-
PDF
- Split View
-
Views
-
Cite
Cite
Narendra Singh Shah, Nirankar Nath, Effect of Calcium Lactate, 4-Hexyl Resorcinol and Vacuum Packing on Physico-chemical, Sensory and Microbiological Qualities of Minimally Processed Litchi (Litchi chinensis Sonn.), International Journal of Food Science and Technology, Volume 41, Issue 9, November 2006, Pages 1073–1081, https://doi.org/10.1111/j.1365-2621.2006.01181.x
Close - Share Icon Share
Abstract
Litchi (Litchi chinensis Sonn.) fruits are very susceptible to pericarp browning which adversely affects consumer acceptability even though the aril portion remains in excellent condition. Litchi arils (litchis) were treated with a solution containing 0–2% (w/v) calcium lactate (CL), 0–0.02% (w/v) 4-hexyl resorcinol (4-HR) and 1% potassium sorbate. The pH of solution was adjusted to 4.0 with citric acid. Treated litchis were packed in polystyrene trays, over-wrapped with polypropylene film, vacuum-packed (0, 47409.3, 94831.9 Pa) and stored at 4 ± 2 °C. Drip losses, pH, total soluble solids (TSS), sensory attributes and microbiological quality of stored samples were estimated. A four-factor, three-level experimental design (D6 Hokes design) with 19 experiments was chosen. Mathematical models were developed to analyse and predict the effect of CL, 4-HR, in-package vacuum and storage time on the responses. TSS, pH and sensory scores decreased significantly (P ≤ 0.01), whereas drip losses and microbial count increased significantly (P ≤ 0.01) with time. Drip loss was significantly (P ≤ 0.1) reduced by addition of CL. 4-HR prevented browning and changes in colour score during storage were significantly less. Vacuum in packages exerted significant (P ≤ 0.01) effect over pH, TSS, sensory and microbiological qualities of minimally processed litchis.
Introduction
Litchi (Litchi chinensis Sonn.) is a fruit of tropical origin, grown in many parts of the world, namely China, India, Thailand, Madagascar, Australia, Taiwan, Israel and USA (Ray, 1998). It is comprised of outer pericarp (11.73–28.7%), aril (37.3–73.6%) and stone (3.2–22.9%); the creamy translucent aril is the edible part of litchis (Revathy & Narasimhan, 1997). Litchis are valued highly in the international market for their deliciously flavoured sweet juicy aril and attractive deep red coloured pericarp. Litchis are harvested during summer months when day temperatures are high upto 36 °C and relative humidities are as low as 60% (Hasan & Chattopadhyay, 1997). Under these conditions, litchi pericarp turns brown within 2–3 days of harvest (Huang & Scott, 1985), which reduces its market acceptabilities (Akamine, 1960; Holcroft & Mitcham, 1996) eventhough the edible aril portion remains in excellent condition (Joubert, 1986). The pericarp browning is associated with dessication, senescence, fruit development, chilling injury, and pest and pathogen attack and is attributed to anthocyanin breakdown, polyphenol oxidase, peroxidase and enzymatic oxidation of ascorbic acid (Holcroft & Mitcham, 1996). Pericarp browning together with a short harvesting season of 2–3 weeks reduces commercial availability of litchis in the international market (Revathy & Narasimhan, 1997). Preservation of arils as a minimally processed food product would be very beneficial for its commercialisation and value addition.
Minimally processed fruits or vegetables have been defined as fresh fruits or vegetables that have been processed to increase their functionality without greatly changing their fresh-like properties (Salunkhe et al., 1991). This produce is characterised by a good degree of freshness, convenience and low levels of chemical preservatives (Shewfelt, 1990). The sale of minimally processed products is increasing throughout the world (Ragaert et al., 2004). Although simplicity in use and convenience are distinguishing features of these products, their shelf-life is generally shorter than that of original raw material because of tissue damage caused by operations such as peeling, cutting and seed removal. Tissue wounding increases the rate of respiration and intermixing of enzymes with substrates resulting in faster texture deterioration and enzymatic browning (Rosen & Kader, 1989; Dong et al., 2000). Minimal processing treatments disrupt surface tissues, expose cytoplasm and provide a potentially richer source of nutrients for micro-organisms than intact produce (Brackett, 1994). The main pathogens of concern with respect to refrigerated minimally processed fruits and vegetables are non-proteolytic Clostridium botulinum, Listeria monocytogenes, Yersinia entercolitica and Aeromonas hydrophila (Francis et al., 1999).
Rate of degradation of minimally processed fruits and vegetables can be reduced and their shelf-life increased through adoption of hurdle technology approaches such as incorporation of preservatives, pH-depressing agents, slight reduction in water activity (aw), mild heat treatments, modified atmosphere packaging (MAP) and refrigeration or other mild preservation techniques (Alzamora et al., 1997; Pereira et al., 2004). Although criteria for evaluation of quality for minimally and traditionally processed foods are similar, there is a greater emphasis on visual characteristics (colour and texture) in the former (Lin & Schyvens, 1995; Kajuna et al., 1998). Maintaining a low oxygen concentration in the package and addition of firming agents like calcium salts have been reported to retard softening of texture and drip losses (Poovaiah, 1986; Rosen & Kader, 1989; Dong et al., 2000; Gorny et al., 2002; Soliva-Fortuny et al., 2002). For controlling browning of cut surfaces, inhibitors like cysteine, ascorbic acid, citric acid, EDTA, sodium erythrobate, 4-hexyl resorcinol (4-HR), calcium salts, etc. are used. Dong et al. (2000) reported 4-HR to be an effective browning inhibitor of fresh-cut pears in the presence of 0.5% ascorbic acid compared with ascorbic acid, calcium lactate, sucrose esters, citric acid, cysteine and pineapple juice alone and their combination thereof. MAP is a food preservation technology whereby the composition of the atmosphere surrounding the product is different from the composition of air. It can be achieved either passively or actively. Most of the MAP approaches for respiring products are based on a reduction in the O2 level and an increase in the CO2 concentration. A low O2 and a high CO2 concentration delays browning and spoilage and offers fresh appearance; however, they may cause development of off-flavours and flavour losses (Gimenez et al., 2003). The aim of the study was to improve the commercial value of litchis through minimal processing of their arils and to evaluate the effect of additives and vacuum packaging on their physicochemical, sensory and microbiological qualities.
Materials
Litchis of Rose Scented variety of optimum maturity (dark red pericarp, total soluble solids ≥18 °Brix, acidity ≤0.3% as malic acid and fruit weight ≥20 g) were obtained from Horticulture Research Centre of G. B. Pant University of Agriculture and Technology, Pantnagar, India in June 2004. The fruits were sorted; the damaged and infested fruits were removed and selected fruits were transferred immediately to a cold storage chamber (4 ± 2 °C, 90% RH) (Cryoscientific, Chennai, India) until use.
Methods
Minimal processing of litchi arils
Wholesome litchis were selected, rinsed with water to reduce the surface microbial load and contaminants, peeled and destoned manually using a sharp stainless steel knife. During the process, care was taken to get the aril portion with minimum damage. Litchi arils or litchis (250 g) were dipped for 2 min in 1 L of the test solution of calcium lactate (CL), 4-HR and 1% potassium sorbate and kept over a plastic sieve for 5 min to drain out excess solution from the surface. Treated samples were placed in plastic trays, over-wrapped with a polypropylene film (600 μ) and sealed in a vacuum-packaging machine (Indvac, Saurabh Engineers, Ahmadabad, India). The pH of the test solutions was adjusted to 4.0 using 2% citric acid solution. Range of CL (0–2%) and 4-HR (0–0.02%) concentration was selected on the basis of preliminary sensory evaluation; at higher levels, the panelists reported undesirable flavour changes in the product. The concentrations of CL and 4-HR in test solution and vacuum in packages (V) were set according to the D6 Hokes experimental design (Thompson, 1982) (Table 1).
| Treatment . | Calcium lactate (% w/v) . | 4-Hexyl resorcinol (% w/v) . | In-package vacuum (Pa) . | Storage time (days) . |
|---|---|---|---|---|
| X1 (x1) . | X2 (x2) . | X3 (x3) . | X4 (x4) . | |
| A | 0 (−1) | 0.01 (0) | 47 409.3 (0) | 10 (0) |
| B | 1 (0) | 0.00 (−1) | 47 409.3 (0) | 10 (0) |
| C | 1 (0) | 0.01 (0) | 94 831.9 (−1) | 10 (0) |
| D | 1 (0) | 0.01 (0) | 47 409.3 (0) | 0 (−1) |
| E | 0 (−1) | 0.00 (−1) | 94 831.9 (−1) | 0 (−1) |
| F | 0 (−1) | 0.02 (1) | 0.00 (1) | 20 (1) |
| G | 2 (1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| H | 2 (1) | 0.02 (1) | 94831.9 (−1) | 20 (1) |
| I | 2 (1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| J | 2 (1) | 0.02 (1) | 94 831.9 (−1) | 0 (−1) |
| K | 2 (1) | 0.00 (−1) | 0.00 (1) | 0 (−1) |
| L | 2 (1) | 0.00 (−1) | 94 831.9 (−1) | 20 (1) |
| M | 0 (−1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| N | 0 (−1) | 0.02 (1) | 94 831.9 (−1) | 20 (1) |
| O | 0 (−1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| P | 1 (0) | 0.02 (1) | 0.00 (1) | 20 (1) |
| Q | 2 (1) | 0.01 (0) | 0.00 (1) | 20 (1) |
| R | 2 (1) | 0.02 (1) | 47 409.3 (0) | 20 (1) |
| S | 2 (1) | 0.02 (1) | 0.00 (1) | 10 (0) |
| Treatment . | Calcium lactate (% w/v) . | 4-Hexyl resorcinol (% w/v) . | In-package vacuum (Pa) . | Storage time (days) . |
|---|---|---|---|---|
| X1 (x1) . | X2 (x2) . | X3 (x3) . | X4 (x4) . | |
| A | 0 (−1) | 0.01 (0) | 47 409.3 (0) | 10 (0) |
| B | 1 (0) | 0.00 (−1) | 47 409.3 (0) | 10 (0) |
| C | 1 (0) | 0.01 (0) | 94 831.9 (−1) | 10 (0) |
| D | 1 (0) | 0.01 (0) | 47 409.3 (0) | 0 (−1) |
| E | 0 (−1) | 0.00 (−1) | 94 831.9 (−1) | 0 (−1) |
| F | 0 (−1) | 0.02 (1) | 0.00 (1) | 20 (1) |
| G | 2 (1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| H | 2 (1) | 0.02 (1) | 94831.9 (−1) | 20 (1) |
| I | 2 (1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| J | 2 (1) | 0.02 (1) | 94 831.9 (−1) | 0 (−1) |
| K | 2 (1) | 0.00 (−1) | 0.00 (1) | 0 (−1) |
| L | 2 (1) | 0.00 (−1) | 94 831.9 (−1) | 20 (1) |
| M | 0 (−1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| N | 0 (−1) | 0.02 (1) | 94 831.9 (−1) | 20 (1) |
| O | 0 (−1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| P | 1 (0) | 0.02 (1) | 0.00 (1) | 20 (1) |
| Q | 2 (1) | 0.01 (0) | 0.00 (1) | 20 (1) |
| R | 2 (1) | 0.02 (1) | 47 409.3 (0) | 20 (1) |
| S | 2 (1) | 0.02 (1) | 0.00 (1) | 10 (0) |
*Xi are the actual values for independent variables and xi are the coded levels of four variables where, 1 = highest level of variable taken for study, 0 = midpoint and −1 = lowest limit of variable taken for study.
| Treatment . | Calcium lactate (% w/v) . | 4-Hexyl resorcinol (% w/v) . | In-package vacuum (Pa) . | Storage time (days) . |
|---|---|---|---|---|
| X1 (x1) . | X2 (x2) . | X3 (x3) . | X4 (x4) . | |
| A | 0 (−1) | 0.01 (0) | 47 409.3 (0) | 10 (0) |
| B | 1 (0) | 0.00 (−1) | 47 409.3 (0) | 10 (0) |
| C | 1 (0) | 0.01 (0) | 94 831.9 (−1) | 10 (0) |
| D | 1 (0) | 0.01 (0) | 47 409.3 (0) | 0 (−1) |
| E | 0 (−1) | 0.00 (−1) | 94 831.9 (−1) | 0 (−1) |
| F | 0 (−1) | 0.02 (1) | 0.00 (1) | 20 (1) |
| G | 2 (1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| H | 2 (1) | 0.02 (1) | 94831.9 (−1) | 20 (1) |
| I | 2 (1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| J | 2 (1) | 0.02 (1) | 94 831.9 (−1) | 0 (−1) |
| K | 2 (1) | 0.00 (−1) | 0.00 (1) | 0 (−1) |
| L | 2 (1) | 0.00 (−1) | 94 831.9 (−1) | 20 (1) |
| M | 0 (−1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| N | 0 (−1) | 0.02 (1) | 94 831.9 (−1) | 20 (1) |
| O | 0 (−1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| P | 1 (0) | 0.02 (1) | 0.00 (1) | 20 (1) |
| Q | 2 (1) | 0.01 (0) | 0.00 (1) | 20 (1) |
| R | 2 (1) | 0.02 (1) | 47 409.3 (0) | 20 (1) |
| S | 2 (1) | 0.02 (1) | 0.00 (1) | 10 (0) |
| Treatment . | Calcium lactate (% w/v) . | 4-Hexyl resorcinol (% w/v) . | In-package vacuum (Pa) . | Storage time (days) . |
|---|---|---|---|---|
| X1 (x1) . | X2 (x2) . | X3 (x3) . | X4 (x4) . | |
| A | 0 (−1) | 0.01 (0) | 47 409.3 (0) | 10 (0) |
| B | 1 (0) | 0.00 (−1) | 47 409.3 (0) | 10 (0) |
| C | 1 (0) | 0.01 (0) | 94 831.9 (−1) | 10 (0) |
| D | 1 (0) | 0.01 (0) | 47 409.3 (0) | 0 (−1) |
| E | 0 (−1) | 0.00 (−1) | 94 831.9 (−1) | 0 (−1) |
| F | 0 (−1) | 0.02 (1) | 0.00 (1) | 20 (1) |
| G | 2 (1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| H | 2 (1) | 0.02 (1) | 94831.9 (−1) | 20 (1) |
| I | 2 (1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| J | 2 (1) | 0.02 (1) | 94 831.9 (−1) | 0 (−1) |
| K | 2 (1) | 0.00 (−1) | 0.00 (1) | 0 (−1) |
| L | 2 (1) | 0.00 (−1) | 94 831.9 (−1) | 20 (1) |
| M | 0 (−1) | 0.02 (1) | 0.00 (1) | 0 (−1) |
| N | 0 (−1) | 0.02 (1) | 94 831.9 (−1) | 20 (1) |
| O | 0 (−1) | 0.00 (−1) | 0.00 (1) | 20 (1) |
| P | 1 (0) | 0.02 (1) | 0.00 (1) | 20 (1) |
| Q | 2 (1) | 0.01 (0) | 0.00 (1) | 20 (1) |
| R | 2 (1) | 0.02 (1) | 47 409.3 (0) | 20 (1) |
| S | 2 (1) | 0.02 (1) | 0.00 (1) | 10 (0) |
*Xi are the actual values for independent variables and xi are the coded levels of four variables where, 1 = highest level of variable taken for study, 0 = midpoint and −1 = lowest limit of variable taken for study.
The trays of minimally processed litchis were stored in a refrigerator at 4 ± 2 °C. Samples were analysed at the 0, 10th and 20th day (T) as per design for drip losses, pH, total soluble solids (TSS), sensory and microbiological qualities (plate count, yeast and mould count and psychrophilic count).
Physicochemical analysis
The internal cellular structure of arils gets damaged during preparation, pretreatments and storage which results in changes in their texture and loss of cellular fluid (juice); greater damage resulted in higher fluid loss. This fluid loss was expressed as drip loss. To measure drip losses (mL/100 g), samples were drained over a 56-mesh sieve for 5 min and volume of the drained liquid was measured. Total soluble solids (°Brix) of litchi juice/drip were measured using a hand refractometer (Erma Japan make; Macro Scientific Works, New Delhi, India). Litchi arils (10 g) were blended with 10 mL of distilled water and pH of the blend was measured using a glass electrode pH meter (Model 5652; ECIL, Hyderabad, India).
Microbiological analysis
Ten grams of litchi arils was ground in a glass mortar and pestle under aseptic conditions using 100 mL of sterile peptone water (0.1% peptone + 0.5% sodium chloride). Further decimal dilutions were made with the same diluent. For total plate count (TPC), plate count agar media (Hi media Laboratories, Mumbai, India) and incubation temperature 30 °C for 48 h were used (Vanderzant & Splittstoesser, 1992). Total yeast and mould count (YMC) was determined using potato dextrose agar (Hi media Laboratories) with pH adjusted to 3.5 by 10% sterile tartaric acid and incubation temperature of 25 °C for 72 h, Total psychrophilic count (PC) was determined on plate count agar (Hi media Laboratories) using an incubation temperature of 7 °C for 7–10 days. All the counts were carried out in duplicate.
Sensory evaluation
The sensory evaluation was used to discriminate between the colour, visual appearance, aroma and overall acceptability of the minimally processed litchi samples. A panel of twenty judges familiar with litchis, selected from the Department of Food Science and Technology of this University were presented coded samples in plastic trays. Samples were evaluated for individual sensory attributes on a nine-point hedonic scale, where a score of 1 indicated poor sensory attribute and a score of 9 indicated excellent sensory attribute. Overall acceptability was calculated by taking arithmetic average of score for all three sensory attributes.
Experimental design
Response surface methodology was used to evaluate the simultaneous effect of CL, 4-HR, vacuum in package (V) and refrigerated storage time (T) on the quality of minimally processed litchis. The experiment was based on a three-level, four-factor D6 Hokes design (Thompson, 1982). It was assumed that n mathematical functions, Fk (k = 1, 2,…, n) existed for each response variable, Yk in terms of m independent processing factors Xi (i = 1, 2,…, m).
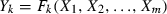
In this case, n = 10 and m = 4. The function was assumed to be approximated by a second-degree polynomial equation
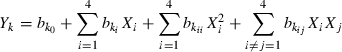
where b0 is the value of the fitted response at the centre point of the design, i.e point (0,0,0,0) and bki, bkii and bkij are the linear, quadratic and cross-product regression terms, respectively. The analysis of variance (Anova) tables were generated and the effect of effect of individual linear (bki), quadratic (bkii) and interaction (bkij) terms were determined (Khuri & Cornell, 1989). The significance of all the terms in the polynomial was judged statistically by computing the F-value. The significance of F-values was judged at a probability level (P) of 0.01, 0.05 and 0.1.
Desirable ranges of the independent variables selected for the present investigation on the basis of preliminary studies were 0–2% for CL (X1), 0–0.02% for 4-HR, 0–94831.9 Pa in-package vacuum (X3) and storage period (X4) of 0–20 days. Coded levels of the four independent variables used in these studies were −1 (lowest level), 0 (intermediate level) and +1 (highest level). Actual values of independent variables along with their coded values (Xi) are given in Table 1. The complete design consisted of nineteen experiments and the experiments were performed in a random order (Table 1).
Data analysis software Minitab 11 (Minitab Inc. UK, 1988) was used to fit the experimental data to the above model (Eqn. 2) to obtain the regression coefficients of equations and for analysis of variance. Contour plots were developed using the fitted quadratic polynomial equations obtained by surfer (Win 32) version 6.04 surface mapping system (Golden Software Inc., Golden, CO, USA).
Results and discussion
Values for various responses were found to vary with treatment (Table 2). The regression models were tested for adequacy and fitness by Anova (Khuri & Cornell, 1989). Table 3 summarises the results for linear, quadratic and cross-product terms. The regression coefficients for the second-order polynomial equations are presented in Table 4.
Physicochemical and sensory characteristics of minimally processed litchis stored at 4 ± 2 °C
| Sample no.* . | Drip loss (%) . | pH . | TSS1 (°Brix) . | Sensory score . | Microbiological counts (log cfu/g) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| A | 15.60 | 4.24 | 14.8 | 7.2 | 7.4 | 7.8 | 7.47 | 4.978 | 5.190 | 4.962 |
| B | 10.50 | 4.19 | 15.2 | 7.1 | 7.5 | 8.0 | 7.53 | 5.000 | 5.288 | 4.463 |
| C | 14.28 | 4.44 | 16.2 | 8.3 | 8.0 | 7.0 | 7.77 | 4.471 | 3.966 | 3.956 |
| D | 0.50 | 4.69 | 17.0 | 8.5 | 8.2 | 8.6 | 8.43 | 3.258 | 3.265 | 2.661 |
| E | 6.20 | 4.68 | 17.0 | 8.2 | 8.5 | 7.6 | 8.10 | 3.570 | 2.946 | 2.441 |
| F | 26.40 | 4.09 | 13.8 | 7.1 | 6.8 | 6.9 | 6.93 | 5.867 | 6.212 | 5.434 |
| G | 24.00 | 4.01 | 13.8 | 6.4 | 7.1 | 6.4 | 6.63 | 5.620 | 6.360 | 5.333 |
| H | 19.60 | 4.27 | 14.6 | 7.8 | 5.8 | 6.3 | 6.62 | 4.219 | 4.688 | 3.625 |
| I | 0.00 | 4.73 | 17.0 | 8.5 | 8.0 | 8.2 | 8.23 | 4.000 | 3.626 | 3.025 |
| J | 5.84 | 4.69 | 17.0 | 8.4 | 7.8 | 7.5 | 7.90 | 4.017 | 2.956 | 2.756 |
| K | 0.00 | 4.71 | 17.0 | 8.3 | 8.2 | 8.5 | 8.35 | 3.659 | 3.869 | 2.642 |
| L | 22.40 | 4.21 | 15.4 | 6.6 | 7.0 | 6.5 | 6.70 | 4.317 | 3.660 | 2.913 |
| M | 0.00 | 4.67 | 17.0 | 8.5 | 7.6 | 8.5 | 8.20 | 3.681 | 3.256 | 2.457 |
| N | 28.45 | 4.25 | 15.2 | 7.6 | 6.8 | 7.0 | 7.13 | 4.778 | 4.945 | 4.431 |
| O | 33.00 | 3.68 | 13.8 | 5.8 | 5.8 | 6.8 | 6.13 | 6.032 | 6.373 | 5.763 |
| P | 25.50 | 3.81 | 14.0 | 6.8 | 6.4 | 7.0 | 6.72 | 5.699 | 6.756 | 4.826 |
| Q | 24.60 | 3.97 | 15.0 | 5.8 | 6.5 | 7.2 | 6.50 | 6.133 | 5.469 | 5.646 |
| R | 26.20 | 4.07 | 14.2 | 7.4 | 7.0 | 7.5 | 7.30 | 5.988 | 5.813 | 5.370 |
| S | 11.20 | 4.21 | 15.6 | 7.6 | 7.1 | 7.8 | 7.50 | 5.535 | 4.895 | 5.038 |
| Sample no.* . | Drip loss (%) . | pH . | TSS1 (°Brix) . | Sensory score . | Microbiological counts (log cfu/g) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| A | 15.60 | 4.24 | 14.8 | 7.2 | 7.4 | 7.8 | 7.47 | 4.978 | 5.190 | 4.962 |
| B | 10.50 | 4.19 | 15.2 | 7.1 | 7.5 | 8.0 | 7.53 | 5.000 | 5.288 | 4.463 |
| C | 14.28 | 4.44 | 16.2 | 8.3 | 8.0 | 7.0 | 7.77 | 4.471 | 3.966 | 3.956 |
| D | 0.50 | 4.69 | 17.0 | 8.5 | 8.2 | 8.6 | 8.43 | 3.258 | 3.265 | 2.661 |
| E | 6.20 | 4.68 | 17.0 | 8.2 | 8.5 | 7.6 | 8.10 | 3.570 | 2.946 | 2.441 |
| F | 26.40 | 4.09 | 13.8 | 7.1 | 6.8 | 6.9 | 6.93 | 5.867 | 6.212 | 5.434 |
| G | 24.00 | 4.01 | 13.8 | 6.4 | 7.1 | 6.4 | 6.63 | 5.620 | 6.360 | 5.333 |
| H | 19.60 | 4.27 | 14.6 | 7.8 | 5.8 | 6.3 | 6.62 | 4.219 | 4.688 | 3.625 |
| I | 0.00 | 4.73 | 17.0 | 8.5 | 8.0 | 8.2 | 8.23 | 4.000 | 3.626 | 3.025 |
| J | 5.84 | 4.69 | 17.0 | 8.4 | 7.8 | 7.5 | 7.90 | 4.017 | 2.956 | 2.756 |
| K | 0.00 | 4.71 | 17.0 | 8.3 | 8.2 | 8.5 | 8.35 | 3.659 | 3.869 | 2.642 |
| L | 22.40 | 4.21 | 15.4 | 6.6 | 7.0 | 6.5 | 6.70 | 4.317 | 3.660 | 2.913 |
| M | 0.00 | 4.67 | 17.0 | 8.5 | 7.6 | 8.5 | 8.20 | 3.681 | 3.256 | 2.457 |
| N | 28.45 | 4.25 | 15.2 | 7.6 | 6.8 | 7.0 | 7.13 | 4.778 | 4.945 | 4.431 |
| O | 33.00 | 3.68 | 13.8 | 5.8 | 5.8 | 6.8 | 6.13 | 6.032 | 6.373 | 5.763 |
| P | 25.50 | 3.81 | 14.0 | 6.8 | 6.4 | 7.0 | 6.72 | 5.699 | 6.756 | 4.826 |
| Q | 24.60 | 3.97 | 15.0 | 5.8 | 6.5 | 7.2 | 6.50 | 6.133 | 5.469 | 5.646 |
| R | 26.20 | 4.07 | 14.2 | 7.4 | 7.0 | 7.5 | 7.30 | 5.988 | 5.813 | 5.370 |
| S | 11.20 | 4.21 | 15.6 | 7.6 | 7.1 | 7.8 | 7.50 | 5.535 | 4.895 | 5.038 |
*Cf. Table 1 for details.
1Total soluble solids; 2overall acceptability; 3total plate count; 4yeast and mould count; 5total psychrophilic count.
Physicochemical and sensory characteristics of minimally processed litchis stored at 4 ± 2 °C
| Sample no.* . | Drip loss (%) . | pH . | TSS1 (°Brix) . | Sensory score . | Microbiological counts (log cfu/g) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| A | 15.60 | 4.24 | 14.8 | 7.2 | 7.4 | 7.8 | 7.47 | 4.978 | 5.190 | 4.962 |
| B | 10.50 | 4.19 | 15.2 | 7.1 | 7.5 | 8.0 | 7.53 | 5.000 | 5.288 | 4.463 |
| C | 14.28 | 4.44 | 16.2 | 8.3 | 8.0 | 7.0 | 7.77 | 4.471 | 3.966 | 3.956 |
| D | 0.50 | 4.69 | 17.0 | 8.5 | 8.2 | 8.6 | 8.43 | 3.258 | 3.265 | 2.661 |
| E | 6.20 | 4.68 | 17.0 | 8.2 | 8.5 | 7.6 | 8.10 | 3.570 | 2.946 | 2.441 |
| F | 26.40 | 4.09 | 13.8 | 7.1 | 6.8 | 6.9 | 6.93 | 5.867 | 6.212 | 5.434 |
| G | 24.00 | 4.01 | 13.8 | 6.4 | 7.1 | 6.4 | 6.63 | 5.620 | 6.360 | 5.333 |
| H | 19.60 | 4.27 | 14.6 | 7.8 | 5.8 | 6.3 | 6.62 | 4.219 | 4.688 | 3.625 |
| I | 0.00 | 4.73 | 17.0 | 8.5 | 8.0 | 8.2 | 8.23 | 4.000 | 3.626 | 3.025 |
| J | 5.84 | 4.69 | 17.0 | 8.4 | 7.8 | 7.5 | 7.90 | 4.017 | 2.956 | 2.756 |
| K | 0.00 | 4.71 | 17.0 | 8.3 | 8.2 | 8.5 | 8.35 | 3.659 | 3.869 | 2.642 |
| L | 22.40 | 4.21 | 15.4 | 6.6 | 7.0 | 6.5 | 6.70 | 4.317 | 3.660 | 2.913 |
| M | 0.00 | 4.67 | 17.0 | 8.5 | 7.6 | 8.5 | 8.20 | 3.681 | 3.256 | 2.457 |
| N | 28.45 | 4.25 | 15.2 | 7.6 | 6.8 | 7.0 | 7.13 | 4.778 | 4.945 | 4.431 |
| O | 33.00 | 3.68 | 13.8 | 5.8 | 5.8 | 6.8 | 6.13 | 6.032 | 6.373 | 5.763 |
| P | 25.50 | 3.81 | 14.0 | 6.8 | 6.4 | 7.0 | 6.72 | 5.699 | 6.756 | 4.826 |
| Q | 24.60 | 3.97 | 15.0 | 5.8 | 6.5 | 7.2 | 6.50 | 6.133 | 5.469 | 5.646 |
| R | 26.20 | 4.07 | 14.2 | 7.4 | 7.0 | 7.5 | 7.30 | 5.988 | 5.813 | 5.370 |
| S | 11.20 | 4.21 | 15.6 | 7.6 | 7.1 | 7.8 | 7.50 | 5.535 | 4.895 | 5.038 |
| Sample no.* . | Drip loss (%) . | pH . | TSS1 (°Brix) . | Sensory score . | Microbiological counts (log cfu/g) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| A | 15.60 | 4.24 | 14.8 | 7.2 | 7.4 | 7.8 | 7.47 | 4.978 | 5.190 | 4.962 |
| B | 10.50 | 4.19 | 15.2 | 7.1 | 7.5 | 8.0 | 7.53 | 5.000 | 5.288 | 4.463 |
| C | 14.28 | 4.44 | 16.2 | 8.3 | 8.0 | 7.0 | 7.77 | 4.471 | 3.966 | 3.956 |
| D | 0.50 | 4.69 | 17.0 | 8.5 | 8.2 | 8.6 | 8.43 | 3.258 | 3.265 | 2.661 |
| E | 6.20 | 4.68 | 17.0 | 8.2 | 8.5 | 7.6 | 8.10 | 3.570 | 2.946 | 2.441 |
| F | 26.40 | 4.09 | 13.8 | 7.1 | 6.8 | 6.9 | 6.93 | 5.867 | 6.212 | 5.434 |
| G | 24.00 | 4.01 | 13.8 | 6.4 | 7.1 | 6.4 | 6.63 | 5.620 | 6.360 | 5.333 |
| H | 19.60 | 4.27 | 14.6 | 7.8 | 5.8 | 6.3 | 6.62 | 4.219 | 4.688 | 3.625 |
| I | 0.00 | 4.73 | 17.0 | 8.5 | 8.0 | 8.2 | 8.23 | 4.000 | 3.626 | 3.025 |
| J | 5.84 | 4.69 | 17.0 | 8.4 | 7.8 | 7.5 | 7.90 | 4.017 | 2.956 | 2.756 |
| K | 0.00 | 4.71 | 17.0 | 8.3 | 8.2 | 8.5 | 8.35 | 3.659 | 3.869 | 2.642 |
| L | 22.40 | 4.21 | 15.4 | 6.6 | 7.0 | 6.5 | 6.70 | 4.317 | 3.660 | 2.913 |
| M | 0.00 | 4.67 | 17.0 | 8.5 | 7.6 | 8.5 | 8.20 | 3.681 | 3.256 | 2.457 |
| N | 28.45 | 4.25 | 15.2 | 7.6 | 6.8 | 7.0 | 7.13 | 4.778 | 4.945 | 4.431 |
| O | 33.00 | 3.68 | 13.8 | 5.8 | 5.8 | 6.8 | 6.13 | 6.032 | 6.373 | 5.763 |
| P | 25.50 | 3.81 | 14.0 | 6.8 | 6.4 | 7.0 | 6.72 | 5.699 | 6.756 | 4.826 |
| Q | 24.60 | 3.97 | 15.0 | 5.8 | 6.5 | 7.2 | 6.50 | 6.133 | 5.469 | 5.646 |
| R | 26.20 | 4.07 | 14.2 | 7.4 | 7.0 | 7.5 | 7.30 | 5.988 | 5.813 | 5.370 |
| S | 11.20 | 4.21 | 15.6 | 7.6 | 7.1 | 7.8 | 7.50 | 5.535 | 4.895 | 5.038 |
*Cf. Table 1 for details.
1Total soluble solids; 2overall acceptability; 3total plate count; 4yeast and mould count; 5total psychrophilic count.
Analysis of variance of regression models for physicochemical and sensory attributes
| Source . | . | DF1 . | Sum of squares . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS2 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||||
| Colour . | Aroma . | Appearance . | OAA3 . | TPC4 . | YMC5 . | PC6 . | ||||||
| Model | 14 | 2169*** | 1.862*** | 27.487*** | 13.478*** | 10.447*** | 8.97*** | 8.822*** | 15.719*** | 27.772*** | 26.438*** | |
| Linear | X1 | 1 | 34.15* | 0.009 | 0.186 | 0 | 0.001 | 0.072 | 0.009 | 0.004 | 0.204 | 0.138 |
| X3 | 1 | 0.14 | 0.012 | 0.004 | 1.652 | 0.442 | 0.026 | 0.066 | 0.106 | 0.022 | 0.096 | |
| X3 | 1 | 0.34 | 0.171** | 1.596* | 1.509 | 0.288* | 0.704* | 0.096 | 2.882*** | 6.310*** | 4.172*** | |
| X4 | 1 | 2040*** | 1.507*** | 23.513*** | 9.166*** | 7.872*** | 6.519*** | 7.849*** | 9.714*** | 17.554*** | 15.194*** | |
| Quadratic | X  | 1 | 9.02 | 0.023 | 0.023 | 0.049 | 0.087 | 0.132 | 0.084 | 0.003 | 0.630 | 0.059 |
X  | 1 | 0.22 | 0.001 | 0.067 | 0.155 | 0.050 | 0.064 | 0.001 | 0.084 | 0.084 | 0.947 | |
X  | 1 | 1.03 | 0.006 | 0.876 | 0.003 | 0.029 | 1.029* | 0.142 | 0.216 | 0.684 | 0.797 | |
X  | 1 | 2.6 | 0.017 | 0.139 | 0.002 | 0.006 | 0.001 | 0.001 | 0.753 | 0.283 | 1.812* | |
| Interaction | X1 · X2 | 1 | 30.35 | 0.086 | 0.272 | 0.281 | 0.521 | 0.102 | 0.278 | 0.658 | 0.339 | 1.026*** |
| X1 · X3 | 1 | 0.97 | 0.005 | 0.099 | 0.062 | 0.536 | 0.108 | 0.190 | 0.047 | 0.215 | 0.337 | |
| X1 · X4 | 1 | 23.85 | 0.001 | 0.021 | 0.002 | 0.003 | 0.004 | 0.001 | 0.076 | 0.282 | 0.414* | |
| X2 · X3 | 1 | 0.29 | 0.002 | 0.166 | 0.051 | 0.352 | 0.021 | 0.028 | 0.004 | 0.460 | 0.223 | |
| X2 · X4 | 1 | 4.28 | 0.006 | 0.03 | 0.285 | 0.001 | 0.032 | 0.058 | 0.027 | 0.077 | 0.033 | |
| X3 · X4 | 1 | 21.93 | 0.017 | 0.495 | 0.264 | 0.259 | 0.158 | 0.019 | 1.146** | 0.622* | 1.184** | |
| Variability explained (R2) | 0.987 | 0.976 | 0.971 | 0.938 | 0.940 | 0.945 | 0.973 | 0.964 | 0.981 | 0.989 | ||
| Source . | . | DF1 . | Sum of squares . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS2 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||||
| Colour . | Aroma . | Appearance . | OAA3 . | TPC4 . | YMC5 . | PC6 . | ||||||
| Model | 14 | 2169*** | 1.862*** | 27.487*** | 13.478*** | 10.447*** | 8.97*** | 8.822*** | 15.719*** | 27.772*** | 26.438*** | |
| Linear | X1 | 1 | 34.15* | 0.009 | 0.186 | 0 | 0.001 | 0.072 | 0.009 | 0.004 | 0.204 | 0.138 |
| X3 | 1 | 0.14 | 0.012 | 0.004 | 1.652 | 0.442 | 0.026 | 0.066 | 0.106 | 0.022 | 0.096 | |
| X3 | 1 | 0.34 | 0.171** | 1.596* | 1.509 | 0.288* | 0.704* | 0.096 | 2.882*** | 6.310*** | 4.172*** | |
| X4 | 1 | 2040*** | 1.507*** | 23.513*** | 9.166*** | 7.872*** | 6.519*** | 7.849*** | 9.714*** | 17.554*** | 15.194*** | |
| Quadratic | X  | 1 | 9.02 | 0.023 | 0.023 | 0.049 | 0.087 | 0.132 | 0.084 | 0.003 | 0.630 | 0.059 |
X  | 1 | 0.22 | 0.001 | 0.067 | 0.155 | 0.050 | 0.064 | 0.001 | 0.084 | 0.084 | 0.947 | |
X  | 1 | 1.03 | 0.006 | 0.876 | 0.003 | 0.029 | 1.029* | 0.142 | 0.216 | 0.684 | 0.797 | |
X  | 1 | 2.6 | 0.017 | 0.139 | 0.002 | 0.006 | 0.001 | 0.001 | 0.753 | 0.283 | 1.812* | |
| Interaction | X1 · X2 | 1 | 30.35 | 0.086 | 0.272 | 0.281 | 0.521 | 0.102 | 0.278 | 0.658 | 0.339 | 1.026*** |
| X1 · X3 | 1 | 0.97 | 0.005 | 0.099 | 0.062 | 0.536 | 0.108 | 0.190 | 0.047 | 0.215 | 0.337 | |
| X1 · X4 | 1 | 23.85 | 0.001 | 0.021 | 0.002 | 0.003 | 0.004 | 0.001 | 0.076 | 0.282 | 0.414* | |
| X2 · X3 | 1 | 0.29 | 0.002 | 0.166 | 0.051 | 0.352 | 0.021 | 0.028 | 0.004 | 0.460 | 0.223 | |
| X2 · X4 | 1 | 4.28 | 0.006 | 0.03 | 0.285 | 0.001 | 0.032 | 0.058 | 0.027 | 0.077 | 0.033 | |
| X3 · X4 | 1 | 21.93 | 0.017 | 0.495 | 0.264 | 0.259 | 0.158 | 0.019 | 1.146** | 0.622* | 1.184** | |
| Variability explained (R2) | 0.987 | 0.976 | 0.971 | 0.938 | 0.940 | 0.945 | 0.973 | 0.964 | 0.981 | 0.989 | ||
***Significant at P ≤ 0.01, **significant at P ≤ 0.05,*significant at P ≤ 0.1.
1DF: degrees of freedom; 2total soluble solids; 3overall acceptability; 4total plate count; 5yeast and mould count; 6total psychrophilic count.
Analysis of variance of regression models for physicochemical and sensory attributes
| Source . | . | DF1 . | Sum of squares . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS2 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||||
| Colour . | Aroma . | Appearance . | OAA3 . | TPC4 . | YMC5 . | PC6 . | ||||||
| Model | 14 | 2169*** | 1.862*** | 27.487*** | 13.478*** | 10.447*** | 8.97*** | 8.822*** | 15.719*** | 27.772*** | 26.438*** | |
| Linear | X1 | 1 | 34.15* | 0.009 | 0.186 | 0 | 0.001 | 0.072 | 0.009 | 0.004 | 0.204 | 0.138 |
| X3 | 1 | 0.14 | 0.012 | 0.004 | 1.652 | 0.442 | 0.026 | 0.066 | 0.106 | 0.022 | 0.096 | |
| X3 | 1 | 0.34 | 0.171** | 1.596* | 1.509 | 0.288* | 0.704* | 0.096 | 2.882*** | 6.310*** | 4.172*** | |
| X4 | 1 | 2040*** | 1.507*** | 23.513*** | 9.166*** | 7.872*** | 6.519*** | 7.849*** | 9.714*** | 17.554*** | 15.194*** | |
| Quadratic | X  | 1 | 9.02 | 0.023 | 0.023 | 0.049 | 0.087 | 0.132 | 0.084 | 0.003 | 0.630 | 0.059 |
X  | 1 | 0.22 | 0.001 | 0.067 | 0.155 | 0.050 | 0.064 | 0.001 | 0.084 | 0.084 | 0.947 | |
X  | 1 | 1.03 | 0.006 | 0.876 | 0.003 | 0.029 | 1.029* | 0.142 | 0.216 | 0.684 | 0.797 | |
X  | 1 | 2.6 | 0.017 | 0.139 | 0.002 | 0.006 | 0.001 | 0.001 | 0.753 | 0.283 | 1.812* | |
| Interaction | X1 · X2 | 1 | 30.35 | 0.086 | 0.272 | 0.281 | 0.521 | 0.102 | 0.278 | 0.658 | 0.339 | 1.026*** |
| X1 · X3 | 1 | 0.97 | 0.005 | 0.099 | 0.062 | 0.536 | 0.108 | 0.190 | 0.047 | 0.215 | 0.337 | |
| X1 · X4 | 1 | 23.85 | 0.001 | 0.021 | 0.002 | 0.003 | 0.004 | 0.001 | 0.076 | 0.282 | 0.414* | |
| X2 · X3 | 1 | 0.29 | 0.002 | 0.166 | 0.051 | 0.352 | 0.021 | 0.028 | 0.004 | 0.460 | 0.223 | |
| X2 · X4 | 1 | 4.28 | 0.006 | 0.03 | 0.285 | 0.001 | 0.032 | 0.058 | 0.027 | 0.077 | 0.033 | |
| X3 · X4 | 1 | 21.93 | 0.017 | 0.495 | 0.264 | 0.259 | 0.158 | 0.019 | 1.146** | 0.622* | 1.184** | |
| Variability explained (R2) | 0.987 | 0.976 | 0.971 | 0.938 | 0.940 | 0.945 | 0.973 | 0.964 | 0.981 | 0.989 | ||
| Source . | . | DF1 . | Sum of squares . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS2 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||||
| Colour . | Aroma . | Appearance . | OAA3 . | TPC4 . | YMC5 . | PC6 . | ||||||
| Model | 14 | 2169*** | 1.862*** | 27.487*** | 13.478*** | 10.447*** | 8.97*** | 8.822*** | 15.719*** | 27.772*** | 26.438*** | |
| Linear | X1 | 1 | 34.15* | 0.009 | 0.186 | 0 | 0.001 | 0.072 | 0.009 | 0.004 | 0.204 | 0.138 |
| X3 | 1 | 0.14 | 0.012 | 0.004 | 1.652 | 0.442 | 0.026 | 0.066 | 0.106 | 0.022 | 0.096 | |
| X3 | 1 | 0.34 | 0.171** | 1.596* | 1.509 | 0.288* | 0.704* | 0.096 | 2.882*** | 6.310*** | 4.172*** | |
| X4 | 1 | 2040*** | 1.507*** | 23.513*** | 9.166*** | 7.872*** | 6.519*** | 7.849*** | 9.714*** | 17.554*** | 15.194*** | |
| Quadratic | X  | 1 | 9.02 | 0.023 | 0.023 | 0.049 | 0.087 | 0.132 | 0.084 | 0.003 | 0.630 | 0.059 |
X  | 1 | 0.22 | 0.001 | 0.067 | 0.155 | 0.050 | 0.064 | 0.001 | 0.084 | 0.084 | 0.947 | |
X  | 1 | 1.03 | 0.006 | 0.876 | 0.003 | 0.029 | 1.029* | 0.142 | 0.216 | 0.684 | 0.797 | |
X  | 1 | 2.6 | 0.017 | 0.139 | 0.002 | 0.006 | 0.001 | 0.001 | 0.753 | 0.283 | 1.812* | |
| Interaction | X1 · X2 | 1 | 30.35 | 0.086 | 0.272 | 0.281 | 0.521 | 0.102 | 0.278 | 0.658 | 0.339 | 1.026*** |
| X1 · X3 | 1 | 0.97 | 0.005 | 0.099 | 0.062 | 0.536 | 0.108 | 0.190 | 0.047 | 0.215 | 0.337 | |
| X1 · X4 | 1 | 23.85 | 0.001 | 0.021 | 0.002 | 0.003 | 0.004 | 0.001 | 0.076 | 0.282 | 0.414* | |
| X2 · X3 | 1 | 0.29 | 0.002 | 0.166 | 0.051 | 0.352 | 0.021 | 0.028 | 0.004 | 0.460 | 0.223 | |
| X2 · X4 | 1 | 4.28 | 0.006 | 0.03 | 0.285 | 0.001 | 0.032 | 0.058 | 0.027 | 0.077 | 0.033 | |
| X3 · X4 | 1 | 21.93 | 0.017 | 0.495 | 0.264 | 0.259 | 0.158 | 0.019 | 1.146** | 0.622* | 1.184** | |
| Variability explained (R2) | 0.987 | 0.976 | 0.971 | 0.938 | 0.940 | 0.945 | 0.973 | 0.964 | 0.981 | 0.989 | ||
***Significant at P ≤ 0.01, **significant at P ≤ 0.05,*significant at P ≤ 0.1.
1DF: degrees of freedom; 2total soluble solids; 3overall acceptability; 4total plate count; 5yeast and mould count; 6total psychrophilic count.
Regression coefficients for full second-order equation of physicochemical and sensory characteristics of minimally processed litchi stored at 4 ± 2 °C
| Coefficient . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS1 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| β0 | 12.177*** | 4.259*** | 15.411*** | 7.557*** | 7.594*** | 7.968*** | 7.706*** | 4.883*** | 4.977*** | 4.662*** |
| β1 | −1.943* | 0.045 | 0.116 | 0.027 | 0.132 | −0.036 | 0.041 | 0.027 | −0.149 | −0.132 |
| β2 | −0.257 | 0.037 | −0.005 | 0.336* | −0.062 | 0.047 | 0.107 | 0.111 | 0.076 | 0.165 |
| β3 | −0.962 | 0.104** | −0.326* | −0.209 | −0.292* | 0.269* | −0.077 | 0.322*** | 0.637*** | 0.449*** |
| β4 | 12.081*** | 0.331*** | −1.184*** | −0.843*** | −0.723*** | −0.607*** | −0.724*** | 0.819*** | 1.045*** | 1.051*** |
| β11 | 1.645 | 0.071 | −0.123 | −0.233 | 0.064 | −0.000 | −0.056 | 0.311 | −0.285 | 0.394 |
| β22 | −0.385 | 0.051 | −0.413 | 0.173 | 0.021 | −0.042 | 0.051 | 0.123 | 0.498 | −0.106 |
| β33 | −0.366 | 0.023 | 0.498 | 0.135 | −0.340 | −0.641* | −0.2827 | −0.302 | −0.447 | −0.485* |
| β44 | 1.536 | 0.076 | 0.377 | −0.038 | −0.024 | 0.061 | −0.001 | −0.546 | −0.438 | −.856*** |
| β12 | 0.869 | −0.067 | −0.037 | −0.076 | −0.304 | −0.028 | −0.136 | 0.066 | 0.043 | 0.123 |
| β13 | 0.641 | 0.008 | 0.127 | −0.019 | 0.232 | 0.071 | 0.094 | 0.096 | 0.092 | 0.207* |
| β14 | −1.406 | 0.001 | −0.027 | 0.046 | −0.084 | −0.043 | −0.027 | −0.094 | −0.045 | −0.115 |
| β23 | −0.185 | 0.014 | 0.142 | −0.083 | 0.209 | 0.050 | 0.059 | 0.024 | −0.237 | −0.164 |
| β24 | −0.72 | 0.026 | −0.063 | 0.198 | −0.006 | 0.061 | 0.085 | −0.056 | 0.099 | −0.062 |
| β34 | 1.649 | −0.046 | −0.248 | −0.185 | 0.179 | −0.139 | −0.048 | 0.377** | 0.277* | 0.383** |
| Coefficient . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS1 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| β0 | 12.177*** | 4.259*** | 15.411*** | 7.557*** | 7.594*** | 7.968*** | 7.706*** | 4.883*** | 4.977*** | 4.662*** |
| β1 | −1.943* | 0.045 | 0.116 | 0.027 | 0.132 | −0.036 | 0.041 | 0.027 | −0.149 | −0.132 |
| β2 | −0.257 | 0.037 | −0.005 | 0.336* | −0.062 | 0.047 | 0.107 | 0.111 | 0.076 | 0.165 |
| β3 | −0.962 | 0.104** | −0.326* | −0.209 | −0.292* | 0.269* | −0.077 | 0.322*** | 0.637*** | 0.449*** |
| β4 | 12.081*** | 0.331*** | −1.184*** | −0.843*** | −0.723*** | −0.607*** | −0.724*** | 0.819*** | 1.045*** | 1.051*** |
| β11 | 1.645 | 0.071 | −0.123 | −0.233 | 0.064 | −0.000 | −0.056 | 0.311 | −0.285 | 0.394 |
| β22 | −0.385 | 0.051 | −0.413 | 0.173 | 0.021 | −0.042 | 0.051 | 0.123 | 0.498 | −0.106 |
| β33 | −0.366 | 0.023 | 0.498 | 0.135 | −0.340 | −0.641* | −0.2827 | −0.302 | −0.447 | −0.485* |
| β44 | 1.536 | 0.076 | 0.377 | −0.038 | −0.024 | 0.061 | −0.001 | −0.546 | −0.438 | −.856*** |
| β12 | 0.869 | −0.067 | −0.037 | −0.076 | −0.304 | −0.028 | −0.136 | 0.066 | 0.043 | 0.123 |
| β13 | 0.641 | 0.008 | 0.127 | −0.019 | 0.232 | 0.071 | 0.094 | 0.096 | 0.092 | 0.207* |
| β14 | −1.406 | 0.001 | −0.027 | 0.046 | −0.084 | −0.043 | −0.027 | −0.094 | −0.045 | −0.115 |
| β23 | −0.185 | 0.014 | 0.142 | −0.083 | 0.209 | 0.050 | 0.059 | 0.024 | −0.237 | −0.164 |
| β24 | −0.72 | 0.026 | −0.063 | 0.198 | −0.006 | 0.061 | 0.085 | −0.056 | 0.099 | −0.062 |
| β34 | 1.649 | −0.046 | −0.248 | −0.185 | 0.179 | −0.139 | −0.048 | 0.377** | 0.277* | 0.383** |
***Significant at P ≤ 0.01; **significant at P ≤ 0.05, *significant at P ≤ 0.1.
1total soluble solids; 2overall acceptability; 3total plate count; 4yeast and mould count; 5total psychrophilic count.
Regression coefficients for full second-order equation of physicochemical and sensory characteristics of minimally processed litchi stored at 4 ± 2 °C
| Coefficient . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS1 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| β0 | 12.177*** | 4.259*** | 15.411*** | 7.557*** | 7.594*** | 7.968*** | 7.706*** | 4.883*** | 4.977*** | 4.662*** |
| β1 | −1.943* | 0.045 | 0.116 | 0.027 | 0.132 | −0.036 | 0.041 | 0.027 | −0.149 | −0.132 |
| β2 | −0.257 | 0.037 | −0.005 | 0.336* | −0.062 | 0.047 | 0.107 | 0.111 | 0.076 | 0.165 |
| β3 | −0.962 | 0.104** | −0.326* | −0.209 | −0.292* | 0.269* | −0.077 | 0.322*** | 0.637*** | 0.449*** |
| β4 | 12.081*** | 0.331*** | −1.184*** | −0.843*** | −0.723*** | −0.607*** | −0.724*** | 0.819*** | 1.045*** | 1.051*** |
| β11 | 1.645 | 0.071 | −0.123 | −0.233 | 0.064 | −0.000 | −0.056 | 0.311 | −0.285 | 0.394 |
| β22 | −0.385 | 0.051 | −0.413 | 0.173 | 0.021 | −0.042 | 0.051 | 0.123 | 0.498 | −0.106 |
| β33 | −0.366 | 0.023 | 0.498 | 0.135 | −0.340 | −0.641* | −0.2827 | −0.302 | −0.447 | −0.485* |
| β44 | 1.536 | 0.076 | 0.377 | −0.038 | −0.024 | 0.061 | −0.001 | −0.546 | −0.438 | −.856*** |
| β12 | 0.869 | −0.067 | −0.037 | −0.076 | −0.304 | −0.028 | −0.136 | 0.066 | 0.043 | 0.123 |
| β13 | 0.641 | 0.008 | 0.127 | −0.019 | 0.232 | 0.071 | 0.094 | 0.096 | 0.092 | 0.207* |
| β14 | −1.406 | 0.001 | −0.027 | 0.046 | −0.084 | −0.043 | −0.027 | −0.094 | −0.045 | −0.115 |
| β23 | −0.185 | 0.014 | 0.142 | −0.083 | 0.209 | 0.050 | 0.059 | 0.024 | −0.237 | −0.164 |
| β24 | −0.72 | 0.026 | −0.063 | 0.198 | −0.006 | 0.061 | 0.085 | −0.056 | 0.099 | −0.062 |
| β34 | 1.649 | −0.046 | −0.248 | −0.185 | 0.179 | −0.139 | −0.048 | 0.377** | 0.277* | 0.383** |
| Coefficient . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drip loss . | pH . | TSS1 . | Sensory score . | Microbiological counts (log cfu/g) . | ||||||
| Colour . | Aroma . | Appearance . | OAA2 . | TPC3 . | YMC4 . | PC5 . | ||||
| β0 | 12.177*** | 4.259*** | 15.411*** | 7.557*** | 7.594*** | 7.968*** | 7.706*** | 4.883*** | 4.977*** | 4.662*** |
| β1 | −1.943* | 0.045 | 0.116 | 0.027 | 0.132 | −0.036 | 0.041 | 0.027 | −0.149 | −0.132 |
| β2 | −0.257 | 0.037 | −0.005 | 0.336* | −0.062 | 0.047 | 0.107 | 0.111 | 0.076 | 0.165 |
| β3 | −0.962 | 0.104** | −0.326* | −0.209 | −0.292* | 0.269* | −0.077 | 0.322*** | 0.637*** | 0.449*** |
| β4 | 12.081*** | 0.331*** | −1.184*** | −0.843*** | −0.723*** | −0.607*** | −0.724*** | 0.819*** | 1.045*** | 1.051*** |
| β11 | 1.645 | 0.071 | −0.123 | −0.233 | 0.064 | −0.000 | −0.056 | 0.311 | −0.285 | 0.394 |
| β22 | −0.385 | 0.051 | −0.413 | 0.173 | 0.021 | −0.042 | 0.051 | 0.123 | 0.498 | −0.106 |
| β33 | −0.366 | 0.023 | 0.498 | 0.135 | −0.340 | −0.641* | −0.2827 | −0.302 | −0.447 | −0.485* |
| β44 | 1.536 | 0.076 | 0.377 | −0.038 | −0.024 | 0.061 | −0.001 | −0.546 | −0.438 | −.856*** |
| β12 | 0.869 | −0.067 | −0.037 | −0.076 | −0.304 | −0.028 | −0.136 | 0.066 | 0.043 | 0.123 |
| β13 | 0.641 | 0.008 | 0.127 | −0.019 | 0.232 | 0.071 | 0.094 | 0.096 | 0.092 | 0.207* |
| β14 | −1.406 | 0.001 | −0.027 | 0.046 | −0.084 | −0.043 | −0.027 | −0.094 | −0.045 | −0.115 |
| β23 | −0.185 | 0.014 | 0.142 | −0.083 | 0.209 | 0.050 | 0.059 | 0.024 | −0.237 | −0.164 |
| β24 | −0.72 | 0.026 | −0.063 | 0.198 | −0.006 | 0.061 | 0.085 | −0.056 | 0.099 | −0.062 |
| β34 | 1.649 | −0.046 | −0.248 | −0.185 | 0.179 | −0.139 | −0.048 | 0.377** | 0.277* | 0.383** |
***Significant at P ≤ 0.01; **significant at P ≤ 0.05, *significant at P ≤ 0.1.
1total soluble solids; 2overall acceptability; 3total plate count; 4yeast and mould count; 5total psychrophilic count.
The Anova of regression models for all the attributes indicated that the models were significant (P ≤ 0.1). The coefficients of determination (R2) was higher than 0.80 and hence the models explained the variability of experimental data satisfactorily (Filmore et al., 1976; Joglekar & May, 1987). Based on all these tests, the prediction models were accepted. The statistical significance of each effect is shown in Table 3.
Physicochemical attributes
Initially drip losses (mL/100 g) associated with minimally processed litchis was 0.0–6.20 mL (Table 2). There was no initial drip loss in samples packed at atmospheric pressure (Exp. I, K and M) but packing under high in-package vacuum caused shrinkage and crushing of arils and hence it resulted losses (Exp. D, E and J) even on 0 days of storage. After 20 days of storage, drip loss was minimum (19.6 mL/100 g) in arils given treatment H and maximum (33.0 mL/100 g) for treatment O (Table 2). Drip losses increased significantly (P ≤ 0.01) with increase in storage time (X4) or decrease in concentration of CL (Table 4). Effect of increase in concentration of X1 (CL) in the dipping solution on reducing drip losses of stored samples was found to be significant (P ≤ 0.1) and negative (Table 4, Fig. 1a) at linear terms. The effect of X2 and X3 was found to be non-significant at all terms. Drip losses may occur because of loss of textural integrity of tissues during storage because of mechanical damage or as a result of biochemical alterations at the cell wall, middle lamella and membrane levels (Garcia & Barrett, 2002). Reduction in drip losses upon increase in calcium lactate concentration has been attributed to the formation of calcium pectate which has firming action upon the tissues of fruits (Poovaiah, 1986). Effect of calcium lactate firming has also been reported in fresh-cut Bosc pears by Dong et al. (2000). The pH of litchis on 0 day was 4.65–4.75 which reduced to 3.65–4.3 after 20 days of storage (Table 2); maximum change was found in samples given treatment O (pH 3.68) and minimum in treatment H (pH 4.27). pH was found to decrease significantly (P ≤ 0.01) with increase in storage time (X4) as shown by positive coefficient of regression at linear terms (Table 4) and negatively to in-package vacuum (P ≤ 0.05) at linear terms (Table 4, Fig. 1b). X1 and X2 showed no significant effect on pH changes either at linear, quadratic or interactive level (Table 4).
Contour plots of physicochemical attributes as related to independent variable (X1, CL; X2, 4-HR, X3, in-package vacuum; X4, storage period) for minimally processed litchis stored at 4 ± 2 °C. Variables are represented in coded forms.
Initial TSS of litchis was 17 °Brix which changed to 13.8–15.8 °Brix during 20 days of storage (Table 2). After 20 days, decrease in TSS was maximum (18.8%) in treatments O, G and F (TSS 13.8 °Brix), whereas it was minimum (9.4%) in samples given treatment L (TSS 15.4 °Brix). With increase in storage time (X4), TSS of litchis decreased significantly (P ≤ 0.01) (Table 4). Increased in-package vacuum reduced these changes significantly (P ≤ 0.1) (Table 4, Fig. 1c). There was no effect of X1 and X2 on changes in TSS either at linear, quadratic or interactive level (Table 4).
Sensory quality
Minimally processed litchis had excellent colour (sensory score 8.5) initially. But this score dropped significantly (P ≤ 0.01) to 8.3–7.1 and 7.8–5.8 after 10 and 20 days of storage, respectively (Table 2). After 20 days, maximum colour score was obtained for treatment H and minimum (5.8) for treatments O and Q (Table 2). Increase in concentration of 4-HR (X2) in dipping solution significantly (P ≤ 0.1) improved the colour scores for litchis during storage as shown by positive coefficient of regression for linear terms (Table 4, Fig. 2a). Colour scores decreased during storage because of browning and incorporation of 4-HR inhibited this change. Dong et al. (2000) had also found 4-HR to be an effective browning inhibitor in fresh-sliced pears.
Contour plots of sensory attributes as related to independent variable (X1, CL; X2, 4-HR, X3, in-package vacuum; X4, storage period) for minimally processed litchis stored at 4 ± 2 °C. Variables are represented in coded forms.
Aroma scores for minimally processed litchi arils were initially 7.6–8.5 which dropped to 5.8–7.1 after 20 days of storage (Table 2) which was found to be significant at P ≤ 0.01 (Table 4). Aroma scores were significantly (P ≤ 0.1) improved with increase in in-package vacuum upto 47409.3 Pa but further increase in vacuum decreased the scores (Fig. 2b). The panelists reported lack of litchi aroma because of loss of aroma but did not notice development of off-odours.
At the start of storage studies (0 day), appearance of the minimally processed litchis was rated as very good and the scores ranged from 7.5 to 8.6 (Table 2) with samples packed under high in-package vacuum getting score <8.0. The appearance scores reduced significantly (P ≤ 0.01) to 6.3–7.5 during 20 days of storage (Tables 2 and 4). The appearance scores were found to improve with increase in in-package vacuum upto 47409.3 Pa but further increase decreased appearance score (Fig 2c). Samples packed under high vacuum (67727.76 Pa) had a crushed appearance.
Minimally processed litchis were highly acceptable [overall acceptability (OAA) 7.9–8.43] on day 0 which reduced significantly (P ≤ 0.01) during storage (Tables 2 and 4). After 20 days storage, OAA was maximum (7.3) for litchis given treatment R and minimum (6.13) for treatment O. Except for storage period (X4), other independent variables (X1, X2 and X3) did not exert any significant effect on OAA (Table 4).
Microbiological quality
Initial total plate count of minimally processed litchis was 3.258–4.017 log cfu/g which increased to 4.219–6.135 log cfu/g during 20 days storage (Table 2); maximum increase was for treatment Q (6.133 log cfu/g) and minimum for treatment H (4.129 log cfu/g) (Table 2). These counts were below the maximum microbial count of 7.7 log cfu/g allowed for minimally processed fruits and vegetables (Francis et al., 1999). Changes in YMC were very similar to TPC and after 20 days of storage maximum YMC (6.756 log cfu/g) was observed in litchis given treatment P and minimum (3.66 log cfu/g) yeast and mould growth was observed in treatment L (Table 2). No visible mould growth was observed in any sample during storage. Psychrophiles increased from an initial count of 2.441–3.025 log cfu/g to 2.913–5.763 log cfu/g during 20 days of storage (Table 2). Minimum PC (2.913 log cfu/g) was observed in litchis given treatment L whereas the maximum count (5.763 log cfu/g) was for treatment O. Lower psychrophilic population is accounted for higher stability of refrigerated cut produce (Garg et al., 1990). Changes in TPC and in PC during storage followed similar pattern as reported earlier by Gimenez et al. (2003) for minimally processed borage. Changes in microbial counts during storage were found to be significant (P ≤ 0.01) and positively correlated to storage period (X4) (Table 4). Increase in in-package vacuum was found to exert significant negative (P ≤ 0.01) effect on the microbial growth (Table 4, Fig 3a–c). PC was also found to be effected by X3 and X4 at quadratic terms (Table 4). X3 and X4 showed a significant (P ≤ 0.05) interactive effect on all the counts. Other factors (X1 and X2) did not show any significant effect on microbial growth. Microbial growth in minimally processed litchis during storage packages was accompanied by decrease in pH, total soluble solids and loss of sensory qualities. Similar results have been reported for minimally processed carrots and apples (Soliva-Fortuny et al., 2002; Tassou & Boziaris, 2002).
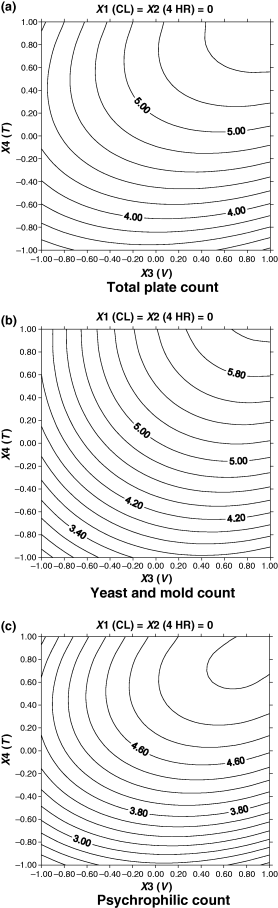
Contour plots of microbiological attributes as related to independent variable (X1, CL; X2, 4-HR, X3, in-package vacuum; X4, storage period) for minimally processed litchis stored at 4 ± 2 °C. Variables are represented in coded forms.
Conclusions
Litchis with brown pericarp but wholesome aril can be minimally processed successfully. The second order polynomial models used to predict the effect of variables (CL, 4-HR, in-package vacuum and storage period) on physico-chemical, sensory and microbiological responses of minimally processed litchis were found to be adequate. Quality of minimally processed litchis decreased during storage; CL reduced drip losses significantly and sensory colour scores were improved with addition of 4-HR. In-package vacuum influenced all the responses except drip losses, sensory colour and overall acceptability. In-package vacuum above 47409.3 Pa impaired the appearance and aroma of minimally processed litchis.



