-
PDF
- Split View
-
Views
-
Cite
Cite
Vladyslav Kushnir, Mahesh Menon, Xavier L. Balducci, Peter Selby, Usoa Busto, Laurie Zawertailo, Enhanced smoking cue salience associated with depression severity in nicotine-dependent individuals: a preliminary fMRI study, International Journal of Neuropsychopharmacology, Volume 16, Issue 5, June 2013, Pages 997–1008, https://doi.org/10.1017/S1461145710000696
Close - Share Icon Share
Abstract
The association between cigarette smoking and depression has been well documented; however, little research has been done to elucidate the neurobiological substrates of this highly prevalent comorbidity. We used multiple linear regression analysis to evaluate the relationship between depression severity as assessed by the Hamilton Depression Rating Scale (HAMD) and blood oxygen level-dependent (BOLD) responses to visual smoking cues in drug-free nicotine-dependent smokers (n=18). Two functional magnetic resonance imaging (fMRI) scans were completed over a single study day, following overnight smoking abstinence (pre-smoking scan) and after cigarette reinstatement (post-smoking scan). During the pre-smoking scan positive correlations between BOLD activity and HAMD scores were observed in areas of the mesocorticolimbic dopaminergic system [inferior frontal gyrus, middle frontal gyrus (MFG), hippocampus (HC), anterior cingulate gyrus] and areas of the visuospatial attention circuit (medial occipital lobe, middle cingulate cortex, superior frontal gyrus, angular gyrus). During the post-smoking scan positive correlations were observed in areas of the brain implicated in drug expectancy (MFG), memory (HC), attentional motivation (posterior cingulate cortex), and visual processing and attention (precuneus). These preliminary findings demonstrate that smokers with higher depression severity attribute greater incentive salience to smoking-related cues and this is especially pronounced during periods of acute abstinence. Such enhanced salience of smoking cues, even after smoking a cigarette, may play a critical role both in the maintenance of smoking in depression and in greater levels of nicotine dependence seen in this patient population.
Introduction
Major depressive disorder (MDD) and nicotine dependence are highly comorbid, with consistent and strong evidence supporting a robust and bidirectional relationship. Smokers are more likely than non-smokers to experience depression in their lifetime (Breslau et al.1991; Kendler et al.1993), and conversely, a history of major depression significantly increases an occasional smoker's risk of progressing to daily smoking (Breslau, 1995; Breslau et al.1998). Furthermore, depressive symptoms are a prominent feature of the withdrawal syndrome during smoking cessation, and in individuals with a past history of depression these symptoms may contribute to an increased risk of developing a major depressive episode (Anda et al.1990; Curtin et al.2000; Glassman et al.2001; Hughes, 2007) and reduce the likelihood of successfully quitting smoking (Covey et al.1997). Although several theories have been proposed to explain the nature of the comorbidity between MDD and nicotine dependence (Audrain-McGovern et al.2004; Balfour & Ridley, 2000; Breslau et al. 1993; Carmody, 1989; Dierker et al.2002; Fowler et al.2003; Kendler et al.1993; Lerman et al.1998; Lewis et al.2007; Markou et al.1998; McClernon et al.2006; Pomerleau & Pomerleau, 1984), the exact neurobiological mechanism responsible for increased smoking behaviour, greater degree of nicotine dependence, and more difficulties quitting in smokers with a history of MDD remains unclear. One possible mechanistic explanation, supported by work from animal studies, implicates the mesocorticolimbic dopamine system. Nestler & Carlezon (2006) suggest that aberrant functioning of this system could be responsible for prominent anhedonia and amotivation seen in depression, which might be partially mitigated by the actions of nicotine.
Nicotine, the major psychoactive component in tobacco, acts on the mesocorticolimbic dopamine system to produce its rewarding and reinforcing effects (Balfour et al.2000; Di Chiara, 2000; Pidoplichko et al.1997; Stein et al.1998; Zubieta et al.2005). With continued use, the transition from drug use to dependence alters the function of the mesocorticolimbic dopaminergic system to facilitate changes in the significance of stimuli associated with drug intake (Volkow et al.2004, 2009). According to the incentive sensitization model, the mesocorticolimbic system becomes sensitized, and drug cues acquire motivational salience and become conditioned reinforcers that perpetuate the desire to consume more drugs (Robinson & Berridge, 1993, 2001). In the case of smokers, smoking-related environmental stimuli are highly attractive and elicit smoking urges and cravings that are a prominent feature of nicotine dependence. Such attentional bias and sensitivity towards these cues is central to the production and modulation of motivational goal-directed behaviour associated with compulsive smoking and relapse (Tiffany, 1990).
The brain circuitry that underlies smoking-cue reactivity has been extensively explored using both PET and functional magnetic resonance imaging (fMRI) methodologies. Most neuroimaging studies examining neural reactivity to smoking-related (compared to neutral) cues have shown that smokers exhibit increased activation in the brain reward and visual attention circuits (Brody et al.2002; David et al.2005; Due et al.2002; Franklin et al.2007; Lee et al.2005; McBride et al.2006; McClernon et al.2005, 2008; Smolka et al.2006; Wilson et al.2005). In particular, a FDG-PET study revealed that when presented with smoking-related (compared to neutral) cues, smokers exhibited higher regional metabolism in bilateral anterior cingulate cortex (ACC), left orbitofrontal cortex (OFC), and left anterior temporal lobe, areas of the mesocorticolimbic dopaminergic system mediating arousal, alertness, motivation, integration of sensory information, and reward monitoring (Brody et al.2002). One of the earliest fMRI studies on cue-induced craving had also shown that exposure to smoking-related images activates parts of the mesocorticolimbic dopaminergic system [right posterior amygdala, posterior hippocampus, ventral tegmental area, dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), medial thalamus], and regions associated with visuospatial attention (posterior fusiform gyrus, intraparietal sulcus) (Due et al.2002). Activation of these networks in response to smoking cues suggested that they may act in concert to increase attention to stimuli of heightened attentional salience.
The only study to examine the association between nicotine dependence and MDD in the context of smoking-cue reactivity has revealed a tendency for smokers with a history of recurrent MDD to respond to smoking-related words with heightened attentional bias while under an acute tryptophan depletion challenge (Hitsman et al.2007). To date, no studies have directly examined smoking-cue reactivity in currently depressed smokers, and only a few neuroimaging studies have attempted to uncover the neurobiological underpinnings of the comorbidity between major depression and nicotine dependence (Brody et al.2009; Busto et al.2009). Therefore, we used fMRI to investigate the neurobiological substrates of smoking-cue reactivity in individuals with comorbid nicotine dependence and MDD. Specifically, we investigated the correlation between depression severity and neural reactivity to smoking-related cues in nicotine-dependent smokers and tested the hypothesis that nicotine-dependent smokers with higher depression severity will exhibit greater incentive salience to smoking-related cues.
MDD is considered in categorical terms in the DSM-IV diagnostic criteria. However, several large studies have shown that dimensional models of depression provide superior predictive validity to MDD when compared to their categorical counterparts (Hankin et al.2005; Prisciandaro & Roberts, 2005, 2009), while other studies have suggested that there are no discontinuities in depression (Franklin et al.2002; Ruscio & Ruscio, 2002; Slade & Andrews, 2005). Although initially we recruited study subjects with and without SCID DSM-IV-diagnosed current major depressive episode where the mean Hamilton Depression Rating Scale (HAMD; Hamilton, 1967) score for subjects meeting DSM-IV criteria for current major depressive disorder was 18.75 (s.d.=5.75, range 14–30), and for subjects not meeting diagnostic criteria the mean HAMD score was 1.3 (s.d.=2.98, range 0–9), we observed the well recognized wide variation in the severity of the disorder among all the study subjects and thus, reanalysed our data using HAMD scores as a continuous variable by which to correlate blood oxygen level-dependent (BOLD) response to smoking cues. In addition, we also investigated whether abstinence from smoking would result in larger brain activation in response to smoking cues. In particular, we attempted to contrast how depression severity influenced cue-induced BOLD activation following overnight abstinence and after having recently smoked one cigarette.
Methods and materials
Subjects
Twenty nicotine-dependent smokers were recruited from the community. In order to participate, subjects had to be aged between 18 and 50 yr, have a minimum score of 4 on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al.1991), smoke at least 10 cigarettes per day, and remain 10–12 h (overnight) abstinent prior to the scan day with an exhaled carbon monoxide (CO) level ≤10 ppm. Subjects were excluded if they had any medical condition requiring immediate attention or treatment, including hypertension or cardiovascular disorders, current DSM-IV Axis I disorder other than MDD, current substance use disorders excluding nicotine, were regular users of any therapeutic or recreational psychoactive drugs (including antidepressant medications) during the last 3 months, were pregnant or lactating, or had conditions for which a MRI would be contraindicated.
Procedures
Potential subjects were recruited by advertisements placed in free weekly newspapers distributed in the Greater Toronto Area. All interested subjects were first screened by telephone based on the inclusion/exclusion criteria listed above. The 21-item HAMD was used to assess depression symptoms and severity. The study was approved by the Centre for Addiction and Mental Health Research Ethics Board and informed written consent was obtained from all participants prior to starting the study. Suitable subjects were invited to attend a clinical screening interview, consisting of a psychiatric assessment using the Structured Clinical Interview Axis I for DSM-IV Disorders (SCID-IV; Spitzer & Williams, 1994), and a medical assessment (physical examination, blood sample for routine biochemistry and haematology screening, and a urine sample for toxicology screening).
Eligible subjects were instructed to refrain from any drug or alcohol use for 24 h and to refrain from smoking for at least 10 h prior to the scan day. Two fMRI sessions were completed during a single scan day, a pre-smoking (abstinence) scan and a post-smoking (cigarette reinstatement) scan. Prior to the pre-smoking scan, compliance with overnight smoking abstinence was verified using breath CO levels (EC50 MicroIII Smokerlyzer, USA) and subjects completed a self-reported measure of craving using the Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991). For all subjects, the first scan (pre-smoking scan) occurred at 10:00 hours in order to minimize exposure to environmental smoking cues after waking up, and increase smoking abstinence compliance. Immediately following the pre-smoking scan, subjective craving was once again assessed. Subjects were taken out of the MRI scanner and smoked one of their own cigarettes before returning for the post-smoking scan. Because cue-induced brain activation has been shown to be largely modulated by expectancy to smoke (McBride et al.2006; Wilson et al.2005), subjects were informed of the opportunity to smoke after the pre-smoking scan. The second scan session took place approximately 30 min after smoking. Subjective craving was assessed immediately after and 1 h following the post-smoking scan.
Visual cue-exposure task
During each functional scan, subjects were presented with a series of smoking-related and neutral photographic cues, adopted from the International Smoking Image Series (Gilbert & Rabinovich, 1999). Smoking cues included photographs of people smoking and smoking-related objects such as packs of cigarettes and ashtrays. Neutral cues consisted of neutral themed pictures that were selected not to elicit strong emotional responses, such as neutral faces, flowers, or furniture. All visual cues were presented in a block design, with four pictures in each block, lasting a total of 20 s (5 s/picture). There were three blocks of neutral cues and eight blocks of smoking cues, and all pictures presented within each block were randomized. Each picture block was interspersed with a rest fixation cross block lasting 18 s. The task was programmed using E-Prime software (version 1.1; Psychology Software Tools Inc., USA) and presented using a set of fMRI compatible VisuaStim Digital presentation system goggles (Resonance Technology Inc., USA) connected to a Dell Latitude D600 laptop.
Imaging parameters
Scanning was conducted at the Toronto Western Hospital Medical Imaging Department on a whole-body GE MRI clinical scanner operating at 3.0 T (GE Medical Systems, USA) using an eight-channel head coil. Each scan session began with a localizer scan and the acquisition of a series of 168 high spatial resolution (1 mm) three-dimensional T1-weighted images for anatomical localization of functional data [TE=5 ms, flip angle=20°, field of view (FOV)=24, matrix size=256×256]. Functional MRI BOLD images were collected using a gradient-echo, echo-planar imaging (EPI) sequence (TE=30 ms, TR=2000 ms, flip angle=85°, FOV=20, matrix size=96×96), for 35 contiguous axial slices (4-mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures and covering the entire brain.
fMRI data analysis
Functional data preprocessing and statistical analysis was conducted using statistical parametric mapping software (SPM5; Wellcome Department of Imaging Neuroscience, UK). The first 15 images were discarded to allow for T1 equilibration effects and because they contained instructions which were non-task-relevant information. For each subject, functional images were realigned to the first task-relevant image to correct for head motion and co-registered to the structural T1-weighted anatomical. Images were spatially normalized (Friston et al.1995 a) using the Montreal Neurological Institute (MNI) template with an isotropic 2×2×2 mm voxel size and smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. Data were high-pass filtered using a 128 s cut-off value.
Each participant's data were entered into a first-level voxel-by-voxel analysis using the general linear model (Friston et al.1995 b) with each cue type (smoking, neutral, rest) coded as a separate regressor using a boxcar design convolved with the haemodynamic response function. Individual contrast images (smoking cues>neutral cues) created from first-level analysis were included in a second-level random-effects analysis. Multiple linear regression analysis was applied to investigate the association between depression severity as determined by HAMD scores and cue-induced brain activity at each of the pre- and post-smoking scans, while treating FTND as a nuisance variable. To investigate how depression severity influences differential activation in response to smoking cues between the two scan sessions (i.e. before and after smoking), for every subject a second-level contrast image was created between pre-scan (smoking cues>neutral cues contrast) and post-scan (smoking cues>neutral cues contrast) using the ImCalc function. The difference between the pre-scan activation and post-scan activation for every subject was then entered into random-effects multiple linear regression analysis with HAMD as a covariate of interest and FTND as a nuisance variable. Activations were considered significant at p<0.005 (uncorrected) with a minimum cluster extent threshold of 10 contiguous voxels.
Results
Subject characteristics
All subjects were in good general health, had no other comorbid psychiatric disorders (with the exception of MDD), and had not used psychoactive drugs or psychotropic medication (including antidepressants) for at least 3 months prior to starting the study. Twenty subjects were enrolled in the study but one subject was excluded due to non-compliance with overnight smoking abstinence (expired CO >10 ppm) and data from one other subject could not be used due to technical difficulties with image reconstruction. Therefore, all subsequent analyses are reported on 18 subjects. The sample of 18 participants consisted of 12 males and had a mean age of 31.1 yr (s.d.=9.7 yr). Subjects smoked an average of 15 cigarettes per day (s.d.=3.6), were moderately dependent on nicotine (mean FTND=5.3, s.d.=1.5), and on the morning of the study day had a mean CO level of 7.3 ppm (s.d.=2.7). The mean HAMD score was 9.1 (s.d.=9.8, range 0–30). Eight subjects met DSM-IV criteria for current major depressive episode; however, these subjects did not differ on any demographic characteristics when compared to the remaining subjects, with the exception of HAMD [t(16)=−9.03, p<0.001].
Subjective craving
For both factors of the QSU a repeated-measures ANOVA showed a main effect of condition (the four craving testing points), such that craving was significantly reduced in the conditions following smoking [F(3, 27)=28.5, p<0.001 with Huyn–Feldt correction]. However, an interaction between smoking condition and HAMD was not found. There were also no significant correlations between depression severity and subjective craving (QSU factor 1 and factor 2) at any of the four testing time-points [baseline (before pre-smoking scan), post-cues (after pre-smoking scan), post-smoking+cues (after post-smoking scan), and 1 h post-smoking+cues (1 h after post-smoking scan)].
fMRI results: cue-induced neural response and depression severity correlations
Brain areas in which the BOLD response to smoking cues>neutral cues contrast was significantly correlated with depression severity during the pre-smoking scan are summarized in Table 1. Positive correlations between HAMD scores and smoking cues>neutral cues contrasts during the pre-smoking scan were found in regions of the mesocorticolimbic system [i.e. the IFG, middle frontal gyrus (MFG), hippocampus (HC), anterior cingulate gyrus (ACG)] and areas of the visuospatial attention system [i.e. medial occipital lobe (MOL), middle cingulate cortex (MCC), superior frontal gyrus (SFG), angular gyrus (AG)]. Conversely, a negative correlation between HAMD and cue reactivity during the pre-smoking scan was found in the supplementary motor area (SMA).
Brain areas where neural reactivity to smoking cues vs. neutral cues as measured by BOLD signal during the pre-smoking scan was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| R | Inferior frontal gyrus | 52 | 32 | 30 | −18 | 4.38 |
| L | Medial occipital lobe | 250 | −38 | −68 | 4 | 4.15 |
| R | Middle frontal gyrus | 17 | 50 | 10 | 50 | 3.99 |
| L | Hippocampus | 114 | −6 | −42 | 4 | 3.82 |
| L | Middle cingulate cortex | 132 | −6 | −54 | 40 | 3.72 |
| L | Superior frontal gyrus | 86 | −26 | 36 | 54 | 3.66 |
| L | Superior medial gyrus | 76 | −10 | 44 | 10 | 3.65 |
| R | Anterior cingulate gyrus | 2 | 46 | 12 | 3.28 | |
| L | Angular gyrus | 26 | −50 | −66 | 36 | 3.29 |
| HAMD negative correlations | ||||||
| R | SMA | 13 | 16 | −6 | 70 | 3.19 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| R | Inferior frontal gyrus | 52 | 32 | 30 | −18 | 4.38 |
| L | Medial occipital lobe | 250 | −38 | −68 | 4 | 4.15 |
| R | Middle frontal gyrus | 17 | 50 | 10 | 50 | 3.99 |
| L | Hippocampus | 114 | −6 | −42 | 4 | 3.82 |
| L | Middle cingulate cortex | 132 | −6 | −54 | 40 | 3.72 |
| L | Superior frontal gyrus | 86 | −26 | 36 | 54 | 3.66 |
| L | Superior medial gyrus | 76 | −10 | 44 | 10 | 3.65 |
| R | Anterior cingulate gyrus | 2 | 46 | 12 | 3.28 | |
| L | Angular gyrus | 26 | −50 | −66 | 36 | 3.29 |
| HAMD negative correlations | ||||||
| R | SMA | 13 | 16 | −6 | 70 | 3.19 |
BOLD, Blood oxygen level-dependent; HAMD, Hamilton Depression Rating Scale; MNI, Montreal Neurological Institute; R, right; L, left; SMA, supplementary motor area.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
Brain areas where neural reactivity to smoking cues vs. neutral cues as measured by BOLD signal during the pre-smoking scan was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| R | Inferior frontal gyrus | 52 | 32 | 30 | −18 | 4.38 |
| L | Medial occipital lobe | 250 | −38 | −68 | 4 | 4.15 |
| R | Middle frontal gyrus | 17 | 50 | 10 | 50 | 3.99 |
| L | Hippocampus | 114 | −6 | −42 | 4 | 3.82 |
| L | Middle cingulate cortex | 132 | −6 | −54 | 40 | 3.72 |
| L | Superior frontal gyrus | 86 | −26 | 36 | 54 | 3.66 |
| L | Superior medial gyrus | 76 | −10 | 44 | 10 | 3.65 |
| R | Anterior cingulate gyrus | 2 | 46 | 12 | 3.28 | |
| L | Angular gyrus | 26 | −50 | −66 | 36 | 3.29 |
| HAMD negative correlations | ||||||
| R | SMA | 13 | 16 | −6 | 70 | 3.19 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| R | Inferior frontal gyrus | 52 | 32 | 30 | −18 | 4.38 |
| L | Medial occipital lobe | 250 | −38 | −68 | 4 | 4.15 |
| R | Middle frontal gyrus | 17 | 50 | 10 | 50 | 3.99 |
| L | Hippocampus | 114 | −6 | −42 | 4 | 3.82 |
| L | Middle cingulate cortex | 132 | −6 | −54 | 40 | 3.72 |
| L | Superior frontal gyrus | 86 | −26 | 36 | 54 | 3.66 |
| L | Superior medial gyrus | 76 | −10 | 44 | 10 | 3.65 |
| R | Anterior cingulate gyrus | 2 | 46 | 12 | 3.28 | |
| L | Angular gyrus | 26 | −50 | −66 | 36 | 3.29 |
| HAMD negative correlations | ||||||
| R | SMA | 13 | 16 | −6 | 70 | 3.19 |
BOLD, Blood oxygen level-dependent; HAMD, Hamilton Depression Rating Scale; MNI, Montreal Neurological Institute; R, right; L, left; SMA, supplementary motor area.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
During the post-smoking scan, positive correlations between HAMD and neural response to cues were found in the MFG, superior temporal gyrus (STG), HC, precuneus (PreCun), posterior cingulate cortex (PCC), and superior occipital cortex (SOC). Significant negative correlations were found in the SFG, superior parietal lobule (SPL), postcentral gyrus (PostCG), precentral gyrus (PreCG), lingual gyrus (LG), temporal pole (TP), and STG (Table 2).
Brain areas where neural reactivity to smoking cues vs. neutral cues as measured by BOLD signal during the post-smoking scan was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| L | Middle frontal gyrus | 80 | −30 | 32 | 56 | 5.71 |
| R | Superior temporal gyrus | 89 | 52 | −40 | 18 | 4.93 |
| R | Middle frontal gyrus | 59 | 34 | 24 | 54 | 4.87 |
| R | Hippocampus | 17 | 40 | −36 | −6 | 3.73 |
| R | Precuneus | 14 | 6 | −74 | 42 | 3.51 |
| R | Posterior cingulate cortex | 19 | 4 | −40 | 38 | 3.38 |
| L | Superior occipital gyrus | 22 | −24 | −90 | 34 | 3.36 |
| HAMD negative correlations | ||||||
| R | Superior frontal gyrus | 102 | 22 | −10 | 72 | 5.29 |
| R | Superior parietal lobule | 170 | 28 | −54 | 72 | 4.36 |
| L | Postcentral gyrus | 77 | −64 | −14 | 20 | 4.01 |
| R | Precentral gyrus | 69 | 58 | 2 | 28 | 3.94 |
| R | Temporal pole | 47 | 38 | 12 | −22 | 3.88 |
| R | Lingual gyrus | 57 | 8 | −72 | 8 | 3.79 |
| L | Superior temporal gyrus | 16 | −42 | −40 | 24 | 3.66 |
| L | Superior parietal lobe | 12 | −26 | −52 | 74 | 3.13 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| L | Middle frontal gyrus | 80 | −30 | 32 | 56 | 5.71 |
| R | Superior temporal gyrus | 89 | 52 | −40 | 18 | 4.93 |
| R | Middle frontal gyrus | 59 | 34 | 24 | 54 | 4.87 |
| R | Hippocampus | 17 | 40 | −36 | −6 | 3.73 |
| R | Precuneus | 14 | 6 | −74 | 42 | 3.51 |
| R | Posterior cingulate cortex | 19 | 4 | −40 | 38 | 3.38 |
| L | Superior occipital gyrus | 22 | −24 | −90 | 34 | 3.36 |
| HAMD negative correlations | ||||||
| R | Superior frontal gyrus | 102 | 22 | −10 | 72 | 5.29 |
| R | Superior parietal lobule | 170 | 28 | −54 | 72 | 4.36 |
| L | Postcentral gyrus | 77 | −64 | −14 | 20 | 4.01 |
| R | Precentral gyrus | 69 | 58 | 2 | 28 | 3.94 |
| R | Temporal pole | 47 | 38 | 12 | −22 | 3.88 |
| R | Lingual gyrus | 57 | 8 | −72 | 8 | 3.79 |
| L | Superior temporal gyrus | 16 | −42 | −40 | 24 | 3.66 |
| L | Superior parietal lobe | 12 | −26 | −52 | 74 | 3.13 |
BOLD, Blood oxygen level-dependent; HAMD, Hamilton Depression Rating Scale; MNI, Montreal Neurological Institute; R, right; L, left.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
Brain areas where neural reactivity to smoking cues vs. neutral cues as measured by BOLD signal during the post-smoking scan was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| L | Middle frontal gyrus | 80 | −30 | 32 | 56 | 5.71 |
| R | Superior temporal gyrus | 89 | 52 | −40 | 18 | 4.93 |
| R | Middle frontal gyrus | 59 | 34 | 24 | 54 | 4.87 |
| R | Hippocampus | 17 | 40 | −36 | −6 | 3.73 |
| R | Precuneus | 14 | 6 | −74 | 42 | 3.51 |
| R | Posterior cingulate cortex | 19 | 4 | −40 | 38 | 3.38 |
| L | Superior occipital gyrus | 22 | −24 | −90 | 34 | 3.36 |
| HAMD negative correlations | ||||||
| R | Superior frontal gyrus | 102 | 22 | −10 | 72 | 5.29 |
| R | Superior parietal lobule | 170 | 28 | −54 | 72 | 4.36 |
| L | Postcentral gyrus | 77 | −64 | −14 | 20 | 4.01 |
| R | Precentral gyrus | 69 | 58 | 2 | 28 | 3.94 |
| R | Temporal pole | 47 | 38 | 12 | −22 | 3.88 |
| R | Lingual gyrus | 57 | 8 | −72 | 8 | 3.79 |
| L | Superior temporal gyrus | 16 | −42 | −40 | 24 | 3.66 |
| L | Superior parietal lobe | 12 | −26 | −52 | 74 | 3.13 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations | ||||||
| L | Middle frontal gyrus | 80 | −30 | 32 | 56 | 5.71 |
| R | Superior temporal gyrus | 89 | 52 | −40 | 18 | 4.93 |
| R | Middle frontal gyrus | 59 | 34 | 24 | 54 | 4.87 |
| R | Hippocampus | 17 | 40 | −36 | −6 | 3.73 |
| R | Precuneus | 14 | 6 | −74 | 42 | 3.51 |
| R | Posterior cingulate cortex | 19 | 4 | −40 | 38 | 3.38 |
| L | Superior occipital gyrus | 22 | −24 | −90 | 34 | 3.36 |
| HAMD negative correlations | ||||||
| R | Superior frontal gyrus | 102 | 22 | −10 | 72 | 5.29 |
| R | Superior parietal lobule | 170 | 28 | −54 | 72 | 4.36 |
| L | Postcentral gyrus | 77 | −64 | −14 | 20 | 4.01 |
| R | Precentral gyrus | 69 | 58 | 2 | 28 | 3.94 |
| R | Temporal pole | 47 | 38 | 12 | −22 | 3.88 |
| R | Lingual gyrus | 57 | 8 | −72 | 8 | 3.79 |
| L | Superior temporal gyrus | 16 | −42 | −40 | 24 | 3.66 |
| L | Superior parietal lobe | 12 | −26 | −52 | 74 | 3.13 |
BOLD, Blood oxygen level-dependent; HAMD, Hamilton Depression Rating Scale; MNI, Montreal Neurological Institute; R, right; L, left.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
When examining the correlation between depression severity and differential BOLD activation between the pre- and post-smoking scans, significant positive correlations were found in the STG, PostCG, SPL, HC, insula, fusiform gyrus (FG), and the PreCG. Significant negative correlation between HAMD and differential neural activation between pre- and post-smoking scans was found in the middle temporal gyrus (MTG) (Table 3). Figure 1 illustrates clusters of significant correlation between depression severity and cue reactivity during the pre-smoking scan, post-smoking scan, and pre–post smoking scans in six representative brain slices. For results of smoking>neutral cue contrasts for each scan session and pre–post smoking scan session difference, see Supplementary Tables S1–S3 (available online).
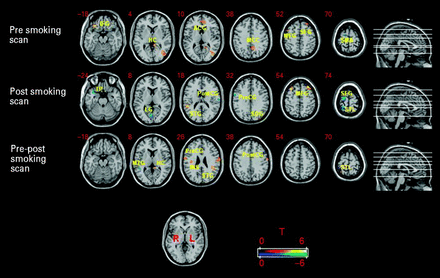
Areas of activation where cue reactivity during each scan session and between the pre- and post-smoking scans was significantly correlated with depression severity (HAMD). Activations in orange represent where cue reactivity was positively correlated whereas areas in blue represent negative correlations. During the pre-smoking scan HAMD was positively correlated with cue reactivity in inferior frontal gyrus (IFG), medial occipital lobe (MOL), middle frontal gyrus (MFG), hippocampus (HC), middle cingulate cortex (MCC), superior frontal gyrus (SFG), and anterior cingulate gyrus (ACG); and negatively correlated in supplementary motor area (SMA). During the post-smoking scan HAMD was positively correlated with cue reactivity in the MFG, superior temporal gyrus (STG), and superior occipital cortex (SOC); and negatively correlated in the superior parietal lobule (SPL), SFG, postcentral gyrus (PostCG), precentral gyrus (PreCG), lingual gyrus (LG), and temporal pole (TP). Differential cue reactivity between the pre-smoking and post-smoking scans was positively correlated with HAMD in the STG, PostCG, PreCG, hippocampus (HC), insula (INS), superior temporal lobe (STL), and fusiform gyrus (FG); and negatively correlated in the middle temporal gyrus (MTG). Numbers in the top left corner of each brain slice represent the z coordinate in MNI space. p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
Brain areas where neural reactivity to smoking cues vs. neutral cues between pre-smoking and post-smoking scans was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations pre–post scans | ||||||
| L | Superior temporal gyrus | 120 | −44 | −40 | 24 | 4.77 |
| R | Postcentral gyrus | 197 | 64 | −4 | 20 | 4.16 |
| L | Superior parietal lobe | 19 | 30 | −48 | 74 | 4.04 |
| L | Postcentral gyrus | 201 | −64 | −8 | 38 | 3.86 |
| L | Hippocampus | 39 | −8 | −38 | 0 | 3.66 |
| R | Insula | 56 | 36 | −16 | 26 | 3.29 |
| L | Fusiform gyrus | 23 | −38 | −64 | −6 | 3.25 |
| R | Precentral gyrus | 22 | 52 | −10 | 34 | 3.24 |
| HAMD Negative correlations pre–post scans | ||||||
| R | Middle temporal gyrus | 24 | 50 | −44 | 10 | 3.37 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations pre–post scans | ||||||
| L | Superior temporal gyrus | 120 | −44 | −40 | 24 | 4.77 |
| R | Postcentral gyrus | 197 | 64 | −4 | 20 | 4.16 |
| L | Superior parietal lobe | 19 | 30 | −48 | 74 | 4.04 |
| L | Postcentral gyrus | 201 | −64 | −8 | 38 | 3.86 |
| L | Hippocampus | 39 | −8 | −38 | 0 | 3.66 |
| R | Insula | 56 | 36 | −16 | 26 | 3.29 |
| L | Fusiform gyrus | 23 | −38 | −64 | −6 | 3.25 |
| R | Precentral gyrus | 22 | 52 | −10 | 34 | 3.24 |
| HAMD Negative correlations pre–post scans | ||||||
| R | Middle temporal gyrus | 24 | 50 | −44 | 10 | 3.37 |
MNI, Montreal Neurological Institute; HAMD, Hamilton Depression Rating Scale; R, right; L, left.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
Brain areas where neural reactivity to smoking cues vs. neutral cues between pre-smoking and post-smoking scans was significantly correlated with HAMD scores
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations pre–post scans | ||||||
| L | Superior temporal gyrus | 120 | −44 | −40 | 24 | 4.77 |
| R | Postcentral gyrus | 197 | 64 | −4 | 20 | 4.16 |
| L | Superior parietal lobe | 19 | 30 | −48 | 74 | 4.04 |
| L | Postcentral gyrus | 201 | −64 | −8 | 38 | 3.86 |
| L | Hippocampus | 39 | −8 | −38 | 0 | 3.66 |
| R | Insula | 56 | 36 | −16 | 26 | 3.29 |
| L | Fusiform gyrus | 23 | −38 | −64 | −6 | 3.25 |
| R | Precentral gyrus | 22 | 52 | −10 | 34 | 3.24 |
| HAMD Negative correlations pre–post scans | ||||||
| R | Middle temporal gyrus | 24 | 50 | −44 | 10 | 3.37 |
| Side . | Brain area . | Cluster size (mm3) . | MNI coordinates . | Tmax . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | ||||
| HAMD positive correlations pre–post scans | ||||||
| L | Superior temporal gyrus | 120 | −44 | −40 | 24 | 4.77 |
| R | Postcentral gyrus | 197 | 64 | −4 | 20 | 4.16 |
| L | Superior parietal lobe | 19 | 30 | −48 | 74 | 4.04 |
| L | Postcentral gyrus | 201 | −64 | −8 | 38 | 3.86 |
| L | Hippocampus | 39 | −8 | −38 | 0 | 3.66 |
| R | Insula | 56 | 36 | −16 | 26 | 3.29 |
| L | Fusiform gyrus | 23 | −38 | −64 | −6 | 3.25 |
| R | Precentral gyrus | 22 | 52 | −10 | 34 | 3.24 |
| HAMD Negative correlations pre–post scans | ||||||
| R | Middle temporal gyrus | 24 | 50 | −44 | 10 | 3.37 |
MNI, Montreal Neurological Institute; HAMD, Hamilton Depression Rating Scale; R, right; L, left.
p≤0.005 (uncorrected), minimum cluster size ⩾10 voxels.
Discussion
To our knowledge, this is the first study to investigate the neural substrates of smoking-cue reactivity in nicotine-dependent individuals with varying degrees of depressive symptomatology. The main finding of this study was that following overnight abstinence greater depression severity was associated with higher fMRI BOLD activation in response to smoking-related cues in areas of the brain that are part of the mesocorticolimbic dopaminergic system and the visuospatial attention circuit. This supports previously reported PET findings which showed that MDD positive smokers exhibited a dysfunctional dopaminergic system whereby smoking caused increased dopamine release compared to control subjects (Brody et al.2009) and amphetamine caused a blunted dopamine response (Busto et al.2009).
In addition, the correlations found in the ACC, DLPFC, IFG, and SFG are all frontal areas that have been previously reported to be correlated with cue-induced craving (McClernon et al.2005) and repeatedly implicated in reward, attention, and motivation (Bonson et al.2002; Brody et al.2002; David et al.2005; Franklin et al.2007). Previous neuroimaging studies have all shown greater ACC activation in response to smoking cues in otherwise healthy subjects, and have attributed the intricate function of both ACC and DLPFC in making smoking cues more salient (Brody et al.2002; McBride et al.2006; McClernon et al.2005). Furthermore, a function of the OFC is to enhance the motivational significance of stimuli and contribute to the production of goal-directed behaviours (Rolls, 2000). Therefore, in concert with other frontal regions, areas of the limbic system (HC), and parts of the visuospatial attention circuit (AG, MOL), this entire network is believed to be responsible for increasing the salience and reward value of drug-related stimuli (Brody et al.2002; Due et al.2002; McBride et al.2006). Our preliminary findings indicate that the presence of greater depressive symptomatology, as well as acute abstinence, further increase the attention given to these stimuli which may enhance the motivation to smoke. The only other study to investigate smoking-cue reactivity in MDD positive smokers showed that under an acute tryptophan depletion challenge individuals with a recurrent history of MDD had a non-significant trend to increased attentional bias in response to smoking words (Hitsman et al.2007). The present study is the first to demonstrate a significant finding of this nature, which suggests that higher levels of depression severity correspond with enhanced sensitivity and incentive salience attribution to smoking cues.
This increased attention to highly salient smoking stimuli among smokers with greater depression severity may explain, at least in part, the nature of the highly prevalent comorbidity between nicotine dependence and MDD. We propose one explanation which may lie in concert with the self-medication hypothesis, i.e. that depressed individuals smoke to modulate their mood symptoms through the actions of nicotine or cigarette smoke (Balfour & Ridley, 2000; Fowler et al.2003; Markou et al.1998; McClernon et al.2006). As animal research has shown that nicotine-induced stimulation of the dopamine system has the potential to alleviate the adverse effects of stressful environmental stimuli on behaviour, with repeated administration, the context in which nicotine is administered becomes a conditioned reinforcer that perpetuates the desire to attenuate stress, or as in the case of humans, also negative affect (Balfour & Ridley, 2000; Benwell & Balfour, 1982; Perkins et al.2008; Piazza & Le Moal, 1997). Indeed, exposure to stressful stimuli as well as induction of negative affect have been shown to enhance the desire to smoke and increase smoking behaviour (Conklin & Perkins, 2005; Doherty et al.1995; Kassel et al.2003; Perkins & Grobe, 1992; Pomerleau & Pomerleau, 1987; Shiffman et al.1996). In depressed individuals, who have been shown to be more sensitive to stress (Bedi, 1999; Ilgen & Hutchison, 2005; Kendler et al.1999), this conditioned response may develop more readily and more powerfully, resulting in increased salience of smoking-related environmental information. However, this concept has not been empirically tested in humans.
When examining how depression severity influences cue-induced neural response after smoking a cigarette of choice, positive correlations were observed in the middle frontal gyrus (DLPFC), STG, HC, PreCun, SOC, and PCC. The majority of these structures have been implicated previously in smoking-cue reactivity studies of satiated smokers (Franklin et al.2007; McBride et al.2006), playing an integral role in expectancy (DLPFC), memory (HC), attentional motivation (PCC), and visual processing and attention (PreCun). With the DLPFC playing a critical role in memory, motivational aspects of drug-seeking behaviour and expectancy of reward, activation of this region, alongside the PCC and HC, may reflect the triggering of memories of smoking as well as an added desire and intent to smoke. Although the motivation to smoke after scanning was not measured, a positive correlation between depression severity and activation of these regions suggests that for individuals with greater depression severity, smoking one cigarette following a period of overnight abstinence may not have been sufficient to relieve withdrawal, thus leaving further motivation and expectancy to smoke. This concept is supported by the observation of negative correlations between HAMD scores and neural responses to cues in primary and somatosensory motor areas, as well as in temporal and parietal regions. As these brain areas are not associated with reward, motivational, or emotional information processing, the results suggest that among less depressed smokers, smoking one cigarette after overnight abstinence may have provided enough relief of craving and withdrawal to no longer attend to smoking cues. As there were no significant differences in severity of tobacco dependence as measured by FTND scores between depressed and non-depressed subjects, an alternative explanation may be that depressed smokers are simply more attentive to smoking cues and find them salient regardless of their state of abstinence.
When examining how depression severity correlated with differential brain activation between the two scan sessions, positive correlations were found in primary and somatosensory motor areas, insula, STG, FG, and HC. The findings are consistent with those of Smolka and colleagues (2006), where in response to smoking cues, positive correlations between severity of nicotine dependence and activation of motor systems were reasoned to mediate motor preparation and imagery. The present findings demonstrate that in individuals with greater depression severity, activation of the motor system in response to smoking cues is significantly greater following overnight abstinence and may highlight a preparation to smoke. The insula has also been implicated in sensory integration and acting as a motor association and somatosensory area (Augustine, 1996), however, unlike the motor areas it has been shown in many fMRI studies to play an integral role in conscious drug urges, where greater activation has been shown to correlate with subjective cue-induced craving (Bonson et al.2002; Brody et al.2002; McClernon et al.2005; Myrick et al.2004). Naqvi and colleagues had found that damage to the insula seems to causes a disruption in nicotine dependence, suggesting that in addition to its role in processing drug-related cues this region is also involved in maintaining smoking behaviour (Naqvi et al.2007). Recent research has confirmed the role of the insula in nicotine self-administration and reinstatement in an animal model (Forget et al.2010). Other research has outlined the function of this region to facilitate interoceptive awareness (Craig, 2009; Janes et al.2010), which, in the context of the present study, suggests that the presentation of smoking cues to individuals with greater depression severity, especially following overnight abstinence, may result in greater attentional bias. Therefore, these findings may underlie a neurobiological link between depression and smoking, where increased attention to internal states may be influenced by mood and cognition. Other areas found to be positively correlated with depression severity are those involved in visual attention (FG) and memory (HC). Taken together, these findings suggest that compared to less depressed smokers, individuals with greater depression severity may be more attentive to smoking cues and have a greater desire and urge to smoke specifically following overnight abstinence than after smoking one cigarette.
Despite the robust positive correlations between depression severity and cue-induced neural activation in areas of the brain that have been previously associated with drug motivation and reward, an association between depression severity and subjective craving was not found. Interpretation of this discrepancy is made difficult due to the very limited and methodologically varied research examining nicotine craving in depression. Thus, these findings may be best accounted for by inherent problems with self-report measures of craving, which are particularly prone to errors due to the influence of the environmental context (i.e. subjects knowing that smoking cues are supposed to make them crave), self-deception, variable interpretation of questions, and differences in response style (Sayette et al.2000). As self-reported cravings also require an individual to reflect on his or her own internal or motivational state (Zinser et al.1992), objective measures obtained by neuroimaging technologies such as fMRI may acquire more subconscious urges not readily disclosed by the subject and in fact may reflect true motivational processes. Therefore, evidence of such disparity between subjective and objective measures emphasizes the need for clinicians to acknowledge the role of subconscious drug cravings when treating addictions, especially in those with comorbid psychiatric illnesses.
Several limitations of this study need to be recognized. First, it is important to note that although there was a wide variation in the severity of depression among study participants, we did observe a bimodal distribution of HAMD scores due to our initial study design. Second, as all subjects received the abstinent scan followed by the smoking/reinstatement scan on the same scan day, this order of scanning sessions may have introduced certain carryover effects of smoking-cue exposure from the first scan of the day. This choice of design does have advantages over a counter-balanced design in that it controls for day-to-day variation in smoking behaviour, cue exposure, and depressive symptomatology, significantly reduces participant dropout and has high degree of face validity since withdrawal, lapse and relapse are necessarily preceded by a period of abstinence. Third, a number of studies have found that greater exposure to smoking cues increases subjective craving. In order to maximize our effect of interest (while being mindful of potential participant fatigue) we included more blocks of smoking-related cues compared to blocks of neutral cues. Fourth, although the effect of cues on subjective craving during the abstinence condition was measured, we did not incorporate an additional assessment of craving immediately after having the subjects smoke one cigarette, thus for the post-smoking condition it was not possible to measure changes in craving as a result of cue presentation. Fifth, due to the exploratory nature of this study, and the a priori regions of interest, an α level of p<0.005 was chosen; however, corrections for multiple comparisons were not implemented. Therefore, these findings should be considered preliminary and replicated with a larger sample size.
In summary, we demonstrated that in response to smoking cues, nicotine-dependent individuals with high depression severity exhibited greater activation of brain regions that are part of the mesocorticolimbic dopaminergic pathway and the visuospatial attention circuit, compared to lesser depressed and non-depressed counterparts. We concluded that while there was no correlation between depression severity and cue-elicited subjective craving, smokers with higher levels of depression subconsciously attributed greater incentive salience and sensitivity to smoking-related environmental stimuli. This was especially pronounced during a period of acute abstinence. Overall, these findings suggest that these smoking-related environmental stimuli may play a critical role in the maintenance of smoking in depression and may help provide important information for appropriate and effective relapse prevention strategies for nicotine-dependent individuals with comorbid MDD.
Note
Supplementary material accompanies this paper on the Journal's website.
Supplementary information supplied by authors.
Acknowledgements
This study was supported by a Canadian Tobacco Control Research Initiative (CTCRI) grant 016031 (L.Z.), the Canadian Institutes of Health Research (CIHR) New Emerging Team grant 79919 (U.B., L.Z.), the CIHR Tobacco Use in Special Populations Training Grant Fellowship (V.K., P.S.), the Ashley Studentship for Research in Tobacco Control (V.K.), and a CTCRI Researcher Travel grant 19837 (V.K.). We thank Eugen Hlasny of the Toronto Western Hospital Medical Imaging Department for technical assistance in performing fMRI scans. We gratefully acknowledge Dr Bruna Brands for assistance with manuscript preparation and appreciate assistance by Alain MacDonald for his help in programming the visual cue-exposure task. We also thank Dr Adrian Crawley and David Kideckel for assistance with fMRI data analysis.
Statement of Interest
Dr Zawertailo has received honoraria from Pfizer Inc. and research funding from Canadian Institutes of Health Research (CIHR) and the Canadian Tobacco Control Research Initiative (CTCRI). Dr Selby has received honoraria and is on advisory boards for Pfizer Inc; he has received funds from Schering Canada to provide buprenorphine training, and received honoraria for consultant work, grant funding, advisory board and/or lectureships from: Johnson & Johnson Consumer Health Care Canada; Pfizer Inc., Canada; Sanofi-Synthelabo, Canada; GlaxoSmithKline, Canada; Genpharm and Prempharm, Canada; Health Canada; Smoke-Free Ontario; Canadian Institutes of Health Research (CIHR); and Evolution Health Systems Inc. Funding was in compliance with the Canadian Medical Association (CMA) and the Canadian Psychiatric Association (CPA) guidelines/recommendations for interaction with the pharmaceutical industry.
References



