-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshihiko Kurimoto, Nobuyoshi Kawaharada, Toshiro Ito, Toshio Baba, Syunsuke Ohori, Atsushi Watanabe, Yasufumi Asai, Tetsuya Higami, Less-invasive management of left subclavian artery in stent-grafting for distal aortic arch disease, Interactive CardioVascular and Thoracic Surgery, Volume 8, Issue 5, May 2009, Pages 548–552, https://doi.org/10.1510/icvts.2008.192484
Close - Share Icon Share
Abstract
Simple coverage of the left subclavian artery (LSA) in thoracic endovascular aortic repair (TEVAR) is still a controversial procedure. We present our modified strategy dealing with LSA in TEVAR. Hand-made stent grafts were placed more proximal beyond the LSA for 104 patients. In elective 76, preoperative LSA occlusion test was performed on 31 patients, and preoperative computed tomographic angiography (CTA) of the vertebro-basilar artery was performed on the remaining 45. Head vessels were planned to be kept patent using fenestrated stent grafts, if possible. Stent grafts were placed from zone 0 in 23, zone 1 in 39, and zone 2 in 42. The LSA occlusion tests revealed harmful effects, such as loss of consciousness and vertigo in two out of 31 patients (6.5%). Vertebro-basilar arterial CTA revealed possible risks, if LSA covered, in three out of 45 patients (6.7%). Fenestrated stent grafts could successfully preserve 131 head vessels, except for one unintentional occlusion of the left carotid artery (0.75%). There was no LSA-related complication in any of the cases. A combination of preoperative vertebro-basilar arterial CTA and fenestrated stent grafts is useful to avoid possible LSA-related complications in TEVAR.
1. Introduction
One of the major limitations to expand the indication of thoracic endovascular aortic repair (TEVAR) is an inadequate length of proximal landing zones because of clinically important head vessels branched from the aortic arch. Because of the unavailability of a commercial device with a fenestration or a branch to keep the head vessels patent after TEVAR, proximal extension of a proximal landing zone has been achieved by a simple coverage of the left subclavian artery (LSA) or revascularization of LSA using LSA bypass-grafting or transposition.
A recent review of TEVAR in which a stent graft was placed from zone 2 reported that revascularization of LSA should be recommended [1]. Although it is well recognized that possible risks following a simple coverage of LSA in TEVAR can be largely prevented by concomitant LSA revascularization [2], we still need to seek an even less-invasive treatment.
2. Material and methods
We began TEVAR by using a hand-made stent graft placed more proximal beyond the LSA (zone 2 [3]) from October 2001. By March 2008, 104 patients underwent TEVAR for distal aortic arch pathology. In elective cases, preoperative assessments were performed to evaluate an influence of possible coverage of LSA. In emergency cases, preoperative evaluation regarding possible LSA coverage was not carried out even if LSA was covered or could be preserved by a fenestrated stent graft.
Until April 2005, preoperative LSA balloon occlusion tests were conducted in cases in which there was a possibility that LSA was covered by a stent graft in elective TEVAR. Since May 2005, preoperative computed tomographic angiography (CTA) of the vertebro-basilar artery has been chosen as an initial screening to predict possible complications if LSA is simply covered. Therefore, a preoperative LSA occlusion test has been considered only for the cases in which preoperative CTA of the vertebro-basilar artery suggests possible complications if LSA is simply covered. The indication of TEVAR for distal aortic arch pathology is that a proximal end of the diseased aortic segment is >15 mm away from the left common carotid artery (LCC) and that a diameter of a proximal landing zone, the aortic arch, is <37 mm (Fig. 1 ).
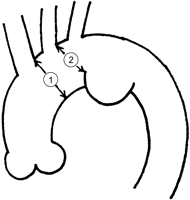
Schema of distal aortic arch aneurysm. The indication of thoracic endovascular aortic repair is that a diameter of a proximal landing zone is <37 mm


The stent graft was custom-made and reconstructed by suturing graft material (Ube Corp, Ube, Japan) to an endoskeleton of Gianturco Z stents (Cook Inc, Bloomington, IN). Z-stents were attached to each other using stainless steel wires with solder, leaving spaces of 8–15 mm between stents so as to fit the configuration of the distal aortic arch based on CTA images. A procedure of TEVAR in our institute was previously reported [13]. In cases in which LSA was located >15 mm away from the diseased aortic segment, LSA was kept patent using a fenestrated stent graft (Fig. 2 ). In cases in which the proximal edge of the diseased aortic segment or primary entry was located fewer than 15 mm away from LSA or in which LSA was involved in the aortic aneurysm, LSA was covered by a stent graft. LSA revascularization was considered if preoperative evaluation suggested a possible risk of neurological complications following a LSA simple coverage. When type II endoleak from LSA was preoperatively expected or seen in digital subtracted angiography (DSA) following stent-graft deployment, LSA was occluded using coil or a stent graft with one end stitched up before or after aortic stent-graft deployment.
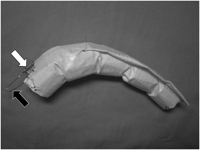
A hand-made fenestrated stent graft. A proximal roof of a stent graft is fenestrated (white arrow) to preserve head vessels. A wire ring (black arrow) prevents distal migration of the device during stent-graft deployment. In a case of aortic dissection, a stent graft is often tapered to fit a size of a true lumen. A stent graft is custom-made to fit the distal aortic arch.
A procedure of LSA occlusion test was previously reported [13]. Briefly, during balloon occlusion of LSA for 20 min, neurological tests, including a finger-to-nose test were repeatedly performed to reveal possible adverse effects, such as cerebellar, brain stem, spinal cord or left arm ischemia.
A case in which the right or left vertebral artery (VA) measured above the posterior inferior cerebellar artery (PICA) was hypoplastic (Fig. 3 ), or in which the right VA was terminated (Fig. 4 ) or stenotic (Fig. 5 ), was considered to have the possibility of neurological complications if LSA is simply covered. In such a case, preoperative LSA balloon occlusion test or LSA revascularization was planned on the same day of TEVAR.
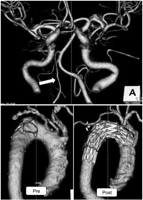
Computed tomographic angiography (CTA) images. CTA of the vertebro-basilar artery revealed hypoplastic right vertebral artery (white arrow) (upper). A fenestrated stent graft well preserved antegrade blood flow into the left subclavian artery as well as the left common carotid artery (lower).
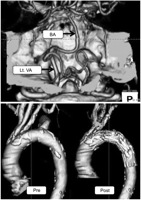
CTA images. CTA of the vertebro-basilar artery suggested terminated right VA (upper). A fenestrated stent graft well preserved antegrade blood flow into LSA as well as LCC (lower). Lt. VA, left vertebral artery; BA, basilar artery.
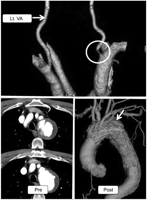
CTA and CT images. CTA of the vertebral artery revealed stenotic orifice (white circle) of right VA (upper). A fenestrated stent graft well preserved antegrade blood flow into LSA as well as LCC (lower). Postoperative CTA shows complete thrombo-occlusion of distal aortic arch aneurysm (lower, white arrow). Lt. VA, left vertebral artery.
3. Results
In 76 elective cases out of 104 subjects, preoperative LSA occlusion tests were conducted on 31 and preoperative CTA of the vertebro-basilar artery was performed on 45. In 28 emergency cases (26.9%), preoperative evaluations regarding an influence of LSA coverage were not carried out regardless if LSA was covered or could be preserved by a fenestrated stent graft. The patients, aged 17–94 years (mean 70.2), consisted of 84 males (80.8%) and 20 females. Distal aortic arch pathology consisted of degenerative thoracic aortic aneurysm in 55 (52.9%), type B aortic dissection in 20 (19.2%), thoracic aortic pseudoaneurysm in 28 (26.9%) and inflammatory thoracic aortic aneurysm in one (Table 1 ).
| Age (years) | 70.2±13.6 (17–94) |
| Male | 84 (80.8%) |
| Pathology | |
| Degenerative aortic aneurysm | 55 (52.9%) |
| Aortic dissection-related | 20 (19.2%) |
| Pseudoaneurysm | 28 (26.9%) |
| Blunt aortic injury | 12 |
| Ruptured degenerative | 7 |
| Ruptured aortic dissection | 3 |
| Anastomotic | 6 |
| Inflammatory | 1 (1.0%) |
| Emergency | 28 (26.9%) |
| Age (years) | 70.2±13.6 (17–94) |
| Male | 84 (80.8%) |
| Pathology | |
| Degenerative aortic aneurysm | 55 (52.9%) |
| Aortic dissection-related | 20 (19.2%) |
| Pseudoaneurysm | 28 (26.9%) |
| Blunt aortic injury | 12 |
| Ruptured degenerative | 7 |
| Ruptured aortic dissection | 3 |
| Anastomotic | 6 |
| Inflammatory | 1 (1.0%) |
| Emergency | 28 (26.9%) |
| Age (years) | 70.2±13.6 (17–94) |
| Male | 84 (80.8%) |
| Pathology | |
| Degenerative aortic aneurysm | 55 (52.9%) |
| Aortic dissection-related | 20 (19.2%) |
| Pseudoaneurysm | 28 (26.9%) |
| Blunt aortic injury | 12 |
| Ruptured degenerative | 7 |
| Ruptured aortic dissection | 3 |
| Anastomotic | 6 |
| Inflammatory | 1 (1.0%) |
| Emergency | 28 (26.9%) |
| Age (years) | 70.2±13.6 (17–94) |
| Male | 84 (80.8%) |
| Pathology | |
| Degenerative aortic aneurysm | 55 (52.9%) |
| Aortic dissection-related | 20 (19.2%) |
| Pseudoaneurysm | 28 (26.9%) |
| Blunt aortic injury | 12 |
| Ruptured degenerative | 7 |
| Ruptured aortic dissection | 3 |
| Anastomotic | 6 |
| Inflammatory | 1 (1.0%) |
| Emergency | 28 (26.9%) |
Stent grafts were placed from zone 0 in 23 patients (22.1%), zone 1 in 39 (37.5%), and zone 2 in 42 (40.4%) (Fig. 6 ). LSA was preserved by a fenestrated stent graft in 53 patients (51.0%) and revascularized by axillo-axillary bypass-grafting in 8 (7.7%) due to LSA occlusion tests being positive in two, patent left internal thoracic artery (LITA) graft in two and patient's discretion in four. LSA was simply covered by a stent graft in the remaining 43 patients (41.3%). Fenestrated stent grafts could successfully preserve 131 head vessels, except for one unintentional occlusion of LCC (0.75%). Fortunately, no neurological complication was observed in this 75-year-old man who additionally underwent LCC bypass-grafting following unintentional coverage of LCC.
![Zone classification of a stent graft deployed from the aortic arch [3].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icvts/8/5/10.1510/icvts.2008.192484/2/m_548fig6.gif?Expires=1750380249&Signature=QXBB8j8tVGcJt-b~P0IfNyeyHWZGHPe7GXWSAlLzbwBdHOL~k~n4XOmRYYgn6X-evISAozAr5X-cRTqq6r4MTUmwzFbfdnSBa3WfJvEvPnAy4Vop0Do0taCuXYKIcQiHjWqitjSRE2b-pwKGgWoLdOD3Gtm2NW1WCyf~-CrdTaikrfhpAXCW41-I0~r7jORl~k2y6jLttM9ZZsmtJ2lNXQbS9-tPvonXvSj51DScH7pkGDOInH5nZMGy6mC~o-9iRnTnJYx366VD1-SCrSeygdM~xxs36no00uLF72ZngGJmD3Ili7JJNl7J-L3CF-W7Hrag5ZgkQm~cMPP5InzZoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Zone classification of a stent graft deployed from the aortic arch [3].
LSA occlusion tests revealed harmful effects in two out of 31 patients (6.5%). A 71-year-old man lost consciousness several seconds after LSA occlusion. As soon as the occlusion balloon was deflated, his consciousness returned. A 76-year-old woman complained of vertigo a few minutes after LSA occlusion. LSA could not be preserved by a fenestrated stent graft due to a lack of distance between LSA and the diseased aortic segment in both cases. TEVAR was performed on these patients following concomitant axillo-axillary bypass-grafting.
CTA of the vertebro-basilar artery revealed possible risks, if LSA covered, in three out of 45 patients (6.7%). A 76-year-old woman with a hypoplastic right VA underwent TEVAR from zone 1 using a fenestrated stent graft (Fig. 3). A 75-year-old woman with a terminated right VA underwent TEVAR using a fenestrated stent graft to preserve LSA although a preoperative LSA occlusion test was negative (Fig. 4). Finally, a 74-year-old man with a stenotic orifice of right VA underwent TEVAR using a fenestrated stent graft to preserve LSA without a preoperative LSA occlusion test (Fig. 5).
There was no LSA-related complication in any of the cases. Postoperative neurological complications included strokes in five patients (4.9%) and spinal cord ischemia in three (2.9%). Among five patients who suffered from strokes as an operative complication, two were treated using a fenestrated stent graft from zone 2 and 0 preserving LSA. The remaining stroke-complicated three patients were treated using a fenestrated stent graft preserving the brachio-cephalic artery (BCA) and LCC, but covering LSA. One of these patients suffered a stroke in the left VA region, but this was due to an embolism, not by LSA coverage. Delayed paraplegia was observed in two emergency rupture cases in which LSA could not be preserved due to the location of the aneurysm, and in one elective case which LSA was preoperatively occluded.
Two patients died due to an aortic aneurysm rupture or acute myocardial infarction in 76 elective cases (early mortality rate, 2.6%) and six patients in 28 emergency cases (21.4%). Excluding these early-death patients, 92 patients (95.8%) could be interviewed about post-TEVAR course, at least six months after operation by telephone or at out-patient clinic. We could not follow one patient until six months after TEVAR due to an unknown reason. Two patients underwent removal of stent grafts and open repair of thoracic aortic diseases three months and four months after TEVAR. One patient died due to cerebral hemorrhage five months after TEVAR. None of the patients, including 43 patients whose LSA was simply covered by a stent graft, have undergone any intervention to restore the blood flow into LSA in the follow-up periods.
4. Discussion
Previously, intentional occlusion of LSA was widely accepted in cases in which a proximal landing zone was too short to exclude distal aortic arch aneurysm in TEVAR [4,5]. However, it is obvious that LSA is anatomically important for some patients. A super-dominant or single left VA or VA that ends in PICA could be considered important anatomy, as could contralateral subclavian arterial disease. This anatomy would be particularly important in the presence of a circle of Willis that is incomplete, reportedly as high as 42.4% [6]. In another example, LSA is also very important for patients with previous coronary artery bypass-grafting with use of LITA. Although left arm ischemia immediately after LSA occlusion is very rare [7] and revascularization of intentionally occluded LSA was reported mainly in the follow-up periods [5], brain stem ischemia and paraplegia as complications following TEVAR [5,8] are different stories, which means these are too late to be treated at the time of diagnosis. According to a report [9] in which the anatomy of the cerebral arteries in 92 forensic medicine autopsies was assessed, the right VA measured above PICA was hypoplastic in 8.7% and the left VA in 7.6%. The right VA terminated to PICA in 3.3%. In 56.5%, either the left or right posterior communicating artery (PComA) was hypoplastic (or absent) and in 7.6%, both PComAs were absent. The authors concluded that there was a substantial risk of neurological complication following a simple coverage of LSA in TEVAR in 5.4% of the cases. This study indicates not only that there is a 5.4% risk following a simple coverage of LSA, but also that >90% of patients do not need LSA revascularization as an additional procedure in TEVAR.
As recent reports recommended [1,2], LSA revascularization is the easiest way to prevent LSA-coverage-related complications. However, LSA revascularization itself also has potential complications, such as vocal cord palsy, spinal cord ischemia, and a chance of infection [10]. Even LSA revascularization alone has a mortality rate of 2.6% [11]. Therefore, we believe that LSA revascularization should also be performed by endovascular techniques, such as a branched [12] or a fenestrated stent graft [13], if possible. Although a fenestrated stent graft cannot allow LSA revascularization for distal aortic arch aneurysm involving LSA, zone 2 cases can be treated using a fenestrated stent graft preserving LSA.
Although the report dealing with the subjects distal from zone 3 showed stroke rates of 2.2% [14], the report dealing with only zone 2 cases showed a rate as high as 8.6% [15], which was reported not to be related to LSA coverage. It seems obvious that more proximal stent-graft placement is related to a high incident rate of stroke. In our experience of 270 TEVAR by March 2008, there was only one patient (0.6%) who showed stroke symptoms following TEVAR in the group of zone 3 or more distal (n=166). However, the stroke incident rate was 4.8% as presented in this study. The prevention of strokes must be one of the important points to extend the indication of TEVAR for distal aortic arch pathology. Spinal cord ischemia also happened in our subjects much like other reports, for example, often in emergency cases. We agree that unprotected LSA coverage increases an incident rate of spinal cord ischemia in TEVAR [2]. Despite two emergency rupture cases, an incident rate of spinal cord ischemia in 76 elective cases was 1.3%, which might mean that spinal cord ischemia does not happen frequently compared to strokes in TEVAR for distal aortic arch pathology.
In conclusion, a combination of preoperative vertebro-basilar arterial computed tomographic angiography and fenestrated stent grafts is useful to avoid possible LSA-related complications in thoracic endovascular aortic repair.
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14–17, 2008.
References
Conference discussion
Dr. B. Zipfel (Berlin, Germany): I wish to congratulate Dr. Kurimoto and his colleagues on their contribution to the problem how to manage the left subclavian artery when it has to be occluded by the stent graft. This remarkable series has good results of stent grafting in distal aortic arch disease.
Dr. Kurimoto and his colleagues were able to preserve the LSA in many cases, and in many cases, also more proximal head vessels by using handmade stent grafts with a scallop fenestration at the proximal end.
In elective cases the authors used an LSA balloon occlusion test to evaluate the cerebral perfusion dependent on patency of the LSA. This procedure was obviously abandoned after computer tomography angiography provided exact anatomical information on the vertebrobasilar artery. Only in cases of known anomalies or stenosis they performed additionally the occlusion test.
Using this policy, in 51% of the patients, the LSA perfusion was preserved by the scallop fenestration and an additional 7.7% with an axio-axillary bypass. In 43% of the patients, the LSA was simply covered with the stent graft. Eight neurological events, five strokes and three spinal cord ischemias are reported. Remarkably six of these events occurred in patients after simple coverage of the LSA.
This is basically the same experience we made. We use a little bit different approach because we are not so confident in fenestrated stent grafts. We use straight stent grafts, and in doubt we perform more general surgical revascularization using extrathoracic left carotid-subclavian bypass.
Now, this is my first question. After simple coverage of the LSA, 6 of 43 patients developed neurological complications that may be related to the LSA occlusion despite sophisticated preoperative workup of the vertebral circulation. How reliable is the balloon occlusion test of the LSA at rest in the cath lab, even if it is restricted to patients with identified abnormalities in the vertebral circulation? Don't you think that transcranial Doppler sonography might provide additional information?
Dr. Kurimoto: You are talking about the two cases of positive by occlusion test?
Dr. Zipfel: My question is whether this test is really reliable to identify potential problems from the LSA circulation, whether you should use additional information like a Doppler sonography.
Dr. Kurimoto: Yes. I really believe the occlusion test is reliable to predict the serious neurological complication. But occlusion test cannot predict possible delayed spinal cord ischemia or delayed claudication of the left arm.
But, yes, really critical neurological complications I think is very important to predict preoperatively.
Dr. Zipfel: You used stent grafts with a proximal scallop fenestration in a significant part of your distal aortic arch implantations. What is your experience in sealing of these grafts at the proximal end? How many Type I endoleaks did you experience?
Dr. Kurimoto: Endoleak?
Dr. Zipfel: Yes.
Dr. Kurimoto: It is one of the major concerns, but we experienced probably 15% of Type I endoleak. But only less than 5 patients underwent open conversion. Just we observed the size of aneurysm. If aneurysm size is increasing, we suggest the patient undergo open conversion.
Dr. E. Saadi (Porto Alegre, Brazil): When you cover the left subclavian artery, you may have Type II endoleak by retrograde flow. Did you have any in your experience, and if yes, how did you treat it?
Dr. Kurimoto: If the left subclavian artery is branched from aneurysm, it is definitely necessary to embolize using the coil or something else.
But if the left subclavian artery is branched from normal arch wall, it isn't necessary to coil-embolize because the stent graft completely seals the left subclavian artery root.
So we don't see any Type II endoleak if the left subclavian artery branched from normal wall.




