-
PDF
- Split View
-
Views
-
Cite
Cite
Karem H. Alzoubi, Marisa Srivareerat, Trinh T. Tran, Karim A. Alkadhi, Role of α7- and α4β2-nAChRs in the neuroprotective effect of nicotine in stress-induced impairment of hippocampus-dependent memory, International Journal of Neuropsychopharmacology, Volume 16, Issue 5, June 2013, Pages 1105–1113, https://doi.org/10.1017/S1461145712001046
Close - Share Icon Share
Abstract
We have previously shown that nicotine prevents stress-induced memory impairment. In this study, we have investigated the role of α7- and α4β2-nicotinic acetylcholine receptors (nAChRs) in the protective effect of nicotine during chronic stress conditions. Chronic psychosocial stress was induced using a form of rat intruder model. During stress, specific antagonist for either α7-nAChRs [methyllycaconitine (MLA)] or α4β2-nAChRs [dihydro-β-erythroidine (DHβE)] was infused into the hippocampus using a 4-wk osmotic pump at a rate of 82 µg/side.d and 41 µg/side.d, respectively. Three weeks after the start of infusion, all rats were subjected to a series of cognitive tests in the radial arm water maze (RAWM) for six consecutive days or until the animal reached days to criterion (DTC) in the fourth acquisition trial and in all memory tests. DTC is defined as the number of days the animal takes to make no more than one error in three consecutive days. In the short-term memory test, MLA-infused stressed/nicotine-treated rats made similar errors to those of stress and significantly more errors compared to those of stress/nicotine, nicotine or control groups. This finding was supported by the DTC values for the short memory tests. Thus, MLA treatment blocked the neuroprotective effect of nicotine during chronic stress. In contrast, DHβE infusion did not affect the RAWM performance of stress/nicotine animals. These results strongly suggest the involvement of α7-nAChRs, but not α4β2-nAChRs, in the neuroprotective effect of chronic nicotine treatment during chronic stress conditions.
Introduction
Nicotine, the major component of tobacco products and the prototypic nicotinic acetylcholine receptor (nAChR) agonist, enhances memory (e.g. Levin, 2002), or attenuates memory impairment associated with several health conditions (Aleisa et al. 2011d; Alzoubi et al.2006; Carrasco et al.2006; Harris et al.2004; Jones et al.1992; Jubelt et al.2008; Kelton et al.2000; Smith et al.2002; Srivareerat et al.2011; White & Levin, 1999). One of these conditions is chronic stress, where nicotine was shown to attenuate stress-induced impairment of short-term memory (Aleisa et al.2006d) and long-term potentiation (LTP; Aleisa et al.2006a, b), the major cellular correlate for learning and memory (Kandel, 2001), in area CA1 of the hippocampus in adult anaesthetized rats. Thus, the increase in tobacco-smoking rate during stress could be interpreted as a form of self-medication to counteract the harmful effect of stress on memory (File et al.2001; Khantzian, 1997). The general effects of nicotine on memory are prevented by mecamylamine, a non-selective nicotinic receptor antagonist, suggesting that nicotine induces its effects by acting on nAChRs (see Levin et al.2006 for review).
Two major subtypes of nAChRs are present, in abundance, in the hippocampus: α7- and α4β2-nAChRs. These subtypes have been shown to be critically involved in spatial memory formation in the hippocampus (e.g. Levin, 2002, 2012; Levin et al.2006). These receptors are calcium-permeable (Bertrand et al.1993; Dajas-Bailador et al.2000; Seguela et al.1993) and may be related to neuroprotective effects of nicotine (Akaike et al.2010; Brandt et al.2011; Kawamata & Shimohama, 2011; Kawamata et al.2012; Yu et al.2011). In this report, we studied the role of α7- and α4β2-nAChR subtypes in the protective effect of nicotine against chronic-stress-induced memory impairment in animals by intrahippocampal infusion of antagonists and cognitive testing in the radial arm water maze (RAWM).
Materials and method
Adult male Wistar rats weighing 200–225 g were housed on a 12:12 h light/dark schedule (lights on 07:00 hours) in Plexiglas cages (six rats per cage) at 24 ± 1 °C with ad libitum access to standard rodent chow and water. All procedures involving animals were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals and on approval of the University of Houston Institutional Animal Care and Use Committee. After arrival at the research facility, all rats were allowed 1 wk to acclimatize before manipulations began.
Treatments
There were six treatment groups in this study: control; stressed (stress); nicotine-treated normal (nicotine); nicotine-treated stressed (nicotine/stress); methyllycaconitine (MLA) and nicotine-treated stressed (MLA/nicotine/stress); dihydro-β-erythroidine (DHβE) and nicotine-treated stressed (DHβE/nicotine/stress) rats. MLA is a specific antagonist for α7-nAChRs, whereas DHβE is specific antagonist for α4β2-nAChRs (e.g. Levin et al.2002). Control and stress groups received normal saline (0.9% NaCl) s.c. twice/d for 4–5 wk. Nicotine, nicotine/stress, MLA/nicotine/stress and DHβE/nicotine/stress groups were treated chronically with nicotine (Sigma, USA); 1 mg/kg s.c. twice/d for 4–5 wk. This nicotine dose is known to produce blood nicotine levels similar to those of chronic smokers (Benowitz, 1986; Benowitz et al.1991; Jacob et al.1988; Le Houezec et al.1993).
Induction of psychosocial stress
Rats in stress, nicotine/stress, MLA/nicotine/stress and DHβE/nicotine/stress groups were stressed by using a form of ‘intruder’ psychosocial model as described (Aleisa et al.2006c; Alzoubi et al.2009; Gerges et al.2001). Briefly, after allowing rats of each group to remain with the same cage mates for at least 1 wk to establish social hierarchy, stress was generated by daily random switching of two animals from one cage to the other, for a period of 4–5 wk. This psychosocial stress model is known to produce stress by disrupting the established social hierarchy, such that rats must continuously adjust to new situations. Psychosocial stress was marked by an increase in serum corticosterone levels (Gerges et al.2001) and elevation of blood pressure (Alkadhi et al.2005).
Osmotic mini-pump implantation
All groups were implanted with 28-d osmotic mini-pumps as described (Alkadhi et al.2010, 2011, 2012; Srivareerat et al.2009, 2011; Tran et al.2010, 2011a, b). Rats were anaesthetized [(i.p. injections of a mixture of ketamine (100 mg/kg), xylazine (2.5 mg/kg) and acepromazine (2.5 mg/kg)] and placed in a stereotaxic frame. The osmotic pump cannulae were inserted into the left and right CA1 areas of the hippocampus through predrilled holes and held in place with dental cement. The 28-d osmotic pumps, containing either MLA or DHβE, dissolved in artificial cerebrospinal fluid, were connected to the brain cannulae by catheters. The scalp wound was closed with wound clips and tincture of iodine and an antibiotic ointment were applied to the wound site to prevent bacterial infection. Animals were allowed to recover. Continuous intrahippocampal infusions of MLA or DHβE were maintained for 4 wk at a rate of 82 µg/side.d and 41 µg/side.d, respectively. Control, stress, nicotine and nicotine/stress groups received vehicle infusion for the same duration.
Radial arm water maze procedure
All animal groups were tested for learning and memory performance on a RAWM immediately after the end of week 3 of the antagonists' infusion. The RAWM is a black circular water tub with six V-shaped stainless steel plates arranged to form a swimming field of an open central area and six arms (arm width 35 cm; Aleisa et al.2006c; Alzoubi et al.2006, 2012; Diamond et al.1999; Gerges et al.2004; Park et al.2001). All experiments were carried out in a dimly lit room. The water temperature was maintained at 24 ± 1 °C. Animals had to find a hidden platform (2 cm under water) at the far end of one of the swim arms (the goal arm). The goal arm was not changed for a particular rat in a single day but cannot be the same for a particular rat on two consecutive days. Rats were allowed a block of four consecutive acquisition (learning) trials followed by a 15-min short-term memory and 5-h and 24-h long-term memory tests per day. Every trial was started in a different start arm (except the goal arm) in a particular day for a particular rat.
In each trial, the rat was allowed to swim freely in the maze to find the hidden platform within 1 min. Once on the platform, the rat was allowed 15 s to observe visual cues before the next trial. Visual cues were in fixed positions throughout the days of the experiments. When a rat was unable to find the platform within the 1-min period allowed, the experimenter guided it toward the platform for the 15-s stay. During the 1-min period, each time the rat entered an arm other than the goal arm, an error was scored. Entry to the arm is defined as the entire body of the rat (except the tail) inside the arm. Memory tests were carried out in a similar manner to acquisition trials. However, in memory tests, animals were neither guided to the hidden platform, nor given the 15-s stay on the hidden platform. Instead, once on the platform, animals were returned to their home cages. During the memory testing, all of the animals were able to find the hidden platform within <1 min. Training continued for six consecutive days or until the animal reached days to criterion (DTC) in the last acquisition trial and in all memory tests. The criterion is defined as a total of no more than one error in a specific trial in three consecutive days of testing. This criterion was used in several previous studies (Aleisa et al.2006c; Alzoubi et al.2005, 2006, 2009; Gerges et al.2004; Khabour et al.2010). Groups were compared based on DTC, number of errors per acquisition trial and number of errors per memory test. All drug treatments (nicotine and antagonists) as well as stress procedure continued during the days of learning and memory testing.
Histology
To identify and ascertain the position of the cannulae in the brain tissue, animals were terminally anaesthetized with sodium pentobarbital (50 mg/kg) after RAWM procedure was completed. Then each cannula was infused with fast green/normal saline solution. Animals were then perfused with phosphate-buffered saline solution, followed by a 4% solution of formaldehyde. After removal, the brains were stored in 4% formaldehyde. To prepare histology slides, brains were frozen on dry ice and sliced on a cryostat. The slides were viewed using a light microscope to determine placement of the cannula. Figure 1 is a schematic diagram showing the cumulative locations of tips of cannulae in CA1 area of rats. Only rats with cannulae placement within the dorsal hippocampus were included in the data analysis for RAWM testing.
Schematic diagram showing positions of bilateral infusion cannulae in the dorsal hippocampus.
Statistical analysis
All statistics were carried out using the GraphPad Prism (4.0) computer program (GraphPad Software, USA). Comparisons of the number of errors were made using two-way analysis of variance (ANOVA), followed by Bonferroni's post-test. Time (repeated measures factor) and treatment (between-subjects factor) groups were the independent variables. Comparisons of DTC were made using one-way ANOVA, followed by Bonferroni's post test. A value of p < 0.05 was considered significant. All values are represented as mean±s.e.m.
Results
To determine spatial memory, animals in all groups were tested in the RAWM task, which measured the hippocampus-dependent spatial memory (Aleisa et al.2006c; Alzoubi et al.2006, 2012; Diamond et al.1999; Gerges et al.2004; Park et al.2001). The results showed that MLA infusion, but not DHβE, blocked the beneficial effect of nicotine on chronic stress-induced memory impairment.
During the RAWM training, animals in all trials attempted to escape the water and find the platform without showing any physical (movement or swimming) disability or reduced motivation (unwilling/unable to climb onto the platform, falling back into the water after climbing, or swimming-still rather than searching for the platform). In the within-day training, all animals showed reduction in the number of errors on all days of training, as they learned during the acquisition (learning) phase. On days 1 and 2 of the radial arm water maze task, there was no significant difference in the number of errors made by rats in all groups in each trials, including short- and long-term memory trials (treatment: F5,79 = 2.01, p > 0.05; time: F6,533 = 51.14, p < 0.05; interaction: F30,533 = 0.65, p > 0.05; Fig. 2a).
![The α7-nicotinic acetylcholine receptors (nAChRs) antagonist [methyllycaconitine (MLA)], but not the α4β2-nAChRs antagonist dihydro-β-erythroidine (DHβE), blocks the effect of nicotine (Nic) on stress-induced short-term memory impairment in the radial arm water maze (RAWM). The first four trials represent the acquisition (learning) phase, followed by the short-term memory test after a 15 min delay and the long-term memory tests after 5 and 24 h delays, respectively. Trials and memory tests were performed every day for a period of 6 d (a, b and c). All groups show within-day improvement in acquisition phase performance in all 6 d. In days 3–4 and 5–6, chronic stress significantly (p < 0.05) impairs short-term memory, which was prevented by chronic nicotine treatment (b and c). The MLA blocked the preventative effect of Nic on stress-induced short-term memory impairment (b and c). * Indicates significant difference from other groups; bars are mean±s.e.m. from 10–18 rats. Inset: sketch of the RAWM.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijnp/16/5/10.1017_S1461145712001046/1/m_s1461145712001046_fig2.gif?Expires=1750247270&Signature=0SaLsoFrdi4c4GgKH4lo6iqhgW0TZLtj75pU1WyKrFqdSTvk8wX8bnCXI-YwojmhkzeiZ4Q6tB7mZVmPiW4EIAktPlNozZ-MV9QFHC78rRWMqrbLO5N5hXvrdrEzwrnMFdJfe8cbPvMs0PhJ19hl~Use8k9GU2-bAEFPe82tdoNOeb3Qe054AzXIFD87LjQrW3RjJZJZkrS~lIq8NWxT1sKOY1h7ltF6FjSeuej0WG~SU5nsDZNNxhmQ7equpL3qrD7sF9EvnFtF60WZYIDquX48Rkwzbj5E7Oy6exxrFxAIhW9sKsIpsCWI4lSUpYReog2v1uZgxGRq3dB~k~ndAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The α7-nicotinic acetylcholine receptors (nAChRs) antagonist [methyllycaconitine (MLA)], but not the α4β2-nAChRs antagonist dihydro-β-erythroidine (DHβE), blocks the effect of nicotine (Nic) on stress-induced short-term memory impairment in the radial arm water maze (RAWM). The first four trials represent the acquisition (learning) phase, followed by the short-term memory test after a 15 min delay and the long-term memory tests after 5 and 24 h delays, respectively. Trials and memory tests were performed every day for a period of 6 d (a, b and c). All groups show within-day improvement in acquisition phase performance in all 6 d. In days 3–4 and 5–6, chronic stress significantly (p < 0.05) impairs short-term memory, which was prevented by chronic nicotine treatment (b and c). The MLA blocked the preventative effect of Nic on stress-induced short-term memory impairment (b and c). * Indicates significant difference from other groups; bars are mean±s.e.m. from 10–18 rats. Inset: sketch of the RAWM.
On days 3–6, all animal groups learned the location of the hidden platform to a similar extent and had similar reduction in the numbers of errors in trials 1–4, indicating that neither stress nor nicotine affects the learning process (Fig. 2b, c). In the 15 min short-term memory test of days 3–6 (for days 3 and 4: treatment: F5,79 = 5.11, p < 0.05; time: F6,533) = 120.74, p < 0.05; interaction: F30,533 = 1.27, p > 0.05; for days 5 and 6: treatment: F5,79 = 3.81, p < 0.05; time: F6,533 = 171.74, p < 0.05; interaction: F30,533 = 2.13, p > 0.05; Fig. 2b, c), animals in the stress group made significantly more errors than the control, nicotine and nicotine/stress groups (control vs. stress: p < 0.05; stress vs. nicotine: p < 0.05; stress vs. nicotine/stress: p < 0.05; nicotine vs. nicotine/stress: p > 0.05), indicating that stress impairs short-term memory. Both the nicotine and nicotine/stress groups made a similar number of errors to that of the control group (control vs. nicotine: p > 0.05; control vs. nicotine/stress: p > 0.05), indicating that chronic nicotine prevented stress-induced short-term memory impairment. However, animals in the MLA/nicotine/stress group made significantly more errors than the control, nicotine and nicotine/stress groups but a similar number of errors to that of the stress group (MLA/nicotine/stress vs. control: p < 0.05; MLA/nicotine/stress vs. nicotine: p < 0.05; MLA/nicotine/stress vs. nicotine/stress: p < 0.05; MLA/nicotine/stress vs. stress: p > 0.05). Thus, MLA blocks the neuroprotective effect of nicotine on stress-induced short-term memory impairment, which indicates the involvement of α7-nAChRs in the beneficial effect of chronic nicotine treatment during stressful conditions.
Interestingly, the results showed that DHβE did not block the effect of nicotine on stress-induced memory impairment. This was indicated by the similar number of errors committed by the DHβE/stress/nicotine, nicotine/stress, nicotine and control groups (DHβE/stress/nicotine vs. control: p > 0.05; DHβE/stress/nicotine vs. nicotine: p > 0.05; DHβE/stress/nicotine vs. nicotine/stress: p > 0.05; Fig. 2b, c). Thus, DHβE does not interfere with the neuroprotective effect of nicotine during chronic stress conditions, which suggests that hippocampal α4β2-nAChRs may not be involved in the neuroprotective effect of chronic nicotine treatment during chronic stress conditions.
To further confirm these results, we also recorded the number of days a rat needed to reach a performance criterion (DTC). In agreement with our previous work (Aleisa et al.2006c; Alzoubi et al.2009; Gerges et al.2004), this test confirmed that chronic nicotine prevented stress-induced impairment of the 15 min short-term memory (F5,65 = 10.97, p < 0.05; Fig. 3b), as indicated by the similar number of days required by the control, nicotine and stress/nicotine group to reach the DTC (control vs. nicotine: p > 0.05; control vs. stress: p < 0.05; control vs. stress/nicotine: p > 0.05; nicotine vs. stress: p < 0.05; stress vs. stress/nicotine: p < 0.05). Chronic intrahippocampal infusion of MLA, but not DHβE, blocked nicotine normalization of stress-induced short-term memory impairment (Fig. 3b). In the MLA/nicotine/stress group, animals required a similar number of days to reach DTC to that of the untreated stress rat group (MLA/nicotine/stress vs. stress: p > 0.05; Fig. 3b). Additionally, the MLA/nicotine/stress group required significantly more days to reach DTC than the control, nicotine and nicotine/stress groups in the short-term memory trial (control vs. MLA/nicotine/stress: p < 0.05; nicotine vs. MLA/nicotine/stress: p < 0.05; nicotine/stress vs. MLA/nicotine/stress: p < 0.05). In the DHβE/nicotine/stress group, however, animals required significantly fewer days to reach the DTC compared to the stress group but a similar number of days to that of the control, nicotine and nicotine/stress groups (control vs. DHβE/nicotine/stress: p > 0.05; nicotine vs. DHβE/nicotine/stress: p > 0.05; stress vs. DHβE/nicotine/stress: p < 0.05; nicotine/stress vs. DHβE/nicotine/stress: p > 0.05). Finally, no significant difference was detected among all groups in the DTC of the learning phase (F5,60) = 0.33, p < 0.05; Fig. 3a) or the 5 h (F5,68 = 1.22, p < 0.05; Fig. 3c), and 24 h (F5,62 = 1.23, p < 0.05; Fig. 3d) long-term memory.
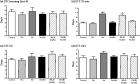
Days to criterion (DTC) measurements show that methyllycaconitine (MLA), but not dihydro-β-erythroidine (DHβE), blocks the effect of nicotine (Nic) on stress (Str)-induced memory impairment. The DTC are plotted for trial 4 (a: learning), test at 15 min (b: short-term memory) and tests at 5 and 24 h (c and d: long-term memory). DTC is a standard criterion, which represents the number of days the animal takes to make no more than one error in three consecutive days. * Indicates significant difference from other groups (p < 0.05).
Discussion
Chronic nicotine treatment was shown to prevent stress-induced impairment of hippocampus-dependent memory (Aleisa et al.2006b, c). The principal finding of the present study is that hippocampal α7-nAChRs, but not α4β2-nAChRs, are required for the neuroprotective effect of chronic nicotine treatment during chronic stress conditions.
Results of this study show that chronic nicotine treatment prevents stress-induced impairment of short-term memory without affecting memory in normal animals, which confirms our previous work (Aleisa et al.2006b, c). Additionally, it has been shown that nicotine attenuates memory deficits associated with a variety of conditions that negatively impact learning and memory including ageing (Carrasco et al.2006), hypothyroidism (Alzoubi et al.2006), Alzheimer's disease (Jones et al.1992; Srivareerat et al.2011; White & Levin, 1999), Parkinson's disease (Kelton et al.2000), schizophrenia (Harris et al.2004; Jubelt et al.2008; Smith et al.2002), attention deficit/hyperactivity disorder (Poltavski & Petros, 2006; Potter & Newhouse, 2008) and sleep deprivation (Aleisa et al.2011a, b). In normal animals, some investigations show memory enhancement (Bancroft & Levin, 2000; Bettany & Levin, 2001; Levin et al.1987, 1993, 1997, 2001; Swan & Lessov-Schlaggar, 2007; Wesnes & Warburton, 1984), whereas others report no effect (Aleisa et al.2006c; Alzoubi et al.2006; Dunne et al.1986; Parrott & Winder, 1989), or even memory impairment (Park et al.2000; Sorenson et al.1991) after nicotine treatment. The inconsistency in the reported effect of nicotine on memory could be due to variations in nicotine dose, treatment duration, route of administration and experimental procedures employed.
Previous studies have shown that nicotine up-regulates multiple subtypes of nAChRs including α7-, and α4β2-nAChRs (Mugnaini et al.2002; Parker et al.2004; Ridley et al.2001) and enhances memory (Bancroft & Levin, 2000; Bettany & Levin, 2001; Levin et al.1987, 1993, 1997, 2001; Swan & Lessov-Schlaggar, 2007; Wesnes & Warburton, 1984). In contrast, stress or stress hormone, down-regulates nAChRs (Pauly & Collins, 1993; Takita et al.1999; Takita & Muramatsu, 1995) and impairs memory (Aleisa et al.2006b; Alzoubi et al.2009; Gerges et al.2004; Park et al.2001; Srivareerat et al.2009; Yun et al.2010). Moreover, chronic nicotine treatment prevents stress-induced down-regulation of central nAChRs (Takita et al.1999), which suggests a mechanism for nicotine prevention of stress-induced impairment of memory. Results of the current study show inhibition of the protective effect of nicotine on stress-induced memory impairment, by the specific α7-nAChRs antagonist, but not by the α4β2-nAChRs antagonist. This indicates the involvement of α7-nAChRs, but not α4β2-nAChRs, in the neuroprotective effect of nicotine against memory impairment during stressful conditions.
In accordance with the results of this study, α7-nAChRs have been implicated in the neuroprotective effect of nicotine in various mental health conditions that have a cognitive effect similar to that caused by stress, such as Alzheimer's disease (Buckingham et al.2009; Kawamata & Shimohama, 2011; Levin, 2012), Parkinson's disease (Kawamata & Shimohama, 2011) and schizophrenia (Levin, 2012; Roncarati et al.2009; Timofeeva & Levin, 2011). These receptors were also involved in the neuroprotective effects of nicotine against neurotoxicity induced by neuro-active agents including glutamate (Shen et al.2010) and amphetamines (Escubedo et al.2009) and other health conditions, such as neuropathy (Pacini et al.2010). Transgenic mice with α7-nAChRs deletion showed impaired memory (Levin, 2012) and nicotine failed to exert neuroprotection in the hippocampal slice ischaemia model from these mice (Egea et al.2007; Rosa et al.2006). Results of these studies, along with our present findings, strongly suggest the pivotal role of α7-nAChRs in the neuroprotection afforded by nicotine against neurodegenerative diseases.
The neuroprotective effect of nicotine is calcium-dependent and can be attributed to the activation of the highly calcium permeable α7-nAChR (Dajas-Bailador et al.2000). Nicotine activates presynaptic α7-nAChR leading to increased calcium influx, which enhances glutamate release from the presynaptic terminal at the pyramidal cells in area CA1 of the hippocampus (Gray et al.1996; Ji et al.2001). Nicotine, thus, increases the probability of coincidence between presynaptic release and post-synaptic depolarization, therefore raising the probability of neuronal firing, LTP induction and memory formation (Ji et al.2001; Mansvelder & McGehee, 2000). In addition, highly localized calcium influx through α7-nAChR activates protein kinases and phosphatases cascades that are known to modulate neurotransmitter release (Shen & Yakel, 2009). Other studies also show that in cultured CA1 neurons, chronic nicotine treatment induces desensitization of α7-nAChR on the GABAergic interneurons (Alkondon et al.2000). The desensitization of α7-nAChR reduces the release of γ-aminobutyric acid (GABA) from these GABAergic interneurons, thus indirectly increasing pyramidal cell excitability (Alkondon et al.2000). This, in turn, facilitates synaptic plasticity by decreasing its threshold of induction (Fujii et al.1999), leading to improved memory formation.
A previous report has shown the importance of both α7- and α4β2-nAChRs for special learning and memory in the dorsal hippocampus (Nott & Levin, 2006). Other studies have reported the involvement of α4β2-nAChRs along with α7-nAChRs in the neuroprotective effects of nicotine in diverse models, such as zebra fish (Bencan & Levin, 2008), the primary culture of mouse dopaminergic neurons model of Parkinson's disease (Akaike et al.2010; Bencan & Levin, 2008; Takeuchi et al.2009) and the cultured cortical neurons model of glutamate cytotoxicity (Akaike et al.2010). However, the present findings indicate that α4β2-nAChRs are not involved in the neuroprotective effects of nicotine during chronic psychosocial stress.
In this study only a single dose of MLA or DHβE was used. The chosen doses of both antagonists were based on previous studies that used the same method/duration of intrahippocampal infusion, or infusion into other brain areas, and examined various aspects of cognitive functions (Cannady et al.2009; Levin et al.2009; Pocivavsek et al.2006). Additionally, intrahippocampal infusion of MLA at the dose used in the current study and for the same duration was shown not to impair hippocampal spatial memory in normal animals (Pocivavsek et al.2006), thus excluding the possibility of a non-specific effect of MLA in the current study. However, the evaluation of the effect of several doses for both antagonists in the beneficial effect of nicotine on chronic stress-induced memory impairment is recommended for future studies.
In conclusion, through the use of intrahippocampus osmotic pump infusion of nAChR antagonists, we have demonstrated the involvement of α7-nAChRs, but not α4β2-nAChRs, in the neuroprotective effect of nicotine during chronic stress-induced memory impairment.
Acknowledgements
This work was partly supported by GEAR and SGP from the University of Houston. We also thank the Department of PPS and Office of the Vice-Dean for Research for partial support of this research.
Statement of Interest
None.
References



