-
PDF
- Split View
-
Views
-
Cite
Cite
Satoshi Iwamoto, Akihiro Higashi, Tetsuya Ueno, Masamichi Goto, Yoshifumi Iguro, Ryuzo Sakata, Protective effect of sivelestat sodium hydrate (ONO-5046) on ischemic spinal cord injury, Interactive CardioVascular and Thoracic Surgery, Volume 8, Issue 6, June 2009, Pages 606–609, https://doi.org/10.1510/icvts.2008.197244
Close - Share Icon Share
Abstract
Prevention of paraplegia remains an important issue in repair of descending thoracic and thoracoabdominal aneurysms. Therefore, we investigated the protective effect of sivelestat sodium hydrate (ONO-5046) on ischemia-induced spinal cord damage in a rabbit model. Twenty New Zealand white rabbits were divided into two equal groups; ONO-5046 (1.6 mg/kg)+isotonic NaCl (30 ml) was administered selectively to the spinal cord via the lumbar arteries for the first 3 min during 30 min of infra-renal aorta clamping in the experimental group (group E), whereas NaCl was given alone in the control group (group C). Motor function of the lower limbs was assessed two days later by Tarlov criteria. The number of intact motor neurons in the anterior segment of the cord (L5 level) was counted after hematoxylin–eosin staining and the number of apoptotic motor neurons after TUNEL staining. Motor function of the lower limbs in group E was significantly better (P=0.003) than that in group C. The number of intact motor neurons was greater and of apoptotic motor neurons was less in group E than C. Selective infusion of sivelestat sodium hydrate directly into the spinal cord via the lumbar arteries significantly attenuated functional and morphological ischemia-induced spinal cord injury.
1. Introduction
Paraplegia remains one of the major complications after operations on descending thoracic or thoracoabdominal aortic aneurysms. During the last decade, several supplemental procedures, including distal perfusion and cerebrospinal fluid drainage, have been developed to help reduce this devastating complication.
Fundamental mechanisms of ischemia–reperfusion injury have also been intensively investigated in neuronal organs such as the brain and spine [1,2]. Several new pharmacologic approaches have been developed to reduce or block activation of neutrophils, which are thought to play a major role in initiation or exacerbation of ischemia–reperfusion injury of the spinal cord [3,4].
In the present study, we examined the protective effect of sivelestat sodium hydrate (ONO-5046), a neutrophil elastase inhibitor, on ischemic spinal cord damage using an established rabbit model.
2. Materials and methods
Thirty-two New Zealand white rabbits (2.75–3.39 kg) were used in these experiments. Animal care and all procedures were performed in compliance with the ‘Guide for the Care and Use of Laboratory Animal’ in Kagoshima University, Graduate School of Medical and Dental Services. Twelve rabbits died before neurological and histological assessments. Consequently, twenty rabbits were included in this study, and divided into a control group (group C) and an experimental group (group E) with ten rabbits in each group. The experimental model is presented in Fig. 1 . After premedication by intramuscular injection of ketamine (25 mg/kg) and medetomidine hydrochloride (0.5 mg/kg), all rabbits were anesthetized with intravenous thiopental (10 mg/kg) and were also treated with cefazolin (20 mg/kg). Lactated Ringer's solution was infused at 25 ml/h via the right ear vein until the end of experiment (∼2 h). After intravenous vecuronium bromide (0.5 mg/kg) was administered, all rabbits underwent tracheostomy and endotracheal intubation and were placed on a Harvard-type ventilator (FiO2 60%, tidal volume 30 ml, respiratory rate 30). An 18-gauge catheter was inserted into the right carotid artery to monitor systemic blood pressure. Under sterile conditions, a midline laparotomy incision was made to expose the abdominal aorta just below the left renal artery and at the level of the iliac bifurcation. Heparin (500 units) was given intravenously. For insertion of an infusion catheter, the aorta was clamped temporarily 1.5 cm above the iliac bifurcation. A transverse incision was made in the aorta just above the bifurcation. A 4-Fr catheter was inserted cranially through the aortotomy, and the aorta was snared just proximal to the aortotomy. After the catheter was connected to the infusion pump, the aorta just distal to the left renal artery and the origin of posterior mesenteric artery (PMA) was clamped for 30 min. Simultaneously with clamping, one of two solutions at 26 °C (room temperature) was infused at a rate of 10 ml/min for 3 min. One solution consisted of 30 ml of isotonic NaCl plus 1.6 mg/kg of ONO-5046 (group E) and the other solution consisted of isotonic NaCl (group C). The aortic incision was sutured with a 7-0 polypropylene after terminating the 3-min infusion. Arterial pressure, heart rate, esophageal temperature, and perfusion pressure were monitored during infusion of isotonic NaCl with or without ONO-5046. The abdominal wound was closed by interrupted 1-0 silk sutures. After weaning from the ventilator, the tracheostomy was closed by a 4-0 polypropylene suture. The skin incision was closed by interrupted 1-0 silk sutures. The rabbits were returned to their cage in a stable condition.
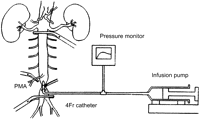
Experimental model. The abdominal aorta below the left renal artery and above the iliac bifurcation, and the posterior mesenteric artery were clamped for 30 min. Intra-aortic infusion of isotonic sodium chloride solution with or without ONO-5046 was performed for the first 3 min.
2.1. Neurologic evaluation
Motor function of hind limbs was assessed 48 h after aortic clamping by an observer who was blinded to experimental protocol using Tarlov's criteria: grade 0, flaccid paraplegia with no movement of the hind limbs; grade I, flaccid paraplegia with slight movement of the hind limbs; grade II, flaccid paraplegia with good movement of the hind limbs but unable to stand; grade III, flaccid paraplegia with good movement of the hind limbs able to stand; grade IV, complete recovery.
2.2. Histopathologic examination
After evaluation of motor function, the rabbits were anesthetized again with thiopental. All rabbits were sacrificed by gravitational infusion (100 cm H2O) of lactated Ringer's solution (500 ml)+heparin (1000 units), followed by 10% buffered formalin (500 ml) through an 18 gauge cannula inserted into the apex of the left ventricle. After lumbar laminectomy, the spinal cord was removed immediately and fixed by immersion in 10% buffered formalin solution. Sections of the lumbar cord were stained with hematoxylin and eosin (HE) and with terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL).
2.3. HE staining and TUNEL staining
After fixation, transverse sections of spinal cord at the L5 level were embedded in paraffin, cut into 5-μm thick sections, and stained with HE. Ten-micrometer sections were also cut from paraffin-embedded sections of the spinal cord and deparaffinized for TUNEL stain. An experienced pathologist (M.G.), who was blinded to the results of motor function of each rabbit, counted the number of intact motor neurons on HE staining and TUNEL positive-stained motor neurons in the anterior segment at the L5 level under ×100 magnification in each rabbit. Necrotic neurons were identified by cytoplasmic eosinophilia with loss of Nissl substance and pyknotic nuclei.
2.4. Statistical analysis
Data are presented as the mean±S.D. of the mean. The Mann–Whitney U-test was used to compare functional and morphologic variables between the two groups. A P-value <0.05 was considered significant.
3. Results
There was no significant difference in the esophageal temperature at the beginning of the experiment and at its lowest value, and in intra-aortic infusion pressure between the two groups (Table 1 ).
| Group C | Group E | ||
| Temperature (°C) | 36.1±0.3a/39.6±0.3b | 36.1±0.3a/39.5±0.4b | N.S. |
| Infusion pressure (mmHg) | 58.4±2.7 | 56.1±4.0 | N.S. |
| Group C | Group E | ||
| Temperature (°C) | 36.1±0.3a/39.6±0.3b | 36.1±0.3a/39.5±0.4b | N.S. |
| Infusion pressure (mmHg) | 58.4±2.7 | 56.1±4.0 | N.S. |
There was no significant difference in the esophageal temperature at the beginning of the experiment (b) and at the lowest value mostly after aortic infusion of solution (a), and aortic infusion pressure between the two groups. N.S., no significance.
| Group C | Group E | ||
| Temperature (°C) | 36.1±0.3a/39.6±0.3b | 36.1±0.3a/39.5±0.4b | N.S. |
| Infusion pressure (mmHg) | 58.4±2.7 | 56.1±4.0 | N.S. |
| Group C | Group E | ||
| Temperature (°C) | 36.1±0.3a/39.6±0.3b | 36.1±0.3a/39.5±0.4b | N.S. |
| Infusion pressure (mmHg) | 58.4±2.7 | 56.1±4.0 | N.S. |
There was no significant difference in the esophageal temperature at the beginning of the experiment (b) and at the lowest value mostly after aortic infusion of solution (a), and aortic infusion pressure between the two groups. N.S., no significance.
3.1. Neurologic function
Table 2 shows the evaluation of motor function of the hind limbs. The majority of rabbits in group C showed grade 0 spastic paraplegia; however, those in group E showed grade III or IV recovery. The Tarlov score in group E (3.1±0.3) was significantly greater (P=0.003) than that in group C (0.8±0.5).
| Group C | Group E | |
| Neurologic status | ||
| Grade 0 | 7 | 0 |
| Grade I | 1 | 1 |
| Grade II | 0 | 1 |
| Grade III | 1 | 4 |
| Grade IV | 1 | 4 |
| 0.8±0.5 | 3.1±0.3* |
| Group C | Group E | |
| Neurologic status | ||
| Grade 0 | 7 | 0 |
| Grade I | 1 | 1 |
| Grade II | 0 | 1 |
| Grade III | 1 | 4 |
| Grade IV | 1 | 4 |
| 0.8±0.5 | 3.1±0.3* |
The Tarlov score in group E was significantly greater than that in group C. *P=0.003.
| Group C | Group E | |
| Neurologic status | ||
| Grade 0 | 7 | 0 |
| Grade I | 1 | 1 |
| Grade II | 0 | 1 |
| Grade III | 1 | 4 |
| Grade IV | 1 | 4 |
| 0.8±0.5 | 3.1±0.3* |
| Group C | Group E | |
| Neurologic status | ||
| Grade 0 | 7 | 0 |
| Grade I | 1 | 1 |
| Grade II | 0 | 1 |
| Grade III | 1 | 4 |
| Grade IV | 1 | 4 |
| 0.8±0.5 | 3.1±0.3* |
The Tarlov score in group E was significantly greater than that in group C. *P=0.003.
3.2. Histopathologic examination
Pathological findings of the lumbar cord at the L5 level of the rabbits in the groups C and E are shown with different magnifications (×100 and ×400) after HE staining in Fig. 2 . Ischemic changes of neurons in the anterior horn of spinal cord were often observed in the group C whereas the neurons in the group E were well preserved. The number of intact motor neurons was greater (P=0.120) in group E (Table 3 ) than in group C. Similarly, the results of TUNEL staining are shown in Fig. 3 . The neurons in the group C showed apoptosis (brown nuclei) whereas the neurons in the group E showed no apoptosis. The number of TUNEL-positive motor neurons in group E was 0 (Table 3).
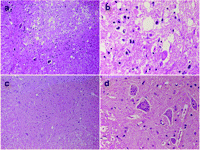
HE staining. The anterior horn of spinal cord at the L5 level in rabbits classified as grade 0 in the group C showed massive ischemic change of neurons (a, b) whereas the anterior horn of spinal cord classified as grade IV in Group E showed well preserved neurons (c, d). Magnifications: a and c, ×100; b and d, ×400; HE, Hematoxylin and Eosin staining.
| Group C | Group E | P-value | |
| HE intact motor neurons | 20.4±17.4 | 35.5±16.5 | 0.120 |
| (median 15) | (median 36) | ||
| TUNEL positive cells | 1.4±2.5 | 0 | 0.078 |
| Group C | Group E | P-value | |
| HE intact motor neurons | 20.4±17.4 | 35.5±16.5 | 0.120 |
| (median 15) | (median 36) | ||
| TUNEL positive cells | 1.4±2.5 | 0 | 0.078 |
In group E, the number of intact motor neurons in the anterior segment at the L5 level was greater on HE staining, associated with no TUNEL-positive motor neurons. HE, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling.
| Group C | Group E | P-value | |
| HE intact motor neurons | 20.4±17.4 | 35.5±16.5 | 0.120 |
| (median 15) | (median 36) | ||
| TUNEL positive cells | 1.4±2.5 | 0 | 0.078 |
| Group C | Group E | P-value | |
| HE intact motor neurons | 20.4±17.4 | 35.5±16.5 | 0.120 |
| (median 15) | (median 36) | ||
| TUNEL positive cells | 1.4±2.5 | 0 | 0.078 |
In group E, the number of intact motor neurons in the anterior segment at the L5 level was greater on HE staining, associated with no TUNEL-positive motor neurons. HE, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling.
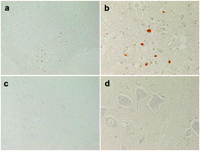
TUNEL staining. The anterior horn of spinal cord at the L5 level in rabbits classified as grade 0 in the group C showed apoptosis presented as brown nuclei (a, b) whereas the anterior horn of spinal cord classified as grade IV in the group E showed no apoptosis (c, d). Magnifications: a and c, ×100; b and d, ×400; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling.
4. Discussion
Neutrophil elastase is one of the most injurious cytotoxic mediators and is released when neutrophils are activated by several kinds of stimuli including ischemia [5]. Neutrophil elastase has been reported to damage endothelial cells and degrade connective tissue components, leading to critical tissue injury and an increase in vascular permeability [6]. A novel neutrophil elastase inhibitor, ONO-5046 (sivelestat sodium hydrate), was investigated initially to reduce neutrophil-mediated acute lung injury, including inflammatory or post-perfusion lung injury [7,8].
Several recent investigations using pharmacological approaches have been performed to attenuate ischemia–reperfusion injury of the spinal cord and to reduce the incidence of paraplegia [3,4]. Yamauchi and co-workers occluded the infra-renal aorta of rabbits for 15 min, and showed that ONO-5046 infused intravenously from anesthetic induction until 60 min of reperfusion attenuated delayed motor neuron death and significantly improved neurological functional scores [4].
In the present study, ONO-5046 was selectively administered via the lumbar arteries to the section of the spinal cord that was jeopardized. This model has been employed in previous studies to examine the effect of several pharmacologic or physiologic strategies on attenuation of ischemia–reperfusion damage of the spinal cord [9,10]. The biggest advantage of our experimental method is that a high concentration of ONO-5046 can be delivered directly to the portion of the spinal cord that is jeopardized by ischemia.
Tonai and co-workers demonstrated a protective effect of pre-treatment with ONO-5046 on inflammatory reactions in response to traumatic injury of the spinal cord [11]. In their investigation, ONO-5046 was thought to attenuate neurologic damage by blocking cytokine-induced neutrophil chemo-attractant (CINC)-1 and preventing neutrophil activation and vascular endothelial cell injury. Although spinal cord damage was due to mechanical trauma in their study rather than ischemia–reperfusion injury, both forms of injury produce an inflammatory response with neutrophil activation and chemotaxis.
Although a 30-min occlusion time of the infra-renal aorta in the present study may be longer than what has been used in previous studies, the neurological score by Tarlov criteria was relatively higher not only in the ONO-5046 group, but also in the control group. It is possible that moderately hypothermic isotonic NaCl (26 °C) infused through a catheter provided a hypothermia-induced protective effect against ischemic injury even in the control group [12]. Excellent neurological recovery in the ONO-5046 group was achieved both by its effect as a neutrophil elastase inhibitor and moderate hypothermia on the jeopardized spinal cord. In the clinical setting, more hypothermic solution containing ONO-5046 could be used, with the goal of achieving a greater protective effect against ischemic spinal cord damage.
Our histological and immunohistological examinations showed that ONO-5046 significantly reduced the number of both necrotic motor neurons and TUNEL-positive neurons in the anterior segment of the spinal cord. Motor neuron death seen on HE staining can be caused by the direct and acute detrimental effect of ischemia on motor neurons. Spinal cord ischemia induces morphological and biochemical changes suggestive of apoptosis [13]. In the present study, ONO-5046 had a significant beneficial effect on apoptotic neuronal injury. This effect may be mediated by a reduced induction of caspase3 [4]. However, it needs to be investigated whether ONO-5046 has a sustained effect on apoptosis or that apoptotic neuronal injury is merely delayed.
There are a couple of limitations of the present study. First, our duration of spinal cord ischemia was 30 min. We do not think this is a sufficiently safe period to perform complicated cases such as repair of Crawford type II or III aneurysms. Second, the number of animals in each group was small and the observation period was not long enough to assess delayed motor neuron death. Third, it remains uncertain that the dose of ONO-5046 used in our study would be absolutely safe in the clinical setting, because of a species difference between humans and rabbits. Fourth, no other objective measurement to evaluate motor function of hind limbs was performed beside Tarlov' score. Fifth, only one pathological section at the L5 level was taken from each rabbit. Despite these limitations, the present study demonstrated a beneficial effect of ONO-5046 on reduction of ischemia–reperfusion injury on spinal cord through its neutrophil elastase inhibition.




