times of day, with at least 2 d (or 1 wk if active tDCS preceded sham tDCS) between visits. At the commencement and completion of each session, subjects completed a visual analogue scale, a self-rating questionnaire evaluating their tDCS experience and any psychological changes or after effects they experienced during or after tDCS. This included ratings of concentration/alertness, tension/anxiety, fatigue/inertia, depression/dejection, vigour/activity, anger/hostility, confusion/bewilderment and sleepiness on a scale from minus to plus 3, where zero represented no change compared to baseline, –3=much worse, +3 = much better.
Subject preparation
Electrode position was determined by the International 10/20 System for EEG Electrodes and used the montage recommended for treatment of depression. Subjects' head measurements were taken and the F3 and F8 locations were marked using a 10/20 cap. Direct currents were applied through a pair of saline-soaked, surface sponge electrodes (7 × 5 cm). For both active and sham tDCS, the anode electrode was placed over F3 (left dorsolateral prefrontal cortex) and the cathode electrode on the contralateral side over F8, a montage recently successfully used in the treatment of depression (Loo et al., 2012). Electrodes were fixed using a rubber head band around the head. Subjects were then positioned in the scanner and lay supine for the duration of the experiment.
A 10 cm diameter 31P surface coil (Pulseteq Ltd, UK) was placed next to the head, centred over the left inferior frontal/left temporal lobe proximate to the anodal electrode and secured using Velcro strips (for anatomical location, see Fig. 1). The coil was not placed directly over the electrode as: (1) surface coils of this design have maximum flux orthogonal to the magnetic field and zero flux along the magnetic field; (2) there may be safety concerns with placing a loop surface coil directly over a working electrode; (3) computer modelling suggests that cerebral effects are diffuse and not limited to the area under the electrode when the electrodes are widely spaced (Sadleir et al., 2010).
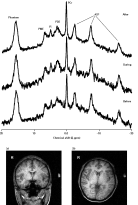
Typical 51.73 MHz 31P spectra. Shown are example spectra from before, during and after conditions (n = 13, active transcranial direct current stimulation). Spectra were acquired with a 10 cm surface coil positioned on the left temporal lobe (a, coronal and b, axial view) using a pulse and collect sequence with a sech pulse centred 300 Hz to high frequency of the resonance from β-adenosine triphosphate (ATP), with TR = 2 s, bandwidth 3500 Hz. The fiducial marker, located in the centre of the coil, can be seen on the left side of the images. Spectra in this diagram have been Fourier transformed with 8 Hz exponential multiplication. PME, Phosphomonoesters; Pi, inorganic phosphate; PDE, phosphodiesters; PCr, phosphocreatine.
MRI protocol
All spectra were acquired using a Philips 3T Achieva TX MRI (Philips, The Netherlands) using the 10 cm diameter 31P circular surface coil. Each spectrum was acquired using a pulse-acquire sequence (TR = 2 s, bandwidth 3500 Hz) with an adiabatic (sech) pulse. Coil localization was verified using scout images to locate the residual signal from the fiducial marker phantom in the centre of the coil. This phantom was filled with a solution of 1 m phenylphosphonate (δ = ∼16 ppm) as an intensity marker. Following volume shimming a scout 31P spectrum was collected and the transmitter frequency set 300 Hz to high frequency of the resonance from β-ATP (∼−16 ppm) in order to optimize and standardize the frequency response window of the adiabatic pulse.
All spectra represented the sum of 16 transients. Spectra were acquired in blocks of five (prior to treatment), 17 (during treatment) or 41 (following treatment). In each case, the first spectrum was used to establish steady-state magnetization and was discarded. Typical spectra from each phase of data collection are shown in Fig. 1.
Anodal transcranial direct current stimulation
A battery-driven, constant-current stimulator (Eldith DC-Stimulator; MR NeuroConn GmbH, Germany) delivered 1 mA (current density of 0.03 µA/cm2) to the brain via the electrodes. For sham stimulation, the current was turned on for 30 s, using a procedure previously found to result in adequate subject blinding (Loo et al., 2012). The quality of data collected during the stimulation period was good (see spectrum in Fig. 1) with no frequency spikes and with no significant difference in measured noise (residuals of AMARES fit) between sham or control or between data collected in the before, during or after stimulation blocks.
Analysis of data
Spectra were processed using jMRUI (http://sermn02.uab.es/mrui/mrui_Overview.shtml; V3.0). Quantification of the reconstructed signals was performed in the time-domain. The AMARES algorithm (Vanhamme et al., 1997) was used to fit decaying sinusoids, corresponding to Lorentzian line shapes in the frequency domain, to the resonances from the phantom, phosphomonoesters (PMEs), Pi, phosphodiesters (PDEs), PCr and the three resonances of ATP (γ, α and β). The line-width of Pi was constrained to not exceed that of PCr, while that of the PDE peak was constrained not to exceed 100 Hz. All other resonances, frequencies and phases were unconstrained. The first 20 points of the free induction decay were multiplied by a quarter sine wave (weighting) to minimize the large anisotropic signal underlying the spectrum. Intracellular pH was calculated by jMRUI using the chemical shift of the Pi peak relative to that of the PCr peak at δ = 0.000 ppm (Iotti et al., 2000).
Statistical analyses using the IBM SPSS Statistics program (version 20) tested the effect of tDCS on six brain bioenergetic measures (phosphomonesters, Pi, PDEs, PCr, β-ATP and pH). In order to measure the effects of tDCS over time, planned contrasts compared the ‘during’ and ‘after’ time-points to baseline, following normalization of the data to the baseline state. A multivariate analysis of variance (MANOVA) was conducted with two within-groups factors: tDCS condition (active or sham); time (before, during and after tDCS). The MANOVA was followed up by separate analyses of variance (ANOVAs) for each brain bioenergetic measure with simple effects testing the direction of any significant interactions. Following the results of a partial least-squares discriminant analysis (PLS-DA), where participants were divided into two distinct bioenergetic groups (described below), further ANOVAs adding a between-groups factor (i.e. tDCS condition × time × group) were also conducted.
Multivariate pattern recognition and data reduction tools are capable of taking into account several predictive variables simultaneously. They are especially useful for analysis of the type of metabolic data presented here as they can objectively distil the major response variables to a few controlled factors, termed latent variables (Wold, 1994). Multivariate data analysis was performed using the program Simca P+ (v11.5; Umetrics, Sweden). Data were imported for each variable as the change in value compared to the baseline mean such that there were two values for each metabolite (during tDCS cf. baseline and after tDCS cf. baseline) in each of the two conditions (sham and active). Data were univariance scaled to standardize variance between the high and low concentration metabolites (Wold et al., 1998) to ensure equal contribution to the model of high and low value variables. A simple principal component analysis model was generated initially that was used objectively to classify subjects into one of two groups (Fig. S1). Data were then subjected to PLS-DA to further test for within-group variability. This approach is useful where data reduction is required and discrimination is the goal (Barker and Rayens, 2003). Variable importance in the projection (VIP) scores were used to determine which variables were discriminatory and which were not (Quintas et al., 2012) and the model was recalculated using only discriminatory variables. Model robustness and predictive strength was verified by cross-validation (Q2).
Results
The quality of spectra obtained before, during and after electrical stimulation in the magnet was similar in each phase of the experiment and in active and sham conditions (Fig. 1), with no change in signal:noise and no significant difference in the noise levels as measured by the residuals following fitting in jMRUI. Careful placement of the external resistor box as close to the MRI room entry point as possible was found in pilot experiments to be adequate to eliminate frequency spikes.
The overall MANOVA revealed no main effect of tDCS condition (F6,7 = 2.469, p = 0.131; Wilks' λ = 0.321) but a significant main effect of time (F12,38 = 3.248, p = 0.003; Wilks' λ = 0.244) and a significant tDCS condition × time interaction (F12,38 = 2.141, p = 0.037; Wilks' λ = 0.356). The follow-up ANOVAs found a significant tDCS condition × time interaction for the measures of PMEs with tDCS condition having an effect during (F1,11 = 25.802, p < 0.001) and after (F1,11 = 7.674, p = 0.018) stimulation. Simple effects showed that PME levels decreased during active tDCS (F1,12 = 8.94, p = 0.011) but not during sham tDCS (F1,12 = 1.04, p = 0.329) and remained decreased after active tDCS (F1,12 = 10.04, p = 0.008) but not after sham tDCS (F1,12 = 0.93, p = 0.354). There was also a significant tDCS condition × time interaction for pH levels, indicating an effect of tDCS during stimulation (F1,11 = 5.978, p = 0.033) but only a trend level effect after stimulation (F1,11 = 3.616, p = 0.084). Simple effects showed that pH levels increased during active tDCS (F1,12 = 20.03, p = 0.001) but not during sham tDCS (F1,12 = 0.02, p = 0.879) and remained increased after active tDCS (F1,12 = 10.06, p = 0.008) but not after sham tDCS (F1,12 = 0.60, p = 0.455). There were also tDCS condition × time interaction trends that suggested decreases in PDE levels during active tDCS (F1,11 = 3.260, p = 0.098) and in Pi after active tDCS (F1,11 = 3.315, p = 0.096) but these did not reach significance. There were no significant tDCS condition × time interactions for PCr or β-ATP (Fig. 2).

31P metabolite levels before, during and after transcranial direct current stimulation for all 13 subjects. Measurements are normalized to each mean baseline measurement (100) made relative to the fiducial marker resonances, phenylphosphonate at δ = ∼15.8 ppm. Active condition is shown in hatched bars and sham condition in white bars. Error bars are s.e.m. and are N = 4 (before), N = 16 (during) and N = 40 (after) repeated measures on 13 subjects. PCr, Phosphocreatine; ATP, adenosine triphosphate.
Simple conservation of mass implies that decreases in PMEs and Pi should be accompanied by alterations in other phosphate moieties, but these were not apparent at the group level. Given that there seemed to be some variability in individual responses to tDCS, particularly in PCr and ATP, which appeared directed rather than random, we undertook an objective, within-group data discrimination process, PLS-DA, to examine the group data for within group variability.
An initial principal components model of all the data was conducted to look for variance in the data and to identify any meaningful eigenvectors related to PCr and ATP. This analysis generated a three component model accounting for 70% of the variance in the data (Fig. S1). This model was poorly cross-validated (Q2 = 39%) but it was used to classify subjects into one of two groups. Group membership was then used as a dummy variable in a PLS-DA model, which accounted for 88% of the variance in the data, and showed good cross-validation (Q2 = 64%). VIP scores for all variables from this model were inspected and those variables that did not contribute significantly to the model were removed (Fig. S2). Finally, a new PLS-DA model was generated using only discriminating variables. This was a two component model accounting for 88% of the variance with a cross-validation score (Q2) of 74% (Fig. 3). This model showed clear separation of the 13 subjects into two groups, where the major discriminating variables were changes in ATP and PCr (Fig. 3). Changes in levels of PMEs during sham stimulation were the only sham condition variables to be included.
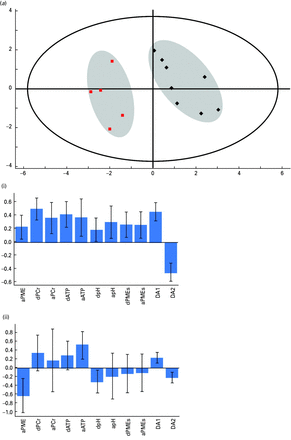
Partial-least squares discriminant analysis (PLS-DA) plot of the effects of transcranial direct current stimulation (tDCS) on all 13 subjects. (a) The plot shows the results of a PLS-DA model where the difference between baseline adenosine triphosphate (ATP), phosphocreatine (PCr) and pH in the during and after states in the active condition were used as inputs, along with phosphomonoesters (PME) in the active state during tDCS and in the sham state in the ‘during’ and ‘after’ conditions. The figure shows clear separation of the two groups of subjects based on their high energy phosphate response to tDCS. The large outer ellipse represents the 95% confidence interval (Hotelling score). The model described a two component model accounting for 88% of the variance in the data with a cross-validation value (Q2) of 74%. (i) and (ii) show the loadings for each variable for each component.
Based on this classification, we subjected the data to a further ANOVA analysis with classification group as a factor in addition to time and tDCS condition. This showed significant three-way interactions in ATP (F1,11 = 6.803, p = 0.024) and PCr levels (F1,11 = 14.608, p = 0.003), with ATP and PCr levels higher during tDCS than in the sham condition in the black group and ATP and PCr levels lower during tDCS than in the sham condition in the red group. These groupwise data are shown in Fig. 4.
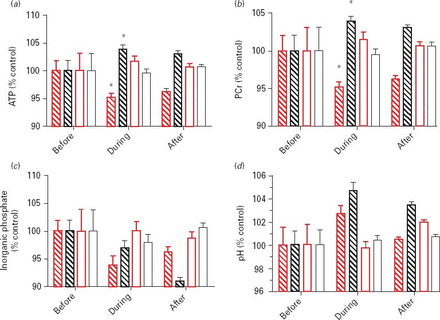
31P metabolite levels before, during and after transcranial direct current stimulation for each of the groups identified by partial-least squares discriminant analysis. Measurements are normalized to each mean baseline measurement (100) made relative to the fiducial marker resonances, phenylphosphonate at δ = ∼15.8 ppm. The two subgroups are coloured as in Fig. 3 (red or black) with the respective sham stimulation shown in white bars with red or black outline. Error bars are s.e.m. and are N = 4 (before), N = 16 (during) and N = 40 (after) repeated measures on 13 subjects. PCr, Phosphocreatine; ATP, adenosine triphosphate.
There were no statistically significant differences in scores on the self-rating questionnaire between sham and active states. Example mean rankings (s.d.) for sham and active conditions respectively for depression/dejection were 0.0 (0.0) and 0.23 (0.73), for tension/anxiety 0.20 (0.42) and 0.23 (1.17) and for concentration/alertness were 0.00 (0.47) and −0.08 (0.95). When asked to rate their experience on a scale of 1–5, where 1 = mildly enjoyable and 5 = extremely unpleasant, subjects undergoing sham treatment reported a mean score (s.d.) of 1.90 (0.57) compared to the active condition 2.08 (0.49).
Finally, we inspected the baseline 31P data in order to determine whether initial raw 31P levels bore any relationship to group membership outcomes. There were no significant between-group differences in baseline 31P data. We built a PLS-DA model containing only the initial baseline data (ratio of PMEs, Pi, PDE, PCr and ATP to the phantom, plus pH). We found that using initial pH and ATP levels only, we could classify the subjects correctly to red or black groups in all but two cases and these latter were only just outside the classification line (Fig. S3). Membership of the black group was associated with lower initial pH and lower initial [ATP]. Initial levels of PCr, PMEs and PDEs were found not to significantly contribute to classification.
Discussion
Here, we show for the first time a significant increase in brain intracellular pH in humans during tDCS, coupled with a significant decrease in PMEs. At the group level, these effects of tDCS were without significant concomitant changes in brain high-energy phosphates.
Why should applied direct current produce an elevation in pH?
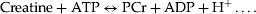
Hydrolysis of PCr requires a proton (H+), resulting in net alkalinization of the milieu. Under conditions of induced workload in the brain, such as that induced by photic stimulation of the visual cortex using a flashing chequerboard, the resultant activity causes a rapid reduction in PCr and a rapid (seconds scale) rise in brain pH (Sappey-Marinier et al., 1992; Kato et al., 1996; Rango et al., 1997). It is generally accepted that ATP levels are not altered by this workload, being strongly buffered via the creatine kinase-catalysed reaction and through increased synthesis of ATP, although the time-scale on which this occurs is not fully explored.
In practice, if increased ATP hydrolysis is the only demanding process going on, the increase in pH should also be accompanied by a net drop in PCr accompanied by either stability of ATP levels or a small drop (due to demand outstripping re-supply). We see at the whole group level no net change in levels of PCr or ATP but we do see a drop in PMEs and possibly Pi. Direct current is known to alter membrane potential (Gross et al., 1986), which will activate the Na + K + -ATPase (Mata et al., 1980). Direct current is also reported to alter Ca2+ influx (Onuma and Hui, 1985) with concomitant activation of the Ca2+ATPase.
Another possible explanation for increase in pH is via electrolysis of water. A number of authors have reported an increase in medium pH after application of direct currents to biological samples (Song et al., 2007), an alkalization that increases with increased applied current and that is also time dependent. Direct current application is a form of electrophoresis and may be expected to induce movement of proteins and other charged species, including phospholipids (Jaffe, 1977), although it is difficult to speculate what the energy cost of protein and phospholipid physical movement to the cell would be as there is a paucity of published data on the subject. Data showing non-synaptic effects of cathodal stimulation support the physical effects argument (Ardolino et al., 2005) with the authors predicting increased pH as one outcome.
In this work, we also saw a drop in the levels of PME during tDCS and, to a lesser extent, of Pi. Under steady-state conditions, the concentration of Pi within the brain is related to the properties of Na+-linked Pi influx and passive Pi efflux and is in equilibrium with the cellular redox states (NAD+/NADH) and the substrates and products of the reaction catalysed by glyceraldehyde-3-phosphate dehydrogenase (Masuda et al., 1990). The amount of Pi is therefore related to its use in the synthesis of ATP and glyceraldehyde-3-phosphate (the latter being relatively small compared to that of ATP), its production through hydrolysis of ATP and its influx and efflux from the cell (Rae et al., 2003). The largest driver of decrease in Pi in this instance is likely to be through increased net synthesis of ATP.
The PME peak is composed of a mix of moieties, including phosphocholine, phosphoethanolamine, AMP and phosphorylated glycolytic intermediates, such as glucose-6-phosphate (G6P) and 3-phosphoglycerate (3PG). It has been shown to change on the time-scale of this experiment in response to various challenges, such as alanine infusion (which stimulates gluconeogenesis and increases the PME resonance by increasing G6P and 3PG levels), by activation of phospholipases, or by stimulation of release of phosphoethanolamine. It is not possible here to say which species are contributing to the loss of PME signal. Movement of protein and phospholipids by electrophoresis with subsequent reorganization of cell membranes may create a demand for the membrane precursors phosphocholine and phosphoethanolamine. There are no reports of loss of the choline signal in 1H spectroscopy studies (Rango et al., 2008; Rae et al., 2009; Clark et al., 2011). This resonance is derived from both phosphorylated and unphosphorylated forms of choline and would be expected to decrease if significant membrane incorporation were occurring due to subsequent significant shortening of relaxation properties with resultant increased signal decay. So along with the evidence of a trend towards decreased Pi levels following tDCS, the loss of PME is suggestive of a general demand elsewhere for phosphate moieties.
A previous study using 31P MRS after imposition of a longer stimulation than that used here (20 min 1 mA anodal stimulation) reported an alteration in the ratio of ATP/Pi and PCr/Pi but did not indicate the degree to which each of these metabolites were responsible for the change (Binkofski et al., 2011). Additional clues about the workload induced by tDCS may come from work subjecting rat skin to an electric current (Cheng et al., 1982). Cheng et al. showed increased ATP production, increased protein synthesis and increased amino acid uptake with applied anodal current up to 1000 µA. tDCS induces an electric potential across a cell; this would have an effect directly on the proton-motive force in the mitochondria (which is the sum of the electrical potential difference and the chemical potential difference; Mitchell, 1976) and would drive the ATP-synthase accordingly. Indeed, it has recently been shown that the F0F1-ATPase is sensitive to applied current with this altering the production rate of ATP with a maximum stimulation around 0.5 mA (Lohrasebi et al., 2008). ATP generated in the mitochondrion equilibrates with mitochondria PCr via the mitochondrial creatine kinase. This reaction takes place in a relatively small compartment and therefore is unlikely to influence intracellular pH measurements made by MRS.
It is at this point that we turn to the PLS-DA analysis of the data to dig further into whether or not there are bioenergetic responses to tDCS. Here, we were able to show division of our 13 subjects into two distinct populations: one where ATP production did not keep pace with ATP loss, resulting in net decreases in ATP and PCr and with a smaller increase in pH (the designated ‘red’ group, Figs 3 and 4); one where ATP production exceeded ATP loss (the black group) and where the pH increase was larger. This suggests that two different biochemical processes were in play: hydrolysis of ATP and activation of the cytosolic creatine kinase system and net synthesis of ATP, which acts to restore ATP levels and also those of PCr.
Group membership was also predicted by baseline levels of ATP and baseline pH. Lower levels of ATP and pH are associated with slower speeds of processing, including performance at the symbol-digit modalities test (Rae et al., 2003), which this electrode montage and anodal tDCS has been shown to improve (Loo et al., 2012).
Although preliminary, it would seem that a more positive mood change score was associated with a higher bioenergetic response. Altered brain bioenergetic levels have been reported in both depression and schizophrenia (Pettegrew et al., 1991; Volz et al., 1998) and bioenergetic levels are known to be related to normal brain function (Vloz et al., 1998; Rae et al., 2003). It remains to be seen whether similar 31P MRS outcomes are seen following anodal tDCS in subjects with psychiatric disorders.
In summary, we find that tDCS is associated with an induced metabolic workload and with an induction in ATP synthesis and an increase in brain pH. The brain pH increase was contributed to by changes in the creatine kinase steady-state equilibrium, created by hydrolysis of PCr due to demand for ATP. The degree of pH change was mediated by the ability to synthesize ATP in mitochondria, with this being influenced by the ability to supply phosphate units from PMEs and PDEs. We provide evidence for individual differences in response to applied direct current and a potential set of biomarkers, baseline pH and ATP, for measuring response to tDCS administered to non-motor areas of the brain.
Supplementary material
Supplementary material accompanies this paper on the Journal's website.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Acknowledgements
The authors thank Dan Moran and Ken Rayner of Diagnostic Medical Services for expert radiography. All scanning was performed at the DMS imaging facility, located at Neuroscience Research Australia. This work was supported by the Australian National Health and Medical Research Council (grants to CR #630516 & CL#510142). The MRUI software package was kindly provided by the participants of the EU Network programmes: Human Capital and Mobility, [CHRX-CT94-0432] and Training and Mobility of Researchers, [ERB-FMRX-CT970160].
Statement of Interest
None.
References



