-
PDF
- Split View
-
Views
-
Cite
Cite
Rui M.S. Almeida, João C. Leal, Eduardo K. Saadi, Domingo M. Braile, Antonio S.T. Rocha, Giuliano Volpiani, Crescencio Centola, Alcides Zago, Thoracic endovascular aortic repair – a Brazilian experience in 255 patients over a period of 112 months, Interactive CardioVascular and Thoracic Surgery, Volume 8, Issue 5, May 2009, Pages 524–528, https://doi.org/10.1510/icvts.2008.192062
Close - Share Icon Share
Abstract
The aim of this study is to analyze the immediate and late evolution for death and reintervention in a thoracic endovascular aortic repair (TEVAR) group, over a follow-up period of 112 months. Retrospective data of 255 patients, from 1998 to 2007, were obtained. The most prevalent diseases were thoracic aortic aneurysms (89), thoracic and abdominal aneurysms (85) and thoracic aortic dissections (61). The mean age was 63.2 years and 67.1% were male. Three hundred and three endoprostheses were used. Causes of morbidity, in the immediate postoperative period, were hyperthermia (45.9%), endoleaks (9.8% – being 7.1% type I), vascular complications (5.2%), renal insufficiency (3.1%) and neurological complications (3.1%). There were two (0.8%) hospital deaths and 17 (6.7%) late deaths. Time of follow-up was up to 112 months (mean of 60 months). The Kaplan–Meier curve analysis showed an increase of reintervention, compared with death, after a follow-up period of 42 months. Freedom from death at 36, 60 and 112 months was 96%, 89.1%, 85.1% and for reintervention, for the same periods was 93.6%, 82.7%, 57.2%, respectively. This study showed low incidence of prostheses related morbidity and immediate mortality. After a period of 42 months there was an increase on the percentual tax of reintervention.
1. Introduction
Surgical treatment of thoracic aortic diseases (TAD) is a challenge due to high morbidity and mortality, associated with the conventional surgical treatment [1,2]. Despite the technical surgical evolutions, such as the medullar protection techniques, co-morbities such as age, complex anatomy and associated diseases have increased and surgery denied to this group of patients, which leads to a decrease in life expectancy and to an increase in the morbidity of TAD.
This retrospect leads to an alternative treatment, the thoracic endovascular aortic repair (TEVAR) with satisfying results published but with no late data regarding patient and endoprosthesis survival.
The objective of this paper is to present the immediate and long-term results of three surgical institutions, in patients submitted to the implant of thoracic endoprosthesis in the descending aorta, related to morbidity and mortality and actuarial curves from freedom of death and reintervention, during a follow-up period of 112 months.
2. Patients and method
In this retrospective study, between November 1998 and November 2007, 255 patients presenting with TAD were submitted to TEVAR, with a mean follow-up of 60 months. Inclusion criteria were consecutive patients with clinical indications for endovascular treatment [3], in which informed consent was obtained, reviewed by at least two authors. There were no exclusion criteria in this report. The participating groups gathered data through a protocol previously established.
Age varied from 16 to 90 years (mean of 63.2 years), and 171 patients were men (67.1%). The most prevalent associated diseases were systemic arterial hypertension, chronic obstructive pulmonary disease and coronary arterial diseases.
Descending aortic aneurysms were present in 34.9% and thoracic and abdominal aneurysms in 33.3%. Table 1 shows all basic diseases of this group.
| Basic disease | Patients | % |
| Descending aortic aneurysm | 89 | 34.90 |
| Thoracic and abdominal aneurysm | 85 | 33.33 |
| Aortic dissection | 61 | 23.92 |
| Aortic coarctation | 9 | 3.53 |
| Penetrating ulcer | 8 | 3.14 |
| Aortic arch aneurysm | 3 | 1.18 |
| Total | 255 | 100.00 |
| Basic disease | Patients | % |
| Descending aortic aneurysm | 89 | 34.90 |
| Thoracic and abdominal aneurysm | 85 | 33.33 |
| Aortic dissection | 61 | 23.92 |
| Aortic coarctation | 9 | 3.53 |
| Penetrating ulcer | 8 | 3.14 |
| Aortic arch aneurysm | 3 | 1.18 |
| Total | 255 | 100.00 |
| Basic disease | Patients | % |
| Descending aortic aneurysm | 89 | 34.90 |
| Thoracic and abdominal aneurysm | 85 | 33.33 |
| Aortic dissection | 61 | 23.92 |
| Aortic coarctation | 9 | 3.53 |
| Penetrating ulcer | 8 | 3.14 |
| Aortic arch aneurysm | 3 | 1.18 |
| Total | 255 | 100.00 |
| Basic disease | Patients | % |
| Descending aortic aneurysm | 89 | 34.90 |
| Thoracic and abdominal aneurysm | 85 | 33.33 |
| Aortic dissection | 61 | 23.92 |
| Aortic coarctation | 9 | 3.53 |
| Penetrating ulcer | 8 | 3.14 |
| Aortic arch aneurysm | 3 | 1.18 |
| Total | 255 | 100.00 |
Two hundred and fifty-five patients underwent implantation of 303 thoracic endoprostheses, being 256 (84.5%) Braile Biomedics® (37.1% with nitinol), 38 (12.5%) Talent®, seven (2.3%) Gore Tag® and two (0.7%) CP Stent™. The choice of each endoprosthesis was done by the individual surgeon, based on their characteristics and of the type of lesions to be treated.
The preoperative image protocol comprised a computed tomography (CT) chest/abdominal scan of multiple detectors. In patients with reduced kidney function, the MRI was used. The examinations were reviewed by at least two of the authors, for diagnosis, obtaining the necessary measures and decision of treatment.
The descending aorta, at the site of treatment, measured from 5.5 to 12.0 cm (mean of 7.0 cm). The mean diameter and length of the prosthesis used was 28 and 95 mm, respectively. An expandable endoprosthesis with the first cage uncoated was used in 52 patients, representing 17.2% of endografts used.
Follow-up was conducted by clinical, laboratory and CT, 30 and 180 days after the procedure, and then annually. The review aimed to assess all types of endoleaks, thrombosis of the false lumen and/or decrease or increase in the size of the aneurysm and the primary failure of the endoprosthesis. Of the total, 29 patients (11.4%) underwent endovascular treatment in emergency conditions and 226 (88.6%) patients were treated electively. In this subgroup, 26 (89.7%) patients had acute dissections of the thoracic aorta and three (10.3%) aortic ruptured aneurysms.
In four (1.6%) patients hybrids/associated procedures were performed – coronary artery bypass grafting (2) and aneurysm of the aortic arch (2).
Spinal anesthesia was used in 66.7% of patients, general 32.9% and 0.4% local, due to a ruptured dissection of the aorta to the left pleura.
Access routes used for the implantation of expanding endoprosthesis were the right femoral artery in 200 (78.4%) patients, the left in 54 (21.2%) and one (0.4%) right iliac artery. All procedures were performed in the catheterization laboratory.
The statistical study to show the curve of events actuarial and death reintervention was conducted by the method of Kaplan–Meier, with the StatsDirect Statistical Software.
3. Results
Results are presented as a unique group, independent of the basic disease, due to the fact that the objective was to analyze the endoprosthesis in late follow-up and its freedom from reintervention.
Average time for the procedures was 110 min, ranging from 60 to 240 min, and stay in the intensive care unit ranged between 24 and 36 h. The length of hospital stay ranged between 2 and 30 days (mean 5 days).
Technical failure, defined as the opening of an endoprosthesis not in place previously established, occurred in 5 cases (2.0%), and in 4 of these (80%), there was the conversion to conventional surgery, via thoracotomy. In another case, with 10 years of follow-up of conventional surgery for correction of chronic dissection of the thoracic aorta, the patient refused any further intervention. Two cases of failure (40%) were due to the diameter of the femoral or iliac artery, being incompatible with the sheath of endoprosthesis. Another two were related to tortuosities in the iliac arteries. There was rupture of the iliac artery in one patient. The fifth conversion was due to an iatrogenic dissection of the aorta, where there was need to replace the ascending aorta and arch, after placing the endoprosthesis in the descending aorta.
In eight (3.1%) patients peripheral vascular complications occurred, related to the site of insertion of the endoprosthesis, six (75%) addressed surgically through femoro-femoral revascularization, one (12.5%) through iliac-femoral revascularization and one (12.5%) with the placement of an endoprosthesis in the iliac artery, at the same time as the endovascular procedure.
Hyperthermia, defined as axillary temperature higher than 38 °C, occurred in 117 (45.9%) patients, but without any sign of infection. Neurological complications occurred in eight (3.13%) patients, and only one patient recovered before hospital discharge. Eight patients had renal failure, and one was referred to dialysis (Table 2 ).
| Morbidity | Patients | % |
| Hyperthermia | 117 | 45.9 |
| Reintervention | 31 | 12.2 |
| Endoleaks | 27 | 10.6 |
| Neurological problems | 8 | 3.1 |
| Renal failure | 8 | 3.1 |
| Morbidity | Patients | % |
| Hyperthermia | 117 | 45.9 |
| Reintervention | 31 | 12.2 |
| Endoleaks | 27 | 10.6 |
| Neurological problems | 8 | 3.1 |
| Renal failure | 8 | 3.1 |
| Morbidity | Patients | % |
| Hyperthermia | 117 | 45.9 |
| Reintervention | 31 | 12.2 |
| Endoleaks | 27 | 10.6 |
| Neurological problems | 8 | 3.1 |
| Renal failure | 8 | 3.1 |
| Morbidity | Patients | % |
| Hyperthermia | 117 | 45.9 |
| Reintervention | 31 | 12.2 |
| Endoleaks | 27 | 10.6 |
| Neurological problems | 8 | 3.1 |
| Renal failure | 8 | 3.1 |
In the immediate postoperative period, defined as the period of hospitalization or until 30 days after the procedure, two patients (0.8%) died, due to causes unrelated to the prosthesis; in one case after conversion into open surgery and in the other due to acute renal failure.
In 17 patients (6.7%) there was a need for endovascular reintervention and three (1.2%) to conventional surgery, as a result of leaks or a non-complete correction of the problem. The immediate postoperative leaks (3.9%) were identified through CT, as type I, in 18 cases (72%) and type II in seven (28%).
In one patient there was structural failure of the endoprosthesis (0.4%), due to the sharp angle of the aortic arch, without clinical complications in the immediate postoperative period, as endoleak type III.
Leak was present in 24 patients, being types I and II the most frequent, occurring in 18 (7.05%) and 6 (2.35%) patients, respectively. Among the natural evolution of TAD, there were four patients with acute dissection of the ascending and aortic arch.
Thirty one (12.15%) reinterventions were performed, with 13 patients undergoing conventional surgery and 18 endovascular. The causes of reinterventions were evolution of the TAD, rupture and leak of endoprosthesis. No mortality occurred in these reinterventions.
The analysis of Kaplan–Meier survival curve showed freedom from reintervention, over 112 month's follow-up period of 57.2±10.4%. From 42 months of follow-up there was an increased rate of reintervention (Fig. 1 ).
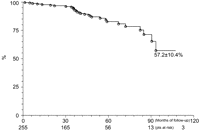
Kaplan–Meier survival curve of patients free from reintervention.
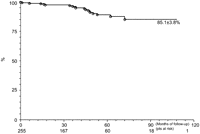
Late mortality occurred in 17 (6.7%) patients, related to the evolution of TAD, breaking the endoprosthesis, endoleaks and cardiovascular diseases associated. The analysis of Kaplan–Meier survival curve showed that freedom from death, over 112 months, was 85.1±3.1% (Fig. 2 ).
4. Discussion
TEVAR is a recent surgical technique which is less invasive compared to conventional open surgery. Long-term results are lacking from the literature.
In our country (Brazil), the experience began in 1998 with the group of the Paulista Medical School. After experimental work with national prostheses [4] placed by endoscopic vision [5], they started implementation of prostheses through peripheral access [6].
Despite the fact that participating centers of this multicenter study have different numbers of patients engaged, all variables and results are similar, as well as standardized data collected.
The etiology of TAD was similar to the French experience [7], Wheatley et al. [8], Fattori et al. [9] and Rodriguez et al. [10]. As a result of the similarities it is possible to compare the results and complications that are presented both in the immediate postoperative period as well as in the long-term.
When comparing the data obtained in this study with the Eggebrecht et al. [11] metanalysis, the success rate of the procedure obtained was 98.1%, compared to 98.5% with a conversion rate for the conventional procedure of 1.6%, compared to 2.3%. With these values and taking into account that this metanalysis originated from the experience in 1007 patients, compiled from 39 studies, is a good point of comparison. If compared to early mortality, peripheral vascular complications, neurological complications, late developments regarding new procedures or endovascular surgery with conventional surgery and survival, the results are similar with the exception of neurological complications, which were observed in seven cases (2.7%), paraplegia, compared with 0.8% achieved in this paper. Yet, when compared with the experience of Criado et al. [12], there was medullar ischemia of 4.3%, immediately in half of the cases and the other after three days. In none of the cases there was regression of the clinical signs. One of the factors observed by these authors was the extensive coverage of the aorta and, as a factor for improvement of neurological complications, the drainage of the brain-spinal fluid, which in this series was not used. However, the literature confirms that if, in cases of dissections, the site of entry as well as a large portion of the aorta, are not covered, the flow may persist in the false lumen leading to the need for a new procedure. Buffolo et al. [13] on 191 patients with TAD have not had any cases of paraplegia, although they treated, in some cases, the entire descending aorta, using one to six endoprostheses per patient. Rodriguez et al. [10], with a similar number of patients and using only one type of prosthesis, obtained an index of paraplegia of 1.5% and 0.9% of paraparesis. However, the presentation of the EuroSTAR results, with 606 patients analyzed, the rate of neurological complications was 5.2%, while the percentage of paraplegia and paraparesis was 2.5% [14].
After the learning curve and the decrease in diameters of the devices, there were no further technical complications, and there was a substantial reduction in peripheral vascular complications.
Long-term survival of these patients, as well as freedom from reintervention, is one of the main points on which the world literature is lacking. Ricco et al. [7], in a series of 160 patients, present an actuarial survival curve free from death and complications, related to endoprosthesis, of 86% and 63%, respectively, at the end of 12 months. Fattori et al. [9], with a follow-up of 60 months in 457 patients, present at the end of 12, 36 and 60 months, a survival freedom from death of 90.1%, 84.6% and 74.1% and freedom from reintervention of 92.4%, 81.3% and 70.0%, respectively. In our environment, Buffolo et al. [13] showed survival freedom from death, including hospital, of 87.4% after 70 months. Freedom from death obtained in this study, after 12, 36, 60 and 108 months, was 98.7%, 96.0%, 89.1% and 85.1%, respectively. When considering the survival free from reintervention, for the same interval of time, it was 98.2%, 93.6%, 82.7% and 57.2%, respectively. Although the data are comparable to the world literature, due to the large number of patients and a follow-up period of 112 months, one can see that at the end of 42 months the two curves are different, with decline in freedom from reintervention.
Prospective clinical trials should be performed, with long-term follow-up, to provide evidence for proper selection of patients, and to determine which patients will benefit from endovascular procedures.
5. Conclusions
TEVAR procedure, despite of multiple limitations of this study, has a low rate of prostheses related complications and mortality in the immediate postoperative period. After 42 months of follow-up, the rate for reintervention increases, mortality remains stable for a total period of 112 months, being 57.2±10.4% and 85.1±3.8% the values for freedom from reintervention and death, respectively.
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14–17, 2008.
To Prof Dr Moacir Fernandes de Godoy, for his statistical analysis.
References
Conference discussion
Dr. M. Schepens (Nieuwegein, Netherlands): This is one of the largest series in the world with excellent results and a very long follow-up.
I have several critical questions and remarks. What I missed in your presentation and also in your paper is the detailed description of the performed procedures. How many of the endoprostheses started into the arch? How many involved the celiac trunk because you are saying in the beginning of your presentation that you were treating 33% of thoracoabdominals. I suppose, correct me if I am wrong, that this statement is a misunderstanding since these are only thoracic aortic aneurysms and not thoracoabdominals.
You also started with saying that it is a multicenter study, involving three centers, but in your paper on page 5 I see that there were three universities and four private hospitals involved in this large series, which, of course, raises the serious question about the uniformity of patient selection, data collection, and probably seven different ways of dealing with aortic problems.
You also said that you treated 26 acute type B aortic dissections. Was this for rupture, for malperfusion, or other complications or were they fully uncomplicated and if this was the case probably a conservative treatment would have been a much better option.
You didn't mention central neurological complications. Probably you didn't have one, but as you heard from the previous speaker, they had 5% stroke. You also mentioned peripheral neurological complications namely about 3.1% paraplegia if I'm correct. This is exactly the same as our percentage in the open surgical repair of thoracoabdominals, so I am interested to hear your explanation of this relatively high percentage of paraplegia.
And finally, I think your results show and underscore once again the weak point of endovascular treatment of aortic problems; namely, that over a period of <9 years, more than 40% of your patients needed a second aortic intervention.
Dr. Almeida: Only two patients had endoprosthesis starting in the arch. This is a recent experience started in one of the institutions.
We considered thoracoabdominal aneurysms when we treated, in the same procedure, descending and abdominal aortic aneurysms, despite not treating the celiac trunk or the nesenteric portion. In these cases or there was not an enlargement of this portion of the aorta, or it was not technically feasible.
On the second question, you are correct on your assumption, but on the three universities plus the four private institutions, the procedure as performed by the same surgeons and the protocols were the same. That's why we embraced this study multicenter with three university centers, plus our private practice, in four other hospitals that had the same protocols. The protocol for the seven centers was the same with no routine differences between our private and our National Health Service patients, performed at the university.
Thirdly, the patients that had emergency, three of them had ruptured aortas, and 10 malperfusion due to compression. On the other 13 cases that had an understanding that they might rupture or cause malperfusion, the types of treatment were discussed with the patients and they made the final decision. This was done in only two of the three centers, because in the third center, we didn't have the prosthesis available at the emergency site. So we had to do, in those cases, the conventional treatment.
Dr. Schepens: Yes, but I asked about the type B dissection, acute type B dissection. Were these uncomplicated type B's or complicated type B's that you have treated?
Dr. Almeida: Well, we think of them as complicated or with high risk to complicate, type B dissection, as we had rupture or malperfusion in those 26 patients presented. That's why they were done as emergency. As I understand, emergencies are cases that arrive and have to be done at the same time, and they will not wait 12 h for the prosthesis to arrive.
So those cases, which are 11.4%, which represent 29 patients.
And for your last question of the patients that we had neurological problems if we didn't have any central. No, we did not have any central, but we had high cases of paresthesia.
And plus, we had on the postoperative still-hospital stay a case of paraplegia that started on the fifth day of the post-op period which recovered partially only.
Dr. Schepens: Can you explain why you didn't have any central neurologic deficit because in the literature it's varying between 5 and 7%. Does it mean that you didn't go into the aortic arch with your prostheses?
Dr. Almeida: I'm sorry, I didn't hear you.
Dr. Schepens: The proximal landing zone your endoprosthesis, did it start into the distal aortic arch in zero patients because you have no central neurologic problem?
Dr. Almeida: Yes, I can understand that. But I think it might be the technique or might be the way to apply the free flow or the beginning of the graft below the subclavian artery and not go above it, that is to say unless by the tip of the catheter that you have to insert. I think that's the only explanation that I can give to you.
Dr. Schepens: So this means that you didn't go into the aortic arch at all with your endoprostheses?
Dr. Almeida: No. You are correct, unless on those cases in which we used free-flow endoprosthesis anchored to the subclavian artery, which represent 17.2%.
Dr. Schepens: That makes a big difference.
Dr. Almeida: It does, yes. Due to the fact that those aortas, they have disease. And when you start placing the tip of catheter or the endoprosthesis near the left carotid artery, you can dislodge a plaque and have central embolization. It may be luck but, I don't believe in it.
Dr. Schepens: And my final point was the weak point of the endovascular repair which we all know is the number of reoperations over time. You have an event-free --
Dr. Almeida: Oh, yes.
Dr. Schepens: -- survival of 57% at 9 years, so it means that a lot of your patients need later aortic reoperations.
Dr. Almeida: It does. We still don't know, and that's why we're separating, in two groups, under review of the Brazilian Journal of Cardiovascular Surgery, the Braile manufactured endoprosthesis group, which represents, 84.5% of the cases that were performed.
We don't know if it's a structural defect or what happens. We have to divide this group into smaller groups to analyze if it also is dependent on the type of material used to manufacture the endoprosthesis.
But one thing that we think is obvious is that at 42 months, those stent grafts start to fail. And we have to be very cautious about what we are going to do because we thought this was a miraculous cure for the descending aortic aneurysms. But we have to be very cautious on what material we use and to thoroughly follow-up our patients and see what happens with them.




