-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Syburra, Oliver Reuthebuch, Kirk Graves, Michele Genoni, Aortic posterior wall perforation with automatic aortic cutter during routine off-pump coronary bypass grafting, Interactive CardioVascular and Thoracic Surgery, Volume 9, Issue 5, November 2009, Pages 840–841, https://doi.org/10.1510/icvts.2009.211078
Close - Share Icon Share
Abstract
Aortic complications are very rare during off-pump coronary artery bypass grafting (OPCAB). When they occur, the mortality is high. We report a case of perforation of the posterior aortic wall after punching out the hole in the ascending aorta with an automatic aortic cutter to avoid clamping for the proximal anastomosis during a routine OPCAB procedure. The consequence was a massive hemorrhage, emergency conversion to cardiopulmonary bypass and replacement of the aortic valve and of the ascending aorta.
1. Introduction
Aortic complications are very rare during off-pump coronary artery bypass grafting (OPCAB) [1,2]. Nevertheless, they carry a high mortality. Emphasizing the importance of a no-touch technique of the aorta during OPCAB, we perform the proximal anastomosis with a no-clamp technique using an aortic cutter (Maquet, Germany; a Getinge Group Company, Sweden) and the Heartstring II Proximal Seal System (Maquet, Germany; a Getinge Group Company, Sweden), in combination with 6/0 Prolene (Ethicon, USA) running suture. We report a case of perforation of the posterior aortic wall that occurred while punching out the hole in the ascending aorta for the proximal anastomosis in a routine OPCAB procedure.
2. Case report
A 68-year-old man with stable angina (CCS II-III), well compensated, was referred to our center for further investigations. The patient's risk factors included dyslipidemia and arterial hypertension, with a standard EuroSCORE of 2. His weight was 86 kg, height 170 cm, body mass index 30 kg/m2 and body surface area 2.04 m2. His medical history was uneventful. Preoperative coronary angiography revealed a three-vessel disease, with a 75% proximal left main (LM) stenosis, a 70% medial and 75% distal left anterior descending (LAD) stenosis, a 70% proximal and medial circumflex (CX) stenosis, and a 75% proximal and medial right coronary artery (RCA) stenosis. The ventriculography showed a lateral and inferior hypokinesia with an akinetic apex. The left ventricular ejection fraction (LVEF) was normal (55%) and the cardiac chambers had normal dimensions. Minimal aortic valve pathology was shown during echocardiographic assessment, with discrete thickening of the cusps, a mean transvalvular gradient of 11 mmHg, a peak gradient of 20 mmHg, and a minimal aortic regurgitation.
Complete OPCAB revascularization was performed. The left internal mammary artery (LIMA) was anastomosed to the LAD. The right internal mammary artery (RIMA) was grafted as T-graft from the LIMA to the CX. One saphenous vein graft (SVG) was anastomosed to the posterior descending artery (PDA), a second SVG was anastomosed to the first diagonal artery (1st DA) of the LAD. Both proximal anastomoses to the ascending aorta were performed with the aortic cutter and the Heartstring II Proximal Seal System without side-clamping of the aorta. The quality of the anastomoses was controlled using transient time flow measurement (Medistim, Norway), which showed good values. The initial intraoperative course was uneventful and all hemodynamic parameters within normal range with 10 μg/min norepinephrine support.
Still in the operating theatre, the patient suffered an acute hemodynamic instability with marked blood volume loss. We identified the origin of the hemorrhage in the posterior wall of the ascending aorta and converted the procedure to emergency cardiopulmonary bypass support, with mild hypothermia to 32 °C. Subsequent inspection revealed damage to the posterior wall of the ascending aorta, that could not be fixed from the outside. We performed a transverse aortotomy, a sharp delimited round hole in the posterior wall of the ascending aorta was identified opposite the second proximal anastomosis (Fig. 1 ). There were no further vascular lesions. Furthermore, the aortic valve was much more severely calcified than described in the echocardiographic findings, and we decided to perform a replacement with a tissue valve (Mosaic Ultra bioprosthesis 25 mm, Medtronic, USA). The valve was sutured using Ti-Cron (Covidien Ltd, Bermuda) 2/0 with single stitches and pledgets. The ascending aorta was replaced with a PTFE graft (Gelweave 26 mm, Vascutek, UK; a Terumo Company, JPN). The SVG to the RCA was re-implanted into the PTFE graft using a 6/0 Prolene running suture. The SVG to the 1st DA was re-implanted as T-graft into the first proximal SVG, using a 7/0 Prolene running suture. The weaning from cardiopulmonary bypass was uneventful. At the end of the procedure, the patient was hemodynamically stable with 200 μg/min dobutamine, and 15 μg/min norepinephrine. He showed normal LVEF in the transesophageal echocardiography, normal valve function without paravalvular leakage, good flows and pulsatility indices in the bypass grafts, and no conduction disturbances. The patient could be discharged from hospital only at the 28th postoperative day, because of sternal wound complications. He was then in a satisfactory condition.
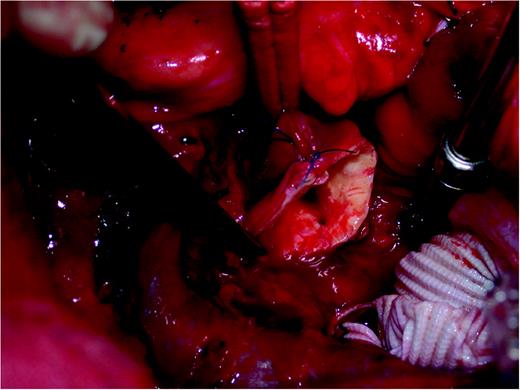
3. Comment
The method of choice to minimize aortic manipulation is the use of arterial conduits for in situ or T-graft configurations. To avoid further aortic manipulations, the industry has developed mechanical devices that allow construction of the proximal coronary bypass graft anastomoses without aortic side-clamping. Since 2003, our department uses the Heartstring Proximal Seal System (Maquet, Germany; a Getinge Group Company, Sweden) together with the aortic cutter from the same company. Since avoiding side-clamping of the aorta for the proximal anastomosis, we could reduce the incidence of neurological complications [3–5].
In this case, all aortic manipulations were conducted in standard manner during punching, with blood pressure values according to our guidelines of systolic <100 mmHg and mean arterial pressure <70 mmHg; also there was no particular hypotension. The palpation of the aorta was smooth, transesophageal echocardiography did not show plaques, and the diameter of the aorta was normal at 2.6 cm. Manipulation and unlocking of the puncher, as well as the use of the release mechanism was without particularities. In absence of technical malfunction of the aortic cutter, one origin of the tearing of the posterior aortic wall may be due to excessive pressure applied to the aortic wall during release of the mechanism. Guidelines of maximal and minimal aortic pressure before releasing the mechanism must be considered. Any pressure on the aortic wall during the punching maneuver must be avoided. The aortic cutter (Fig. 2 ) was returned to the manufacturer for examination in order to rule out a malfunction. Our clinic has continued to use the Heartstring II Proximal Seal System for performing the proximal seal of the anastomotic site after briefing of our surgical staff for the appropriate punching maneuver, emphasizing the no-pressure perpendicular apposition of the device on the aortic wall during puncher release and visual cross-check from the first assistant. It is likely that negative events are under-reported. We strongly recommend the surgical community to report their negative events, particularly in association with innovative technology, in order to improve the patient's safety during surgery.





