-
PDF
- Split View
-
Views
-
Cite
Cite
Iosif Gulkarov, Subroto Paul, Nasser K. Altorki, Paul C. Lee, Use of Amplatzer device for endobronchial closure of bronchopleural fistulas, Interactive CardioVascular and Thoracic Surgery, Volume 9, Issue 5, November 2009, Pages 901–902, https://doi.org/10.1510/icvts.2009.215202
Close - Share Icon Share
Abstract
Postpneumonectomy bronchopleural fistulas (BPFs) remain difficult management problems associated with considerable morbidity and mortality. Traditional therapies have included primary repair or delayed closure with the creation of an Eloesser cavity and tissue flap reinforcement. New bronchoscopic modalities have included the use of bioglues, stents, and coils. We describe another additional, less invasive bronchoscopic modality – the use of an atrial septal closure device.
1. Introduction
Postpneumonectomy bronchopleural fistulas (BPFs) represent difficult management problems and are associated with very high mortality and morbidity. The treatment of BPF is varied and dependent on the size of the BPF as well as its time course in relation to surgery. Traditional surgical therapies have included primary or delayed closure with creation of an Eloesser cavity with or without omental or muscle flap reinforcement. Newer less invasive techniques utilize bronchoscopy to deliver bioadhesives, stents, or coils for BPF closure. Use of the Amplatzer device, which is commonly used for transcatheter closure of atrial septal defects (ASDs), is one such technique that has been recently described [1,2]. We highlight another case in which use of the Amplatzer device was instrumental in weaning a patient with a large BPF after a right pneumonectomy off the ventilator.
2. Case report
Our patient (T.Z.) is a 68-year-old male with a history of T2N0 squamous cell carcinoma of the right upper and middle lobes treated by a sleeve bilobectomy in 1984, head and neck cancer for which he received chemotherapy and radiation in 1995, who underwent right completion pneumonectomy for recurrent squamous cell carcinoma. The recurrent tumor was ∼1.5 cm distal to the right carina into the right lower lobe. His postoperative course was complicated by persistent leukocytosis. Two weeks after surgery, chest X-ray revealed a decrease in the right chest air fluid level. Bronchoscopy revealed a 3-mm opening in the middle of the right bronchial stump. Initial treatment with endobronchial alcohol injection, chest tube drainage, and antibiotic irrigation failed and the patient underwent the creation of an Eloesser flap. Despite dressing changes for six weeks and nutritional support with jejunal feedings, the bronchial stump remained widely patent (Fig. 1a ). The patient developed pneumonia and respiratory failure. He was emergently intubated and was septic on vasopressors for hemodynamic support. With worsening oxygenation and higher peak pressures, the patient was unable to be ventilated with a single lumen tube due to volume loss from the BPF. A double lumen endotracheal tube was placed but the patient remained septic. As the position of the double lumen was precarious with need for constant adjustment, a decision was made to attempt to seal the BPF, at least temporarily, to simplify respiratory management. A 10-mm Amplatzer septal occluder device (AGA Medical, Golden Valley, MN) was placed through the Eloesser flap with a guidewire and deployed. Bioglue surgical adhesive (Cryolife, Inc, Kennesaw, GA) was then used to obtain a complete seal of the BPF (Fig. 1b). Intra-operatively, there was no evidence of an airleak and the patient underwent tracheostomy. Within the ensuing week, the patient was weaned to tracheostomy collar and had resolution of his leukocytosis and need for vasopressor support. Three weeks later, the patient required another bronchoscopy with application of the bioglue to re-establish his seal. The patient's BPF remains completely closed with the granulation tissue developing around the device. With continued nutritional support and development of granulation tissue, omental and lattismus muscle flap closure of the Eloesser cavity is planned within the ensuing year. He was then subsequently discharged from the hospital a month from the insertion of the device to a rehabilitation facility.
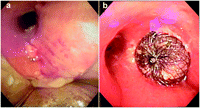
(a) Endobronchial view of bronchopleural fistula. (b) Endobronchial view of the deployed Amplatzer septal occluder device.
3. Discussion
This is the third report published to date demonstrating the use of a novel technique for endobronchial closure of BPF by the implantation of an Amplatzer ASD occlude [1,2]. The Amplatzer device has traditionally been employed by interventional cardiologists to close atrial septal defects. The Amplatzer is a self-expanding double disk made from nitinol wire mesh. The disks are joined together by a connecting mesh tube, which acts to stent the defect. Polyester patches are sewn within the disks and central stent, which serve to occlude blood flow through the device. The waist portion also serves to self-center the device during deployment. The waist size varies from 4 to 40 mm so that the occluder can be appropriately matched to the size of the defect.
The technique described appears to be very useful in patients who fail conservative therapy. Other devices are available for attempted BPF closure such as collagen plugs and endobronchial valves and stents [3–5]. However, such devices are either not available commercially, not large enough to entirely cover a large mainstem bronchial breakdown, or have complicated delivery systems.
In contrast to two previous case reports [1,2], we applied the occluder device in an intubated patient and therefore, the technique had to be slightly modified. Positive pressure ventilation led to an air leak through the device; and hence ineffective ventilation. The addition of bioglue resolved this issue. The device provided the framework for bioglue preventing it from spilling into the pleural space or the contralateral bronchi. The procedure was well tolerated by the patient and facilitated his weaning from the ventilator. As the patient continued to develop granulation tissue around the device, flap closure of the cavity is planned once the nutritional status of the patient improves. The long-term results of this application of the device are unknown.
4. Conclusion
This case report highlights the utility of the Amplatzer device to facilitate the closure of BPFs in marginal patients who fail conservative therapy.




