-
PDF
- Split View
-
Views
-
Cite
Cite
Jade Claessens, Loren Packlé, Hanne Oosterbos, Elke Smeets, Jelena Geens, Jens Gielen, Silke Van Genechten, Samuel Heuts, Jos G Maessen, Alaaddin Yilmaz, Totally endoscopic coronary artery bypass grafting: experience in 1500 patients, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 39, Issue 3, September 2024, ivae159, https://doi.org/10.1093/icvts/ivae159
Close - Share Icon Share
Abstract
Totally endoscopic coronary artery bypass grafting (TECAB) is a minimally invasive approach to achieve surgical revascularization through a minimally invasive approach. Still, data regarding non-robotic TECAB are limited. This report presents the results of a TECAB technique using long-shafted instruments, defined as Endo-CABG, from a single-centre experience in 1500 consecutive patients.
One thousand and five hundred patients underwent Endo-CABG between January 2016 and February 2023. Data were collected retrospectively, and patients were followed up for 1 year. The primary outcome of this study was major adverse cardiac and cerebrovascular events (MACCE)-free survival. Secondary efficacy outcomes were graft failure and mortality. Furthermore, we analysed factors influencing long-term freedom from MACCE and all-cause mortality.
The mean age was 68 [61–75] years, of which 193 (12.87%) were octogenarians. Multivessel disease was present in 1409 (93.93%) patients, and the mean EuroSCORE II was 1.64 [1.09–2.92] %. All patients underwent full arterial revascularization with bilateral internal mammary grafting in 88.47%. Graft failure occurred in 1.80% of cases after 1 year (n = 27). Thirty-day mortality was 1.73% (n = 26), 1-year survival was 94.7% (95% CI: 93.5–95.9%; n = 26) and 1-year MACCE-free survival was 91.7% (95% CI: 90.2–93.2%). Age, left ventricular ejection fraction, arterial hypertension and urgency were significantly associated with 1-year MACCE-free survival.
Endo-CABG appears to be a safe procedure, achieves surgical revascularization and provides good outcomes regarding graft failure and MACCE at 1 year, while age, left ventricular ejection fraction, arterial hypertension and urgency were associated with 1-year outcomes.
INTRODUCTION
Less invasive coronary artery bypass grafting (CABG) procedures emerged in the mid-1990s and developed significantly in the next 25 years. Early results of minimally invasive direct coronary artery bypass grafting (MIDCAB) using an anterior mini-thoracotomy with rib spread approach showed promising results with good patency and low mortality rates [1–3]. In 1998, further development in the field of cardiac surgery led to the first totally endoscopic coronary artery bypass grafting (TECAB), in which not only the harvesting of the bilateral internal mammary arteries (BIMA) was performed endoscopically but also the anastomoses, followed by the first robotic TECAB in 1999 [4, 5]. In contrast to robotic TECAB, non-robotic TECAB is conducted with long-shafted instruments under endoscopic guidance.
In 2020, a new TECAB procedure, defined as Endo-CABG, was described by Yilmaz et al. [6], documenting the results of the first 423 patients. During this procedure, the IMAs are harvested using 3 5 mm ports (bilateral) in the 2nd, 3rd and 4th intercostal space and a utility port of 3–4 cm in the 2nd intercostal space to perform the anastomosis. In that preliminary series, no perioperative mortality was reported, and no conversion to (mini-)sternotomy was required. As such, Endo-CABG seems a promising procedure; however, data regarding non-robotic TECAB are generally scarce, and data from more extensive series are lacking. Therefore, this report presents an extensive series of 1500 consecutive Endo-CABG patients with a 1-year follow-up.
PATIENTS AND METHODS
Ethical statement
On 16 November 2022, the local ethics committee of the Jessa Hospital, Belgium, approved this retrospective cohort study (2022/152). Due to the retrospective nature of this study, the local ethics committee waived the need for written informed consent.
Patients and procedure
In this single-centre retrospective study, an unselected group of consecutive CABG patients was included, all undergoing an Endo-CABG procedure between January 2016 and February 2023. There was no patient selection. All patients were discussed at the heart team meeting, consisting of cardiologists, cardiac surgeons and anaesthesiologists, to make a multidisciplinary decision for percutaneous coronary intervention (PCI) or surgery. The criteria for selection of interventional modality were based on the ESC/EACTS guidelines on myocardial revascularization [7]. No distinction in patient inclusion was made regarding the learning curve. This series also constitutes all patients undergoing primary surgical revascularization from this centre. However, in the absence of the leading surgeon, 55 patients underwent conventional CABG through sternotomy in this period. Patients who had undergone previous cardiac surgery or underwent concomitant surgeries were excluded. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was used to determine surgical risk, and all variables required to calculate EuroSCORE II were presented separately.
Surgical technique
The Endo-CABG procedure was previously described by Yilmaz et al. in 2020 [6]. Three endoscopic ports (5 mm) were bilaterally placed in the 2nd, 3rd and 4th intercostal spaces to harvest the internal mammary arteries. The complete harvested internal mammary arteries were used to perform the anastomoses using a utility port of 3 cm in the 2nd intercostal space through continuous 8–0 sutures. All patients underwent full arterial revascularization. Although endoscopic coronary intervention is often associated with coronary artery anastomosis in an easy-to-reach anatomical location and not in the ideal anatomical location, we believe that our technique allows the identification of the ideal anatomical location. To do so and allow exposure of all coronary territories, an endoscopic grasper covered in surgical gauze (making the tip blunt) is introduced through a subxyphoidal trocar. This blunt instrument is subsequently used to manipulate and tilt the heart into a position that exposes the ideal anastomosis location, similar to gauze-slings used in open cardiac surgery. In the case of extensively diseased, calcified coronary vessels, an endarterectomy is also possible totally endoscopically. When dealing with cases of adhesions due to pericarditis, pleuritis or redo cases, the first step is adhesiolysis, which is performed without cardiopulmonary bypass (CPB) to create a large enough surgical working space. However, the largest challenge is locating and visualizing the intramural coronaries with a high risk of perforating the ventricle. Techniques to localize are the same as in conventional surgery: good insight on the images of the coronary angiograms, gentile soft longitudinal small incisions in the muscle, and, when necessary, multiple incisions close to each other. Anastomoses on intramural coronaries are known for their difficulty, even after locating them. More details about the procedure can also be found elsewhere [8].
Outcomes
The primary objective of this study is major adverse cardiac and cerebrovascular events (MACCE)-free survival 1 year postoperatively. The composite end-point MACCE includes cardiac death, myocardial infarction (MI), stroke [cerebrovascular accident (CVA) and transient ischaemic attack (TIA)] and target lesion revascularization; defined as a reintervention in the target vessel. Myocardial injury is defined as type 5 MI according to the 4th Universal definition of MI and covers CABG-related MI within the first 48 hr of the procedure [9]. In patients with normal baseline cTn values, cTn values must be elevated by >10 times the 99th percentile upper reference level, while in patients with abnormal baseline levels, a >20% increase is needed. Additionally, one of the following criteria is required: development of new pathological Q-waves, angiographically documented new graft occlusion or new native coronary artery occlusion, or imaging evidence of new loss of viable myocardium consistent with an ischaemic aetiology [9]. As a secondary outcome, graft failure is defined as a clinical suspicion of ischaemia [persistent angina and/or electrocardiogram (ECG)-abnormalities] with impaired flow of the graft or target vessel or asymptomatic stenosis of the graft or anastomosis >75% [10, 11], is investigated. Other outcomes encompassed the hospital and intensive care unit (ICU) lengths of stay (LOS), ventilation time, 24 hr postoperative bleeding (in mL) and complications, including surgical revisions (<48 hr, <1 week), neurological complications, new-onset atrial fibrillation and pacemaker implantation. The urgency of the surgery is defined according to the criteria of the EuroSCORE II as elective (routine admission), urgent (patients who are not electively admitted and require intervention during the current admission for medical reasons), emergency (operation needed before the next working day) and salvage (patients requiring cardiopulmonary resuscitation) [12]. Data are collected through the patient’s medical file and through the online portal of the Belgian Government to collect and share healthcare data.
Data analysis
Normality was tested using the Kolmogorov-Smirnov test. Data are expressed as frequencies (%), numbers (n), and as mean ± standard deviation or median interquartile ranges [p25–p75]. The survival was assessed using a Kaplan–Meier analysis for all-cause mortality and MACCE-free survival (defined as alive without MACCE). Furthermore, a uni- and multi-variable Cox-regression model was constructed to evaluate important covariates for MACCE-free survival. These variables were selected based on either statistical significance (P < 0.05) or clinical relevance. For all analyses, a P-value < 0.05 was considered statistically significant. All data were analysed using an intention-to-treat principle in R Core Team (2021), R: A language and environment for statistical computing, Vienna, Austria.
RESULTS
Demographics
A total of 1500 consecutive patients presented themselves for a CABG procedure, of which all patients underwent endo-CABG, with a median follow-up of 365 [247–365] days and a follow-up index of 1.00 [0.84–1.00]. The median age was 68 [61–75] years, including 193 (12.87%) octogenarians. The majority of patients were males (n = 1243, 82.87%). The EuroSCORE II was 1.64% [1.09–2.92], corresponding to a low-intermediate mortality risk. Most patients (93.93%) had multivessel disease, and left main disease was present in 35.83% of cases. All demographic variables are displayed in Table 1.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Age (years) | 68 [61–75] |
| Octogenarians | 193 (12.87) |
| Male patients | 1243 (82.87) |
| BMI (kg/m2) | 27.13 [24.73–30.12] |
| NYHA class | |
| − I | 536 (35.76) |
| − II | 704 (46.96) |
| − III | 218 (14.54) |
| − IV | 41 (2.74) |
| LVEF (%) | |
| − Good (>50%) | 1113 (80.65) |
| − Moderate (31–50%) | 224 (16.23) |
| − Poor (21–30%) | 38 (2.75) |
| − Very poor (<20%) | 5 (0.36) |
| EuroSCORE II (%) | 1.64 [1.09–2.92] |
| Arterial hypertension | 1012 (67.47) |
| Diabetes mellitus | |
| − Type 1 | 21 (1.40) |
| − Type 2 | 444 (29.60) |
| Hyperlipidaemia | 1214 (80.93) |
| Smoking history | |
| − Active | 368 (24.58) |
| − Ceased | 379 (25.32) |
| Chronic obstructive pulmonary disease | 107 (7.13) |
| Neurologic history | |
| − CVA | 29 (1.93) |
| − TIA | 18 (1.20) |
| Peripheral vascular disease | 225 (15.00) |
| Impaired renal function | 319 (21.27) |
| Atrial fibrillation | 142 (9.47) |
| Symptoms | |
| − Stable angina | 691 (46.28) |
| − Unstable angina | 243 (16.28) |
| − STEMI | 71 (4.76) |
| − NSTEMI | 233 (15.61) |
| − Dyspnoea | 287 (19.13) |
| − Cardiogenic shock | 8 (0.54) |
| − No symptoms | 173 (11.59) |
| − Others | 97 (0.54) |
| Use of DAPT | 254 (16.93) |
| Coronary vessel disease | |
| − Single vessel disease | 91 (6.07) |
| − Double vessel disease | 440 (29.33) |
| − Three vessel disease | 969 (64.60) |
| − Left main disease | 535 (35.83) |
| Urgency | |
| - Elective | 1117 (74.47) |
| - Urgent | 322 (21.47) |
| - Emergency | 55 (3.67) |
| - Salvage | 6 (0.40) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Age (years) | 68 [61–75] |
| Octogenarians | 193 (12.87) |
| Male patients | 1243 (82.87) |
| BMI (kg/m2) | 27.13 [24.73–30.12] |
| NYHA class | |
| − I | 536 (35.76) |
| − II | 704 (46.96) |
| − III | 218 (14.54) |
| − IV | 41 (2.74) |
| LVEF (%) | |
| − Good (>50%) | 1113 (80.65) |
| − Moderate (31–50%) | 224 (16.23) |
| − Poor (21–30%) | 38 (2.75) |
| − Very poor (<20%) | 5 (0.36) |
| EuroSCORE II (%) | 1.64 [1.09–2.92] |
| Arterial hypertension | 1012 (67.47) |
| Diabetes mellitus | |
| − Type 1 | 21 (1.40) |
| − Type 2 | 444 (29.60) |
| Hyperlipidaemia | 1214 (80.93) |
| Smoking history | |
| − Active | 368 (24.58) |
| − Ceased | 379 (25.32) |
| Chronic obstructive pulmonary disease | 107 (7.13) |
| Neurologic history | |
| − CVA | 29 (1.93) |
| − TIA | 18 (1.20) |
| Peripheral vascular disease | 225 (15.00) |
| Impaired renal function | 319 (21.27) |
| Atrial fibrillation | 142 (9.47) |
| Symptoms | |
| − Stable angina | 691 (46.28) |
| − Unstable angina | 243 (16.28) |
| − STEMI | 71 (4.76) |
| − NSTEMI | 233 (15.61) |
| − Dyspnoea | 287 (19.13) |
| − Cardiogenic shock | 8 (0.54) |
| − No symptoms | 173 (11.59) |
| − Others | 97 (0.54) |
| Use of DAPT | 254 (16.93) |
| Coronary vessel disease | |
| − Single vessel disease | 91 (6.07) |
| − Double vessel disease | 440 (29.33) |
| − Three vessel disease | 969 (64.60) |
| − Left main disease | 535 (35.83) |
| Urgency | |
| - Elective | 1117 (74.47) |
| - Urgent | 322 (21.47) |
| - Emergency | 55 (3.67) |
| - Salvage | 6 (0.40) |
BMI: body mass index; CVA: cerebrovascular event; DAPT: dual antiplatelet therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; (N)STEMI: (non-)ST-elevation myocardial infarction; TIA: transient ischaemic attack.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Age (years) | 68 [61–75] |
| Octogenarians | 193 (12.87) |
| Male patients | 1243 (82.87) |
| BMI (kg/m2) | 27.13 [24.73–30.12] |
| NYHA class | |
| − I | 536 (35.76) |
| − II | 704 (46.96) |
| − III | 218 (14.54) |
| − IV | 41 (2.74) |
| LVEF (%) | |
| − Good (>50%) | 1113 (80.65) |
| − Moderate (31–50%) | 224 (16.23) |
| − Poor (21–30%) | 38 (2.75) |
| − Very poor (<20%) | 5 (0.36) |
| EuroSCORE II (%) | 1.64 [1.09–2.92] |
| Arterial hypertension | 1012 (67.47) |
| Diabetes mellitus | |
| − Type 1 | 21 (1.40) |
| − Type 2 | 444 (29.60) |
| Hyperlipidaemia | 1214 (80.93) |
| Smoking history | |
| − Active | 368 (24.58) |
| − Ceased | 379 (25.32) |
| Chronic obstructive pulmonary disease | 107 (7.13) |
| Neurologic history | |
| − CVA | 29 (1.93) |
| − TIA | 18 (1.20) |
| Peripheral vascular disease | 225 (15.00) |
| Impaired renal function | 319 (21.27) |
| Atrial fibrillation | 142 (9.47) |
| Symptoms | |
| − Stable angina | 691 (46.28) |
| − Unstable angina | 243 (16.28) |
| − STEMI | 71 (4.76) |
| − NSTEMI | 233 (15.61) |
| − Dyspnoea | 287 (19.13) |
| − Cardiogenic shock | 8 (0.54) |
| − No symptoms | 173 (11.59) |
| − Others | 97 (0.54) |
| Use of DAPT | 254 (16.93) |
| Coronary vessel disease | |
| − Single vessel disease | 91 (6.07) |
| − Double vessel disease | 440 (29.33) |
| − Three vessel disease | 969 (64.60) |
| − Left main disease | 535 (35.83) |
| Urgency | |
| - Elective | 1117 (74.47) |
| - Urgent | 322 (21.47) |
| - Emergency | 55 (3.67) |
| - Salvage | 6 (0.40) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Age (years) | 68 [61–75] |
| Octogenarians | 193 (12.87) |
| Male patients | 1243 (82.87) |
| BMI (kg/m2) | 27.13 [24.73–30.12] |
| NYHA class | |
| − I | 536 (35.76) |
| − II | 704 (46.96) |
| − III | 218 (14.54) |
| − IV | 41 (2.74) |
| LVEF (%) | |
| − Good (>50%) | 1113 (80.65) |
| − Moderate (31–50%) | 224 (16.23) |
| − Poor (21–30%) | 38 (2.75) |
| − Very poor (<20%) | 5 (0.36) |
| EuroSCORE II (%) | 1.64 [1.09–2.92] |
| Arterial hypertension | 1012 (67.47) |
| Diabetes mellitus | |
| − Type 1 | 21 (1.40) |
| − Type 2 | 444 (29.60) |
| Hyperlipidaemia | 1214 (80.93) |
| Smoking history | |
| − Active | 368 (24.58) |
| − Ceased | 379 (25.32) |
| Chronic obstructive pulmonary disease | 107 (7.13) |
| Neurologic history | |
| − CVA | 29 (1.93) |
| − TIA | 18 (1.20) |
| Peripheral vascular disease | 225 (15.00) |
| Impaired renal function | 319 (21.27) |
| Atrial fibrillation | 142 (9.47) |
| Symptoms | |
| − Stable angina | 691 (46.28) |
| − Unstable angina | 243 (16.28) |
| − STEMI | 71 (4.76) |
| − NSTEMI | 233 (15.61) |
| − Dyspnoea | 287 (19.13) |
| − Cardiogenic shock | 8 (0.54) |
| − No symptoms | 173 (11.59) |
| − Others | 97 (0.54) |
| Use of DAPT | 254 (16.93) |
| Coronary vessel disease | |
| − Single vessel disease | 91 (6.07) |
| − Double vessel disease | 440 (29.33) |
| − Three vessel disease | 969 (64.60) |
| − Left main disease | 535 (35.83) |
| Urgency | |
| - Elective | 1117 (74.47) |
| - Urgent | 322 (21.47) |
| - Emergency | 55 (3.67) |
| - Salvage | 6 (0.40) |
BMI: body mass index; CVA: cerebrovascular event; DAPT: dual antiplatelet therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; (N)STEMI: (non-)ST-elevation myocardial infarction; TIA: transient ischaemic attack.
Intraoperative outcomes
The total operating room (OR) time was 228 [189–272] min, with a CPB time of 98 [78–125] min and an aortic cross-clamping (AoXC) time of 57 [45–71] min. An additional mini-sternotomy was needed in 12 (0.80%) patients when peripheral cannulation was not possible through femoral or subclavian access, while a conversion rate to a median sternotomy of 0.40% was observed (n = 6). The median number of bypasses performed was 3 [2–3]. The most common target vessel was the left anterior descending (LAD) artery (96.86%), followed by the marginal obtuse (MO) (68.09%) and diagonal branches (39.92%). BIMA grafting was performed in 88.47% of patients (n = 1327), for which a Y-graft was used in 634 patients and BIMA in situ in 693 patients. Intraoperative outcomes are described in Table 2.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| CPB time (min) | 98 [78–125] |
| AoXC time (min) | 57.00 [45–71] |
| OR time (min) | 228.00 [189–272] |
| Conversion to sternotomy | 6 (0.40) |
| Cannulation site | |
| − Femoralis | 1438 (95.87) |
| − Subclavian | 44 (2.93) |
| − Aorta | 18 (1.20) |
| Target vessel | |
| − LAD | 1451 (96.86) |
| − D | 598 (39.92) |
| − MO | 1020 (68.09) |
| − PLCX | 126 (8.41) |
| − AL | 213 (14.22) |
| − RDP | 292 (19.49) |
| − RCA | 88 (5.87) |
| − PLR | 69 (4.61) |
| Number of bypasses | 3.00 [2.00–3.00] |
| Single LIMA | 162 (10.80) |
| Single RIMA | 11 (0.73) |
| BIMA in-situ | 693 (46.20) |
| Y-graft | 634 (42.27) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| CPB time (min) | 98 [78–125] |
| AoXC time (min) | 57.00 [45–71] |
| OR time (min) | 228.00 [189–272] |
| Conversion to sternotomy | 6 (0.40) |
| Cannulation site | |
| − Femoralis | 1438 (95.87) |
| − Subclavian | 44 (2.93) |
| − Aorta | 18 (1.20) |
| Target vessel | |
| − LAD | 1451 (96.86) |
| − D | 598 (39.92) |
| − MO | 1020 (68.09) |
| − PLCX | 126 (8.41) |
| − AL | 213 (14.22) |
| − RDP | 292 (19.49) |
| − RCA | 88 (5.87) |
| − PLR | 69 (4.61) |
| Number of bypasses | 3.00 [2.00–3.00] |
| Single LIMA | 162 (10.80) |
| Single RIMA | 11 (0.73) |
| BIMA in-situ | 693 (46.20) |
| Y-graft | 634 (42.27) |
AL: anterolateral; AoXC: aortic cross-clamping; BIMA: bilateral internal mammary arteries; D: diagonal; LAD: left anterior descending; LIMA: left internal mammary artery; MO: marginal obtuse; OR: operating room; PLCX: proximal left circumflex; PLR: posterolateral right artery; RCA: right coronary artery; RDP: right descending posterior; RIMA: right internal mammary artery.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| CPB time (min) | 98 [78–125] |
| AoXC time (min) | 57.00 [45–71] |
| OR time (min) | 228.00 [189–272] |
| Conversion to sternotomy | 6 (0.40) |
| Cannulation site | |
| − Femoralis | 1438 (95.87) |
| − Subclavian | 44 (2.93) |
| − Aorta | 18 (1.20) |
| Target vessel | |
| − LAD | 1451 (96.86) |
| − D | 598 (39.92) |
| − MO | 1020 (68.09) |
| − PLCX | 126 (8.41) |
| − AL | 213 (14.22) |
| − RDP | 292 (19.49) |
| − RCA | 88 (5.87) |
| − PLR | 69 (4.61) |
| Number of bypasses | 3.00 [2.00–3.00] |
| Single LIMA | 162 (10.80) |
| Single RIMA | 11 (0.73) |
| BIMA in-situ | 693 (46.20) |
| Y-graft | 634 (42.27) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| CPB time (min) | 98 [78–125] |
| AoXC time (min) | 57.00 [45–71] |
| OR time (min) | 228.00 [189–272] |
| Conversion to sternotomy | 6 (0.40) |
| Cannulation site | |
| − Femoralis | 1438 (95.87) |
| − Subclavian | 44 (2.93) |
| − Aorta | 18 (1.20) |
| Target vessel | |
| − LAD | 1451 (96.86) |
| − D | 598 (39.92) |
| − MO | 1020 (68.09) |
| − PLCX | 126 (8.41) |
| − AL | 213 (14.22) |
| − RDP | 292 (19.49) |
| − RCA | 88 (5.87) |
| − PLR | 69 (4.61) |
| Number of bypasses | 3.00 [2.00–3.00] |
| Single LIMA | 162 (10.80) |
| Single RIMA | 11 (0.73) |
| BIMA in-situ | 693 (46.20) |
| Y-graft | 634 (42.27) |
AL: anterolateral; AoXC: aortic cross-clamping; BIMA: bilateral internal mammary arteries; D: diagonal; LAD: left anterior descending; LIMA: left internal mammary artery; MO: marginal obtuse; OR: operating room; PLCX: proximal left circumflex; PLR: posterolateral right artery; RCA: right coronary artery; RDP: right descending posterior; RIMA: right internal mammary artery.
Postoperative outcomes
The median ICU stay was 48 [25–88] hr, with a ventilation time of 5.5 [3.5–9.5] hr. The 24-hr blood loss was 460 [240–820] mL, with the need for thoracoscopic re-exploration for bleeding in 4.73% (n = 71). In 92.96% of these early revisions, active bleeding was found. A late surgical re-exploration was needed in 1.00% of patients (n = 15).
Eighty-three patients (5.53%) experienced neurological symptoms, of which 19 (1.27%) were diagnosed with a CVA and 8 (0.53%) with a TIA through imaging. Fourteen of the patients who endured a CVA fully recovered afterwards. Additionally, 5 other patients (0.33%) developed epilepsy, while 51 (3.40%) experienced delirium.
Graft failure occurred in 11 (0.73%) patients during the hospital stay and was solved through endoscopic re-exploration. After 1 year of follow-up, graft failure was 1.80% (n = 27). There was no systematic follow-up of graft failure, and all cases were discovered after clinical symptoms or incidental findings. A direct post-operative re-anastomosis was needed in 5 patients, and excessive blood loss in the ICU occurred in 3 patients. One patient had increased perfusion pressure at the ICU. Moreover, graft failure was discovered in 5 patients during the postoperative PCI in the context of hybrid revascularization. Another 5 patients endured an MI, and 8 patients had recurrent angina that led to the discovery. In 7 patients where graft failure occurred in-hospital, there was a rupture of the graft, while during follow-up, 10 patients had an occluded graft. In the remaining 10 patients, the grafts were afunctional due to, e.g. competitive flow. Of the 27 patients with graft failure, 23 required a target lesion revascularization. In 15 patients, the revascularization was performed through PCI; a surgical revision was needed in 8 patients. Here, the surgical revisions were also performed endoscopically, except in 1 case where a mini-sternotomy was required. All postoperative outcomes are presented in Table 3.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Ventilation time (h) | 5.5 [3.5–9.5] |
| Bleeding 24 hr (ml) | 460 [240–820] |
| ICU LOS (h) | 48 [25–88] |
| Hospital LOS (days) | 6 [4–8] |
| Permanent pacemaker implantation (in hospital) | 11 (0.73) |
| Early revision (<48 hr) | 97 (6.47) |
| − Bleeding | 71 (4.73) |
| − Active bleeding | 66 (4.40) |
| − Tamponade | 25 (1.67) |
| − Empyema | 1 (0.07) |
| Late revision (>48 hr) | 15 (1.00) |
| − Bleeding | 5 (0.33) |
| − Active bleeding | 2 (0.13) |
| − Tamponade | 2 (0.13) |
| − Graft failure | 2 (0.13) |
| − Air leakage | 2 (0.13) |
| − Empyema | 2 (0.13) |
| − Other | 2 (0.13) |
| Neurological complications | |
| − CVA | 19 (1.27) |
| − TIA | 8 (0.53) |
| − Neurological insult | 5 (0.33) |
| − Delirium | 51 (3.40) |
| New onset atrial fibrillation | 319 (21.27) |
| Graft failure | |
| − In-hospital | 11 (0.73) |
| − Follow-up | 27 (1.80) |
| Target lesion revascularization | 16 (1.07) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Ventilation time (h) | 5.5 [3.5–9.5] |
| Bleeding 24 hr (ml) | 460 [240–820] |
| ICU LOS (h) | 48 [25–88] |
| Hospital LOS (days) | 6 [4–8] |
| Permanent pacemaker implantation (in hospital) | 11 (0.73) |
| Early revision (<48 hr) | 97 (6.47) |
| − Bleeding | 71 (4.73) |
| − Active bleeding | 66 (4.40) |
| − Tamponade | 25 (1.67) |
| − Empyema | 1 (0.07) |
| Late revision (>48 hr) | 15 (1.00) |
| − Bleeding | 5 (0.33) |
| − Active bleeding | 2 (0.13) |
| − Tamponade | 2 (0.13) |
| − Graft failure | 2 (0.13) |
| − Air leakage | 2 (0.13) |
| − Empyema | 2 (0.13) |
| − Other | 2 (0.13) |
| Neurological complications | |
| − CVA | 19 (1.27) |
| − TIA | 8 (0.53) |
| − Neurological insult | 5 (0.33) |
| − Delirium | 51 (3.40) |
| New onset atrial fibrillation | 319 (21.27) |
| Graft failure | |
| − In-hospital | 11 (0.73) |
| − Follow-up | 27 (1.80) |
| Target lesion revascularization | 16 (1.07) |
CVA: cerebrovascular accident; ICU: intensive care unit: LOS: lengths of stay; TIA: transient ischaemic attack.
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Ventilation time (h) | 5.5 [3.5–9.5] |
| Bleeding 24 hr (ml) | 460 [240–820] |
| ICU LOS (h) | 48 [25–88] |
| Hospital LOS (days) | 6 [4–8] |
| Permanent pacemaker implantation (in hospital) | 11 (0.73) |
| Early revision (<48 hr) | 97 (6.47) |
| − Bleeding | 71 (4.73) |
| − Active bleeding | 66 (4.40) |
| − Tamponade | 25 (1.67) |
| − Empyema | 1 (0.07) |
| Late revision (>48 hr) | 15 (1.00) |
| − Bleeding | 5 (0.33) |
| − Active bleeding | 2 (0.13) |
| − Tamponade | 2 (0.13) |
| − Graft failure | 2 (0.13) |
| − Air leakage | 2 (0.13) |
| − Empyema | 2 (0.13) |
| − Other | 2 (0.13) |
| Neurological complications | |
| − CVA | 19 (1.27) |
| − TIA | 8 (0.53) |
| − Neurological insult | 5 (0.33) |
| − Delirium | 51 (3.40) |
| New onset atrial fibrillation | 319 (21.27) |
| Graft failure | |
| − In-hospital | 11 (0.73) |
| − Follow-up | 27 (1.80) |
| Target lesion revascularization | 16 (1.07) |
| Variables . | Median [p25–p75] or n (%) . |
|---|---|
| Ventilation time (h) | 5.5 [3.5–9.5] |
| Bleeding 24 hr (ml) | 460 [240–820] |
| ICU LOS (h) | 48 [25–88] |
| Hospital LOS (days) | 6 [4–8] |
| Permanent pacemaker implantation (in hospital) | 11 (0.73) |
| Early revision (<48 hr) | 97 (6.47) |
| − Bleeding | 71 (4.73) |
| − Active bleeding | 66 (4.40) |
| − Tamponade | 25 (1.67) |
| − Empyema | 1 (0.07) |
| Late revision (>48 hr) | 15 (1.00) |
| − Bleeding | 5 (0.33) |
| − Active bleeding | 2 (0.13) |
| − Tamponade | 2 (0.13) |
| − Graft failure | 2 (0.13) |
| − Air leakage | 2 (0.13) |
| − Empyema | 2 (0.13) |
| − Other | 2 (0.13) |
| Neurological complications | |
| − CVA | 19 (1.27) |
| − TIA | 8 (0.53) |
| − Neurological insult | 5 (0.33) |
| − Delirium | 51 (3.40) |
| New onset atrial fibrillation | 319 (21.27) |
| Graft failure | |
| − In-hospital | 11 (0.73) |
| − Follow-up | 27 (1.80) |
| Target lesion revascularization | 16 (1.07) |
CVA: cerebrovascular accident; ICU: intensive care unit: LOS: lengths of stay; TIA: transient ischaemic attack.
Major adverse cardiac and cerebrovascular events and all-cause mortality
The in-hospital and 30-day mortality rate was 1.73% (n = 26), while the overall survival after 1 year was 94.7% (95% confidence interval [CI]: 93.5–95.9%; Fig. 2). The 1-year MACCE-free survival was 91.7% (95% CI: 90.2–93.2%; Fig. 1). In total, 83 (5.53%) patients endured a MACCE, 43 (2.87%) during the first 30 days. Thirty-two (2.13%) patients died due to a cardiac-related cause, 12 patients endured an MI and 23 suffered a stroke during follow-up. Lastly, 16 patients required a target lesion revascularization.
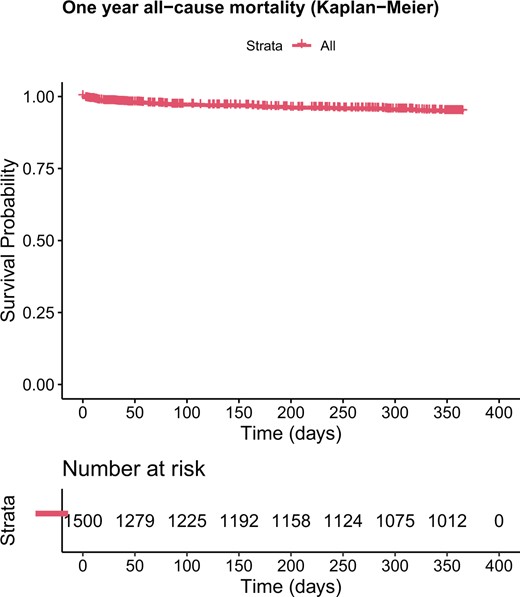
Estimated survival of the all-cause mortality (Kaplan–Meier) after 1 year.

Estimated major adverse cardiac and cerebrovascular events-free survival (Kaplan–Meier) after 1 year.
Predictors of major adverse cardiac and cerebrovascular events-free survival
All uni-and multivariable factors that were tested can be found in Supplementary Material, Table S1. Our final model showed that age, left ventricular ejection fraction (LVEF), arterial hypertension and urgency were significantly associated with 1-year MACCE-free survival (Table 4).
| . | Coefficient . | SE . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Age | 0.051 | 0.011 | 1.052 | 1.029–1.075 | <0.001 |
| Sex | 0.193 | 0.263 | 1.213 | 0.724–2.031 | 0.463 |
| LVEF | |||||
| − Good (>50%) | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Moderate (31–50%) | 0.596 | 0.225 | 1.814 | 1.168–2.819 | 0.008 |
| − Poor (21–30%) | 0.440 | 0.468 | 1.557 | 0.623–3.895 | 0.344 |
| − Very poor (<20%) | −14.810 | 202.080 | <0.001 | 0 Inf | 0.995 |
| Arterial hypertension | 0.518 | 0.243 | 1.678 | 1.043–2.700 | 0.033 |
| Urgency | |||||
| − Elective | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Urgent | 0.523 | 0.220 | 1.687 | 1.096–2.599 | 0.018 |
| − Emergency | 1.463 | 0.348 | 4.320 | 2.184–8.547 | <0.001 |
| − Salvage | 1.854 | 0.773 | 6.383 | 1.404–29.025 | 0.016 |
| . | Coefficient . | SE . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Age | 0.051 | 0.011 | 1.052 | 1.029–1.075 | <0.001 |
| Sex | 0.193 | 0.263 | 1.213 | 0.724–2.031 | 0.463 |
| LVEF | |||||
| − Good (>50%) | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Moderate (31–50%) | 0.596 | 0.225 | 1.814 | 1.168–2.819 | 0.008 |
| − Poor (21–30%) | 0.440 | 0.468 | 1.557 | 0.623–3.895 | 0.344 |
| − Very poor (<20%) | −14.810 | 202.080 | <0.001 | 0 Inf | 0.995 |
| Arterial hypertension | 0.518 | 0.243 | 1.678 | 1.043–2.700 | 0.033 |
| Urgency | |||||
| − Elective | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Urgent | 0.523 | 0.220 | 1.687 | 1.096–2.599 | 0.018 |
| − Emergency | 1.463 | 0.348 | 4.320 | 2.184–8.547 | <0.001 |
| − Salvage | 1.854 | 0.773 | 6.383 | 1.404–29.025 | 0.016 |
CI: confidence interval; HR: hazard ratio; LVEF: left ventricular ejection fraction; MACCE: major adverse cardiac and cerebrovascular events; SE: standard error.
| . | Coefficient . | SE . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Age | 0.051 | 0.011 | 1.052 | 1.029–1.075 | <0.001 |
| Sex | 0.193 | 0.263 | 1.213 | 0.724–2.031 | 0.463 |
| LVEF | |||||
| − Good (>50%) | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Moderate (31–50%) | 0.596 | 0.225 | 1.814 | 1.168–2.819 | 0.008 |
| − Poor (21–30%) | 0.440 | 0.468 | 1.557 | 0.623–3.895 | 0.344 |
| − Very poor (<20%) | −14.810 | 202.080 | <0.001 | 0 Inf | 0.995 |
| Arterial hypertension | 0.518 | 0.243 | 1.678 | 1.043–2.700 | 0.033 |
| Urgency | |||||
| − Elective | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Urgent | 0.523 | 0.220 | 1.687 | 1.096–2.599 | 0.018 |
| − Emergency | 1.463 | 0.348 | 4.320 | 2.184–8.547 | <0.001 |
| − Salvage | 1.854 | 0.773 | 6.383 | 1.404–29.025 | 0.016 |
| . | Coefficient . | SE . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Age | 0.051 | 0.011 | 1.052 | 1.029–1.075 | <0.001 |
| Sex | 0.193 | 0.263 | 1.213 | 0.724–2.031 | 0.463 |
| LVEF | |||||
| − Good (>50%) | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Moderate (31–50%) | 0.596 | 0.225 | 1.814 | 1.168–2.819 | 0.008 |
| − Poor (21–30%) | 0.440 | 0.468 | 1.557 | 0.623–3.895 | 0.344 |
| − Very poor (<20%) | −14.810 | 202.080 | <0.001 | 0 Inf | 0.995 |
| Arterial hypertension | 0.518 | 0.243 | 1.678 | 1.043–2.700 | 0.033 |
| Urgency | |||||
| − Elective | Ref. | Ref. | Ref. | Ref. | Ref. |
| − Urgent | 0.523 | 0.220 | 1.687 | 1.096–2.599 | 0.018 |
| − Emergency | 1.463 | 0.348 | 4.320 | 2.184–8.547 | <0.001 |
| − Salvage | 1.854 | 0.773 | 6.383 | 1.404–29.025 | 0.016 |
CI: confidence interval; HR: hazard ratio; LVEF: left ventricular ejection fraction; MACCE: major adverse cardiac and cerebrovascular events; SE: standard error.
Older patients are at greater risk for enduring a MACCE or mortality with a hazard ratio (HR) of 1.052 (95% CI: 1.029–1.075%). Additionally, LVEF influences MACCE-free survival. Patients with a moderate (31–50%) LVEF are at higher risk compared to patients with a good (>50%) LVEF (HR: 1.814; 95% CI: 1.168–2.819%). Arterial hypertension also increases the risk with an HR of 1.678 (95% CI: 1.043–2.700%). Lastly, as the urgency of the surgery increases, the higher the risk for MACCE or mortality. The HR increases from 1.687 (95% CI: 1.096–2.599%) in urgent patients to 4.320 (95% CI: 2.184–8.547%) in emergency patients and 6.383 (95% CI: 1.404–29.025%) in salvage patients.
DISCUSSION
This retrospective trial of 1500 consecutive patients that underwent Endo-CABG showed (i) a MACCE-free survival after 1 year of 91.7% with age, LVEF, arterial hypertension and urgency as significant predictors (ii) a graft failure rate of 1.80% after a 1-year follow-up period, (iii) in the case of multivessel coronary disease, BIMA was used, effectively grafting 88.67% of patients fully arterial (iv) a 30-day mortality rate of 1.73%, with an overall survival after 1 year of 94.7%.
Since the introduction of TECAB in the late 90s, research has focused mainly on robotic TECAB. Due to the technical challenge of using long-shafted instruments, the implementation of this technique is not yet widespread. However, an advantage of Endo-CABG over robotic TECAB is that it may be less expensive, and patient selection is less of an issue, making this a suitable technique for patients with increased risk factors, including obesity and diabetes [13]. Furthermore, robotic assistance has specific logistic requirements, including OR availability and dedicated personnel. Additionally, robotic TECAB is mainly performed without the use of CPB. Previous trials have demonstrated off-pump CABG to be beneficial for short-term outcomes. Still, it may be subjected to incomplete revascularization and reduced graft patency, potentially leading to impaired prognosis [14]. Indeed, incomplete revascularization could lead to residual ischaemia, which requires repeat revascularization and has an eventual adverse effect on the long-term MACCE [15, 16].
To our knowledge, we evaluated the results of 1500 non-robotic TECAB cases, which is the most extensive series reported thus far. When comparing our data to literature, it is essential to note that no patient selection has been made. All patients with an indication for CABG underwent Endo-CABG at our centre, except for 55 patients who needed emergency surgery in the absence of the leading surgeon. Still, the observed graft failure of 1.80% cannot be translated entirely to graft patency since not all patients underwent coronary angiography or computed tomographic angiography post-surgery/during follow-up. The patients with graft failure reported symptoms or were incidental findings. Therefore, asymptomatic patients with possible graft failure may have remained undetected. A meta-analysis performed by Gaudino et al. [17] showed a pooled graft failure of 9.7% of left internal mammary artery (LIMA), 23% of right internal mammary artery (RIMA) and 13.8% of radial grafts, which is higher than our observations. However, the abovementioned disclaimer of possibly undetected graft failure due to the lack of systematic graft failure follow-up should be borne in mind. In our cohort, all patients received total arterial revascularization. The median number of bypasses was 3.00 [2.00–3.00], and a Y-graft was created in 42.27% of cases. A Y-graft was constructed in 50% of the patients with graft failure, while in 25%, more than 3 anastomoses were created. Every additional anastomosis may increase the probability of graft failure. Of note, in 330 patients, a hybrid procedure was performed. A reverse (PCI first) hybrid procedure was done in 82 patients, while in 244 patients, the PCI followed after the surgery. There were various reasons to choose a hybrid. In most cases, the left side (LAD and Cx) was surgically revascularized while a PCI was done on the right. These 330 patients were all pre-planned hybrids.
Of the 20 patients with graft failure, a conservative approach was chosen in 4, while 16 (1.07%) patients required target lesion revascularization. Leonard et al. [18] reported a pooled event rate for repeated revascularization of 2.99%.
Central cannulation was needed in 18 patients (1.20%), of which 6 (0.4%) were converted to a median sternotomy. These results are lower compared to the 1.6% after MIDCAB surgery, according to the systematic review of Bonatti et al. [19]. For TECAB, conversion rates vary widely from 0.2% to 10% in literature [20–22].
A consequence of avoiding a median sternotomy during Endo-CABG is that CPB is connected peripherally using the femoral artery and vein, leading to retrograde arterial perfusion. In theory, retrograde arterial perfusion is associated with a higher incidence of neurological complications [23]. However, a recent study showed that the incidence of poor neurological outcomes after Endo-CABG is low and comparable to PCI [24]. A CVA occurred in 19 (1.27%) patients in our patient cohort, while a TIA was observed in 8 (0.53%). In TECAB patients, the pooled event rate of a meta-analysis for perioperative stroke was 1.50% [18]. Computed tomography angiography is not routinely performed in all patients pre-operatively to minimize the adverse effects of contrast-enhancing agents and radiation. During pre-operative evaluation, patients are screened to determine individuals who are unsuited for retrograde femoral artery perfusion. A computed tomography angiography was performed if patients had a history of central or peripheral vascular intervention or presented themselves with signs of peripheral vascular disease or during physical examination.
A revision for bleeding was needed in 4.73% of patients, which is at the upper end of the spectrum found in literature. After conventional cardiac surgery, a reintervention seems necessary in 2–6% of cases, while after off-pump TECAB, revision rates as low as 1.1% have been reported [22]. An active bleeding was found in 92.96% of revisions in our experience. An explanation for the higher occurrence of bleeding is that dual antiplatelet therapy (DAPT) was not ceased preoperatively in 16.93% of patients due to vital indications, such as recent PCI. Although re-exploration is traditionally associated with postoperative infection [25], in endoscopic surgery, the small incision reduces the risk of infection and mediastinitis, lowering the re-exploration threshold. We observed no postoperative mediastinitis, and empyema occurred in 3 patients, indicating a reduced infection rate after endoscopic surgery.
In our patient cohort, the median hospital LOS was 6.00 [4.00–8.00] days, which is slightly higher compared to different studies regarding TECAB (Balkhy et al.: 2.72 ± 1.29 days; Bonaros et al.: mean of 6 (min: 2–max: 54) days; Argenziano et al.: 5.1 ± 3.4 days) [20–22]. The extended hospital stay is probably due to the longer ICU LOS of 48.00 [25.00–88.00] hr, which is longer than the reported TECAB ICU LOS (Balkhy et al.: 1.30 ± 0.69 days; Bonaros et al.: 35 ± 37 hr; Argenziano et al.: 23 (min: 11−max: 1048) hr) [20–22]. Additionally, differences in LOS can be explained by variances in government payment systems and a variety in postoperative protocols, as our hospital lacks an intermediate care unit (MCU). Consequently, the total time spent in the ICU and MCU is reflected in the ICU LOS.
Limitations
It is crucial to recognize that this single-centre study has some inherent limitations, mainly related to the retrospective nature. This may lead to missing data when the follow-up of the patients is conducted in a different hospital. Additionally, it is difficult to determine whether complete revascularization is achieved. The single-centre design may be less generalizable to other institutions, but it does reflect a complete overview of all consecutive Endo-CABG procedures, ensuring high internal validity. Additionally, no standard coronary angiography or computed tomographic angiography was undertaken to assess the graft patency, which may lead to missing data. Lastly, since all elective patients in our centre are referred to Endo-CABG, no comparator was available to compare the results.
CONCLUSION
In conclusion, we present a large single-centre series of 1500 patients who underwent Endo-CABG using long-shafted instruments. This technique shows good results regarding graft failure and 1-year mortality rate. However, long-term results are to be awaited.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
FUNDING
No external funding was granted.
Conflict of interest: The authors report no conflict of interest.
DATA AVAILABILITY
The article’s data will be shared on reasonable request to the corresponding author.
Author contributions
Jade Claessens: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing—original draft. Loren Packlé: Data curation; Formal analysis; Investigation; Writing—review and editing. Hanne Oosterbos: Data curation; Investigation; Writing—review and editing. Elke Smeets: Investigation. Jelena Geens: Investigation. Jens Gielen: Investigation. Silke Van Genechten: Methodology; Supervision; Writing—review and editing. Samuel Heuts: Writing—review and editing. Jos G. Maessen: Supervision; Writing—review and editing. Alaaddin Yilmaz: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing—review and editing
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Eric J. Lehr, Kerem M. Vural and the other anonymous reviewers for their contribution to the peer review process of this article.
This study was approved by the local ethics committee of the Jessa Hospital, Belgium (registration number 2020/152).
REFERENCES
ABBREVIATIONS
- BIMA
Bilateral internal mammary arteries
- CABG
Coronary artery bypass grafting
- CPB
Cardiopulmonary bypass
- CVA
Cerebrovascular accident
- D
Diagonal
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- ICU
Intensive care unit
- LAD
Left anterior descending
- LOS
Lengths of stay
- LVEF
Left ventricular ejection fraction
- MACCE
Major adverse cardiac and cerebrovascular events
- MI
Myocardial infarction
- PCI
Percutaneous coronary intervention
- TECAB
Totally endoscopic coronary artery bypass grafting
- TIA
Transient ischaemic attack
- left ventricular ejection fraction
- hypertension
- coronary artery bypass surgery
- surgical procedures, minimally invasive
- endoscopy
- surgical procedures, operative
- tissue transplants
- heart
- mortality
- transplantation
- revascularization
- total endoscopic coronary artery bypass
- octogenarians
- european system for cardiac operative risk evaluation
- multi vessel coronary artery disease





