-
PDF
- Split View
-
Views
-
Cite
Cite
Petra Beatriz Navas, Armando Carrasquero-Durán, The influence of pH and digestion with commercial enzymes on calcium adsorption in casabe, International Journal of Food Science and Technology, Volume 41, Issue 9, November 2006, Pages 991–996, https://doi.org/10.1111/j.1365-2621.2006.01055.x
Close - Share Icon Share
Abstract
The Ca2+ binding capacity of a sample of casabe made from cassava (Manihot esculenta) was evaluated by using adsorption isotherms after a digestion process with commercial enzymes. It was found that enzymatic treatment increased the ability of vegetable material to retain calcium and to release endogenous mineral ions as a consequence of possible modifications to the carbohydrate matrix. Untreated casabe did not release mineral ions. pH also influenced the retention of Ca2+: at pH 4.5 release was the main process but adsorption increases with alkalinity up to pH 8.5. The Ca concentrations at which neither adsorption nor release occurred [Ca2+]e were as follows: 5.2 mm (pH 4.5), 3.5 mm (pH 7.1), and 0.63 mm (pH 8.5). The pH effect was explained by an increase in the density of negatively charged functional groups produced by ionization reactions at pH below the point of zero net charge (pHo) which was evaluated by using the Gouy–Chapman double layer model. Values of pHo were 6.4 for raw material and 4.1 after digestion with enzymes. In both cases, the density of positively charged sites below pHo was much higher that the density of negatively charged sites above pHo.
Introduction
The plant cassava, known in Venezuela as yucca, has been one of the most important resources for nutrition for Venezuelan ethnic groups for the last 2000 years (Lathrap, 1970). Ecological conditions and soils in the wet tropics are ideal for this crop which can supply high quantities of low cost carbohydrates from roots for human food and proteins from foliage for animal feeding. Also, yucca is ideal as a raw material for agro industrial systems in developing countries to produce starch, alcohol, beer, concentrates of proteins and casabe.
Casabe is a round flat bread made from cassava flour (manihot esculenta) and widely consumed in the Caribbean countries, particularly Venezuela. It is highly recommended by nutritionists and physicians for the prevention of gastrointestinal diseases due to its high fibre content that varies between 7 and 19% depending on the cassava variety used during preparation (Cova, 1987; Blanco et al., 1994). This food is produced as follows: first, yucca is grated and pressed to eliminate water and water-soluble substances such as cyanide present in bitter varieties. The flour is sieved and spread on a hot circular stone plate. The excess water is thus evaporated to allow the flour particles to adhere to each other to form a cake or thin bread (Quiñones et al., 1982).
The main limitation of casabe is its low protein content, but it is usually consumed with artisan-made cheese or milk. However, little research has studied the bioavailability of essential minerals such as calcium ions in casabe. Rendelman (1982) has pointed out that vegetable fibre can reduce the absorption of minerals and Camire & Clydesdale (1989) reported that lignin and pectin present in most vegetable fibres have a high capacity to bind calcium in alkaline media. In contrast, Joshi & Agte (1995) found in the case of rice fibre, that treatment with digestive enzymes reduced the ability to bind exogenous calcium.
In previous work, it has been proposed that calcium binding by a vegetable fibre can be represented as a cationic exchange reaction (Navas & Carrasquero-Duran, 2003) between dissolved calcium ions and reversibly adsorbed Na+, as represented in the following chemical equation:
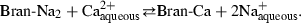
Calcium and sodium binding by the fibre is interpreted as a complexing reaction between cations and anions originating from ionization of functional groups such as hydroxyls. The density of negatively charged sites on the fibre is therefore, dependent on the pH because functional groups may dissociate or be protonated in an aqueous solution according to the following equation:
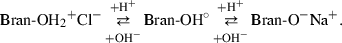
In acidic solutions, close to stomach pH, it is possible that vegetable material exhibits a net positive charge with a reduced binding capacity for cations. On the other hand, above a certain pH value, negative charges increase with the ability of the fibre to retain calcium ions, which may explain the effect of intestinal pH on the mucosal absorption of metallic ions found by Lopez et al. (2002).
An intermediate situation in which the surface has no net charge is predicted by the ionic equilibrium; in this case neither adsorption nor release of ions occurs. In order to establish the corresponding pH value, the Gouy–Chapman model (Sparks, 1985) may be used and the effect of pH on the surface density charge can be calculated by using the following equation:
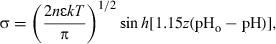
where, σ is the surface electric charge (C m−2); n the counter ion concentration in equilibrium solution (ions m−3); ɛ the dielectric constant; k the Boltzmann's constant; T the absolute temperature (K) and z the counter ion valence. pHo is the pH value of zero net surface charge and depends on both the type of vegetable fibre and the previous treatment to which it is subjected and pH quantifies H3O+ activity in aqueous media.
The procedure for determining pHo consists of the titration of a sample with H+ and OH− which are known as potential-determining ions, and by running blank titrations; the amount of protons and hydroxyls adsorbed over a range of pH can be calculated. A net adsorption of H+ indicates a net negative charge, while adsorption of OH− is produced in the presence of positively charged groups. Therefore, pHo is the pH value at which σ equals zero.
The action of enzymes on the carbohydrates during the digestion process could change the relative densities of electric charges thereby modifying the adsorbing properties of the vegetable material. For this reason, commercial digestive enzymes micro-encapsulated in pellets can be employed. These pellets can be made stable at pH 5.5 so that they are not activated in the stomach but in the duodenum where the enzymes are dispersed and digest fats, proteins and carbohydrates.
The aims of this work were to evaluate the effects of pH and an in vitro digestion on the calcium binding capacity of a sample of casabe by using adsorption isotherms and on the surface electric change because of the dissociation of functional groups.
Materials and methods
Sample
Casabe was obtained locally, milled to a particle size of 2 mm and stored in plastic bags. The composition determined by the AOAC (1990) official methods were: moisture (method 925.10) 12.5%, fat (method 920.85) 0%, protein (micro Kjeldhal) 1%, fibre (985.29) 10.2%. Starch content was 60% (McCready et al., 1950) and calcium was 0.06%, analysed by atomic absorption spectrometry after acid dissolution of the ashes. The 2 mm fraction was sieved again to 75 μm to evaluate the effect of particle size on calcium adsorption.
In vitro enzymatic digestion
Ten grams of milled casabe (2 mm or 75 μm) were placed in 100 mL beakers with 50 mL of distilled water and 1.5 g of commercial digestive enzyme powder with the following composition per gram: 20 mg bromelain, 750 UFIP protease; 4000 UFIP lipase, 8000 UFIP amylase and 40 mg ox bile. The mixture was macerated, its pH was adjusted to 7 with 0.1 m NaOH or 0.1 m HCl. using a pH meter (Metrohm, Bern, Switzerland) and placed in a water bath at 38 °C for 2 h with continuous agitation. The supernatant was separated by centrifugation and the residue was washed with three portions of 50 mL of distilled water and dried in an electric oven for 3 days at 40 °C. The dried residue was crushed in a porcelain mortar and stored in plastic bags at 5 °C.
Adsorption isotherms for enzyme-treated and untreated casabe
Standard solutions of 0.1 m CaCl2 and 0.5 m NaCl were prepared using analytical grade reagents. The amounts of enzyme-treated casabe (2 mm) and calcium were added in eight flasks as indicated in Table 1 plus 2 mL of 0.5 m NaCl. The pH was adjusted to 8.5 with 0.1 mol L−1 HCl and distilled water was added to give 20 mL. Mixtures were shaken for 1 h at 31 °C and centrifuged at 3000 × g for 10 min and the clear supernatant analysed for gain or loss in calcium (ΔCa). Ca concentration in solution ([Ca2+]sol) was determined by atomic absorption spectrometry. ΔCa was calculated by calculating [Ca2+]initial −[Ca2+]sol and plotted against [Ca2+]sol. A positive value of ΔCa indicated adsorption, while a negative result was obtained when endogenous calcium was released from the fibre. The procedure was repeated using 2 mm untreated casabe. Experiments were carried out in triplicate.
| Flask . | Casabe mass (g) . | Volume CaCl2 (mL) . | [Ca2+]initial (mmol L−1) . | Total volume (mL) . |
|---|---|---|---|---|
| 1 | 0.3 | 0.0 | 0.00 | 20 |
| 2 | 0.4 | 0.0 | 0.00 | 20 |
| 3 | 0.5 | 0.0 | 0.00 | 20 |
| 4 | 0.5 | 0.5 | 2.50 | 20 |
| 5 | 0.5 | 1.0 | 5.00 | 20 |
| 6 | 0.5 | 2.0 | 10.00 | 20 |
| 7 | 0.5 | 3.0 | 15.00 | 20 |
| 8 | 0.5 | 12 | 60.00 | 20 |
| Flask . | Casabe mass (g) . | Volume CaCl2 (mL) . | [Ca2+]initial (mmol L−1) . | Total volume (mL) . |
|---|---|---|---|---|
| 1 | 0.3 | 0.0 | 0.00 | 20 |
| 2 | 0.4 | 0.0 | 0.00 | 20 |
| 3 | 0.5 | 0.0 | 0.00 | 20 |
| 4 | 0.5 | 0.5 | 2.50 | 20 |
| 5 | 0.5 | 1.0 | 5.00 | 20 |
| 6 | 0.5 | 2.0 | 10.00 | 20 |
| 7 | 0.5 | 3.0 | 15.00 | 20 |
| 8 | 0.5 | 12 | 60.00 | 20 |
| Flask . | Casabe mass (g) . | Volume CaCl2 (mL) . | [Ca2+]initial (mmol L−1) . | Total volume (mL) . |
|---|---|---|---|---|
| 1 | 0.3 | 0.0 | 0.00 | 20 |
| 2 | 0.4 | 0.0 | 0.00 | 20 |
| 3 | 0.5 | 0.0 | 0.00 | 20 |
| 4 | 0.5 | 0.5 | 2.50 | 20 |
| 5 | 0.5 | 1.0 | 5.00 | 20 |
| 6 | 0.5 | 2.0 | 10.00 | 20 |
| 7 | 0.5 | 3.0 | 15.00 | 20 |
| 8 | 0.5 | 12 | 60.00 | 20 |
| Flask . | Casabe mass (g) . | Volume CaCl2 (mL) . | [Ca2+]initial (mmol L−1) . | Total volume (mL) . |
|---|---|---|---|---|
| 1 | 0.3 | 0.0 | 0.00 | 20 |
| 2 | 0.4 | 0.0 | 0.00 | 20 |
| 3 | 0.5 | 0.0 | 0.00 | 20 |
| 4 | 0.5 | 0.5 | 2.50 | 20 |
| 5 | 0.5 | 1.0 | 5.00 | 20 |
| 6 | 0.5 | 2.0 | 10.00 | 20 |
| 7 | 0.5 | 3.0 | 15.00 | 20 |
| 8 | 0.5 | 12 | 60.00 | 20 |
Effect of pH on adsorption of Ca2+ by enzyme-treated casabe (75 μm)
Three groups of eight flasks were formed and the amounts of enzyme-treated casabe and calcium were added to each as indicated in Table 1. The pH of the suspensions were adjusted with 0.1 m HCl to 4.5; 7.1 and 8.5 for groups I, II and III, respectively. Water was added to make up to 20 mL and pH was checked again. The mixtures were shaken and the procedure followed was the same as that for adsorption isotherm.
Characterization of surface charge
To seven flasks aliquots of NaOH and HCl were added as indicated in Table 2. Volumes were made up to 20 mL with distilled water and the pH was measured (pHinitial). One gram of enzyme-treated or untreated casabe was added to each flask and the suspension agitated for 1 h, after which pH was measured again (pHequilibrium). By using pH and pOH values and the equations (H+) = 10−pH and (OH−) = 10(14−pH), initial activities of H+ and OH− can be calculated. The amount of H+ adsorbed was obtained from: (H+)adsorbed = (H+)initial − (H+)equilibrium. In a similar way, the amount of hydroxyl retained was (OH−)adsorbed = (OH−)initial − (OH−)equilibrium.
Aliquots of HCl (0.1 m) and NaOH (0.1 m) for the determination of pH effect on casabe surface charge
| Flask . | Volume HCl (mL) . | Volume NaOH (mL) . |
|---|---|---|
| 1 | 2.0 | 0.0 |
| 2 | 1.0 | 0.0 |
| 3 | 0.5 | 0.0 |
| 4 | 0.0 | 0.0 |
| 5 | 0.0 | 0.2 |
| 6 | 0.0 | 1.0 |
| 7 | 0.0 | 2.0 |
| Flask . | Volume HCl (mL) . | Volume NaOH (mL) . |
|---|---|---|
| 1 | 2.0 | 0.0 |
| 2 | 1.0 | 0.0 |
| 3 | 0.5 | 0.0 |
| 4 | 0.0 | 0.0 |
| 5 | 0.0 | 0.2 |
| 6 | 0.0 | 1.0 |
| 7 | 0.0 | 2.0 |
Aliquots of HCl (0.1 m) and NaOH (0.1 m) for the determination of pH effect on casabe surface charge
| Flask . | Volume HCl (mL) . | Volume NaOH (mL) . |
|---|---|---|
| 1 | 2.0 | 0.0 |
| 2 | 1.0 | 0.0 |
| 3 | 0.5 | 0.0 |
| 4 | 0.0 | 0.0 |
| 5 | 0.0 | 0.2 |
| 6 | 0.0 | 1.0 |
| 7 | 0.0 | 2.0 |
| Flask . | Volume HCl (mL) . | Volume NaOH (mL) . |
|---|---|---|
| 1 | 2.0 | 0.0 |
| 2 | 1.0 | 0.0 |
| 3 | 0.5 | 0.0 |
| 4 | 0.0 | 0.0 |
| 5 | 0.0 | 0.2 |
| 6 | 0.0 | 1.0 |
| 7 | 0.0 | 2.0 |
Microsoft Excel software was employed to obtain equations and regression coefficients for Ca2+ binding by enzyme-treated and untreated casabe.
Results and discussion
Ca2+ binding by enzyme-treated and untreated casabe
Adsorption isotherms for 2 mm untreated casabe (Fig. 1) showed that adsorption of exogenous Ca2+ took place at ionic concentrations higher than 5 mmol L−1, while at lower solution concentrations the vegetable material did not release endogenous calcium, suggesting that the nutrient is trapped as a non-exchangeable form by untreated carbohydrates in casabe. The treatment with enzymes produced modifications in the carbohydrate matrix shown by changes in the adsorption isotherm, which now exhibited positive and negative values of ΔCa indicating both adsorption and desorption. Another important observation was that for enzyme-treated casabe, the point of equilibrium ([Ca2+]e) was 4.1 mmol L−1 compared with the untreated casabe.
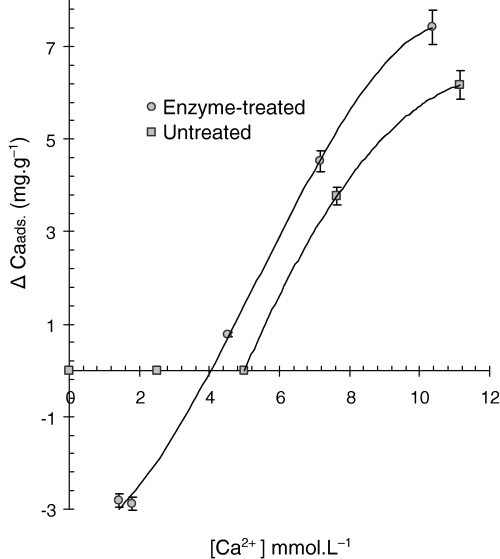
Adsorption isotherm of Ca by untreated and enzyme-treated Casabe (2 mm). Each point is the mean ± 1 SD of three measurements.
It was also observed that the quantity of calcium retained by the enzyme-treated material was higher than in untreated one, shown by the additional 1.4 mg g−1 for the enzyme-treated casabe at a given calcium ion concentration. This may have been a consequence of a higher density of negatively charged functional groups because of the action of enzymes on the vegetable material, thus facilitating processes of adsorption of Ca2+ and exchange by other cations such as monovalent sodium.
Effect of pH on the adsorption of Ca by enzyme-treated casabe
Casabe samples of 75 μm particle size were treated with digestive enzymes in order to evaluate the effect of pH and particle size on calcium retention by the vegetable material. Adsorption isotherms (Fig. 2) showed that adsorption and release of Ca2+ was dependent on pH and calcium concentration in equilibrium with the solid material. For instance, in acid solution (pH 4.5) as in the stomach, [Ca2+]e is 5.2 mmol L−1: above this value a slight adsorption of calcium was observed reaching a maximum of 2 mg g−1. If the pH was increased to 7.1, [Ca2+]e falls to 3.6 mmol L−1 and the amount of Ca complexed is higher than that in the acid condition, with a maximum absorption of about 6 mg g−1. In both cases, adsorption isotherms at ionic concentrations above [Ca2+]e resemble Langmuir's model (Glasstone, 1960) in which adsorption occurs as a monolayer because of the interaction between divalent cations and negatively charged adsorption sites. When those sites are saturated the adsorption curve reaches a plateau indicating the maximum adsorption capacity of the vegetable material.
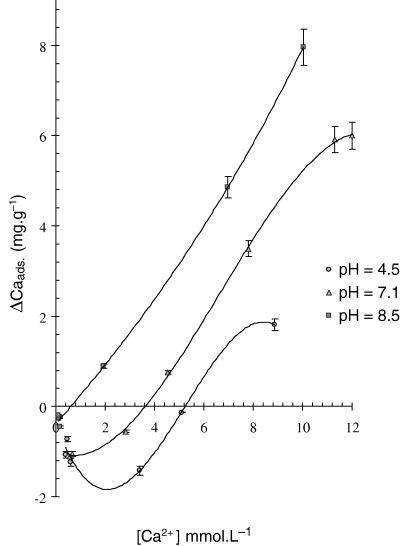
Adsorption isotherm of Ca by Casabe (75 μm). Each point is the mean ± 1 SD of three measurements.
The most significant change was observed in the isotherm at pH 8.5, which reproduces the alkaline environment in the small intestine: the calcium equilibrium concentration was very low (0.63 mmol L−1) and Ca2+ retention increased without reaching a maximum absorption in the range of concentrations evaluated. Similar results were reported by Lönnerdal (2002) who found that calcium was released by phytic acid at stomach pH, but the higher pH in the duodenum favoured calcium complexation by anion phytate. At this pH commercial enzymes have dissolved and are ready to break down carbohydrates, therefore, it is possible calcium is retained in the food matrix.
The amount of calcium that must be provided in the diet to obtain a specific concentration of dissolved Ca2+ can be calculated, as an approximation, by using the cubic regression equations of Table 3 derived from curve-fitting using the adsorption isotherms. For instance, at pH 8.5 to have a calcium concentration of 8 mmol L−1 (6.53 mg/20 mL) the diet must provide an amount 12.34 mg, because 1 g of enzyme-treated casabe will retain 5.81 mg. Whereas, the equation for pH 4.5 showed that the enzyme-treated casabe retains as much as 1.84 mg g−1, therefore only 8.37 mg is required to have the same calcium concentration in solution.
| Particle size . | pH . | Regression equation . | R 2 . |
|---|---|---|---|
| 75 μm | 8.5 | ΔCa = 0.0008[Ca2+]3 + 0.0116[Ca2+]2 + 0.631[Ca2+] − 0.9387* | 0.9992 |
| 75 μm | 7.1 | ΔCa = −0.0086[Ca2+]3 + 0.1709[Ca2+]2 − 0.2278[Ca2+] − 1.0099* | 0.9996 |
| 75 μm | 4.5 | ΔCa = −0.0292[Ca2+]3 + 0.4606[Ca2+]2 − 1.5413[Ca2+] − 0.3615* | 0.9871 |
| 2 mm | 8.5 | ΔCa = −0.0162[Ca2+]3 + 0.2591[Ca2+]2 + 0.1161[Ca2+] − 3.6253* | 0.9989 |
| 2 mm | 8.5 | ΔCa = −0.1217[Ca2+]2 + 2.9686[Ca2+] − 11.8** | 1.0000 |
| Particle size . | pH . | Regression equation . | R 2 . |
|---|---|---|---|
| 75 μm | 8.5 | ΔCa = 0.0008[Ca2+]3 + 0.0116[Ca2+]2 + 0.631[Ca2+] − 0.9387* | 0.9992 |
| 75 μm | 7.1 | ΔCa = −0.0086[Ca2+]3 + 0.1709[Ca2+]2 − 0.2278[Ca2+] − 1.0099* | 0.9996 |
| 75 μm | 4.5 | ΔCa = −0.0292[Ca2+]3 + 0.4606[Ca2+]2 − 1.5413[Ca2+] − 0.3615* | 0.9871 |
| 2 mm | 8.5 | ΔCa = −0.0162[Ca2+]3 + 0.2591[Ca2+]2 + 0.1161[Ca2+] − 3.6253* | 0.9989 |
| 2 mm | 8.5 | ΔCa = −0.1217[Ca2+]2 + 2.9686[Ca2+] − 11.8** | 1.0000 |
[Ca2+] in mmol L−1; ΔCa in mg g−1.
*Enzyme-treated casabe.
**Untreated casabe.
| Particle size . | pH . | Regression equation . | R 2 . |
|---|---|---|---|
| 75 μm | 8.5 | ΔCa = 0.0008[Ca2+]3 + 0.0116[Ca2+]2 + 0.631[Ca2+] − 0.9387* | 0.9992 |
| 75 μm | 7.1 | ΔCa = −0.0086[Ca2+]3 + 0.1709[Ca2+]2 − 0.2278[Ca2+] − 1.0099* | 0.9996 |
| 75 μm | 4.5 | ΔCa = −0.0292[Ca2+]3 + 0.4606[Ca2+]2 − 1.5413[Ca2+] − 0.3615* | 0.9871 |
| 2 mm | 8.5 | ΔCa = −0.0162[Ca2+]3 + 0.2591[Ca2+]2 + 0.1161[Ca2+] − 3.6253* | 0.9989 |
| 2 mm | 8.5 | ΔCa = −0.1217[Ca2+]2 + 2.9686[Ca2+] − 11.8** | 1.0000 |
| Particle size . | pH . | Regression equation . | R 2 . |
|---|---|---|---|
| 75 μm | 8.5 | ΔCa = 0.0008[Ca2+]3 + 0.0116[Ca2+]2 + 0.631[Ca2+] − 0.9387* | 0.9992 |
| 75 μm | 7.1 | ΔCa = −0.0086[Ca2+]3 + 0.1709[Ca2+]2 − 0.2278[Ca2+] − 1.0099* | 0.9996 |
| 75 μm | 4.5 | ΔCa = −0.0292[Ca2+]3 + 0.4606[Ca2+]2 − 1.5413[Ca2+] − 0.3615* | 0.9871 |
| 2 mm | 8.5 | ΔCa = −0.0162[Ca2+]3 + 0.2591[Ca2+]2 + 0.1161[Ca2+] − 3.6253* | 0.9989 |
| 2 mm | 8.5 | ΔCa = −0.1217[Ca2+]2 + 2.9686[Ca2+] − 11.8** | 1.0000 |
[Ca2+] in mmol L−1; ΔCa in mg g−1.
*Enzyme-treated casabe.
**Untreated casabe.
Equations for the two particle sizes of casabe at pH 8.5 showed that the 75 μm fraction retained the highest amount of Ca2+ at a given calcium concentration. The value of [Ca2+]e also depends on the particle size, being lesser for the finest fraction, which is a consequence of the greater surface exposed to the enzyme and calcium ions. Therefore, the degree of subdivision of casabe particles will affect its ability to retain the nutrient.
pH dependent surface charge
The adsorption of potential-determining ions (H+ and OH−) by vegetable material (Fig. 3), showed the small amounts of H+ required to neutralize negative charges in enzyme-treated casabe. This provide evidence of a very low density of negatively charged sites, which are practically absent in the untreated casabe. In contrast, rice bran has a high density of negative charges leading to a much greater capacity to retain calcium ions (Navas & Carrasquero-Duran, 2003). On the other hand, the high adsorption of OH−, much higher than the corresponding values for H+, in enzyme-treated and untreated casabe, suggested that in both materials the retention of anions prevailed affecting eventually, the bioavailability of other nutrients such as phosphate anions.
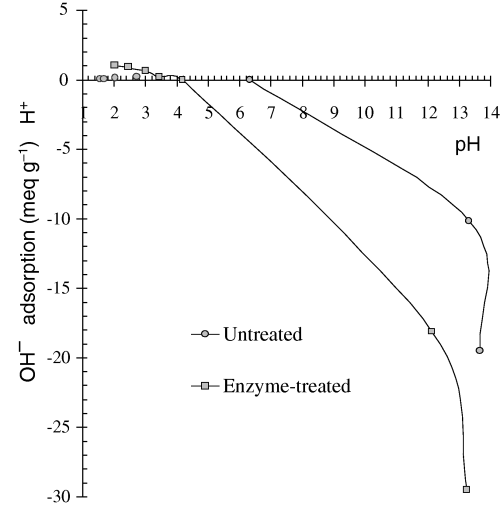
To know the value of pHo it is necessary to establish the nature of the surface electric charges at a fixed value of pH. In this case untreated casabe had a pHo of 6.4 which indicated the presence of net negative charges in the more alkaline solutions. However, in solutions with pH below pHo, this material did not exhibit appreciable quantities of positive charges. Once the casabe had been treated with digestive enzymes, pHo fell to 4.1 and a small but identifiable amount of protonated functional groups was observed. The negatively charged sites increased when the pH was greater than 4.1 which would explain the adsorption of Ca2+ for enzyme-treated casabe at the pH in stomach.
Conclusions
Casabe exhibits an ability to bind Ca2+ thereby reducing its bioavailability; however, the amount of calcium retained is low compared with other vegetable fibres such as rice bran. Treatment with commercial digestive enzymes increases calcium adsorption which also depends on Ca2+ concentration. In acid solution, calcium release with low adsorption predominates, but Ca2+ binding increases when the pH is higher than pHo which suggests that enzymatic treatment modifies the extent of negative charge density.
Casabe has been an important source of low cost energy and fibre for Venezuelan people. When it is consumed with other foods that supply calcium for an adequate nutrition, mineral retention is relatively low without negative effects on mineral bioavailability, and fibre may contribute to the prevention of illness of the digestive system. Production of casabe must be promoted not only in Venezuela, but also in the Caribbean countries to improve the diet for poor people and this economic activity may have positive effects in countries’ economies by promoting employment and reducing the imports of other fibre sources from foreign countries.



