-
PDF
- Split View
-
Views
-
Cite
Cite
Chen-Sung Lin, Liang-Shun Wang, Chun-Ming Tsai, Yau-Huei Wei, Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy, Interactive CardioVascular and Thoracic Surgery, Volume 7, Issue 6, December 2008, Pages 954–958, https://doi.org/10.1510/icvts.2008.177006
Close - Share Icon Share
Abstract
The decrease in the copy number of mitochondrial DNA (mtDNA) in cancer tissues might be associated with a decrease in oxidative mtDNA damage to achieve cancer immortalization and progression. Lung cancer specimens were collected from 29 patients with stage III non-small cell lung cancer (NSCLC) after neoadjuvant chemotherapy followed by surgical resection. The relative mtDNA copy number and the oxidative mtDNA damage (formation of 8-OHdG in mtDNA) of each cancer tissue were measured by quantitative real-time PCR. Seven female and 22 male lung cancer patients, with a mean age of 63.5 years were evaluated. Tumors of five patients became progressive, 13 stable, and 11 partially responsive after preoperative chemotherapy. Low mtDNA copy number (P=0.089) and low degree of oxidative mtDNA damage (P=0.036) were found to associate with tumor progression. Moreover, mtDNA copy number was significantly related to the degree of oxidative mtDNA damage (P=0.031). The mtDNA copy number and oxidative mtDNA damage were lower in advanced NSCLC after chemotherapy. This finding suggests that a decrease in the content of mtDNA may result in a decrease of mitochondrial density in cancer cells, which leads to a decrease of endogenous ROS production and reduction of ROS-triggered DNA damage to achieve immortalization.
1. Introduction
Reactive oxygen species (ROS) are essential for cell differentiation, however, excess ROS may damage DNA and lead to subsequent carcinogenesis [1]. Very few studies have addressed the relationship between oxidative DNA damage and tumor progression. Mitochondria are not only the power house responsible for ATP production via the Krebs cycle and oxidative phosphorylation to keep cell alive but also responsible for production of about 85% intracellular ROS during the electron transport to promote cell differentiation or induce apoptosis [2]. The dual roles of mitochondria are responsible for life and death of the cell. The number of mitochondria represents not only the capacity of ATP production but also reflects the oxidative stress to which the cell is exposed.
Each human cell contains several hundreds to thousands of mitochondria and each mitochondrion has 2 to 10 copies of mtDNA. The copy number of mitochandrial DNA (mtDNA) may reflect the abundance of mitochondria in a human cell [2]. The characteristics, including intron-less, without binding to histones, and inefficient mtDNA proof-reading and DNA repair system, render mtDNA more susceptible to oxidative damage than nuclear DNA (nDNA) [3]. Among the many types of DNA damage caused by ROS, the formation of 8-OHdG is the most common one [4]. Thus, the amount of 8-OHdG in mtDNA may be an index for cellular oxidative damage.
Lung cancer is an important malignant disease and has been the leading cause of cancer-related death in Taiwan since 2005. Despite improvements in diagnosis and treatment, the overall 5-year survival rate remains below 15%. In recent 10 years, some studies suggested that neoadjuvant chemotherapy followed by surgical resection offers a better prognosis of patients with stage III NSCLC [5].
In this study, we measured the copy number and 8-OHdG content in mtDNA to reflect cellular oxidative damage in cancer tissues from patients with stage III NSCLC after neoadjuvant chemotherapy followed by surgical resection. Based on the patients' response to drug treatment, we evaluated their relationships to oxidative damage in cancer tissues. We expect that the results from this study are of clinical relevance.
2. Materials and methods
2.1. Patient selection and collection of cancer tissues
From January 1997 to December 2004, 29 consecutive patients with N2 positive stage III NSCLC, confirmed by mediastinal scope lymph nodes biopsies received complete neoadjuvant chemotherapy followed by surgical resection, were retrospectively collected. Neoadjuvant chemotherapy regimen consisting of gemcitabine plus cisplantin were performed for three courses within three months (one course means gemcitabine on day 1, day 8, and day 15, and cisplantin on day 15, and then rest between days 16 to 30) [6]. Exploratory thoracotomy was performed to remove tumor one month after completion of the neoadjuvant chemotherapy. All the patients signed the informed consent before chemotherapy. All the pathological slides of the resected tumors were reviewed by an experienced pathologist to locate the distinct cancerous parts and dissect cancer tissues from corresponding paraffin block for DNA extraction with a QIAamp DNA Mini Kit (Qiagen Ltd., Valencia, CA). This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB-95-11-15A).
2.2. Definition of the treatment response after neoadjuvant chemotherapy
The primary tumor size and its association to the surrounding conditions were evaluated by chest CT-scan. All the CT-scans before chemotherapy and one month after completion of chemotherapy were compared in detail to determine the treatment response. According to the RECIST (response evaluation criteria in solid tumors), we divided the patients into three groups including partial response (PR), stable disease (SD) and progressive disease (PD) [7].
2.3. Quantitative real-time PCR
Quantitative real-time PCR was performed using the LightCycler Instrument (Roche, Mannheim, Germany). The sequences of primers used for amplification of ND1 gene in mtDNA were: mtF3212, 5′-CACCCAAGAACAGGGTTTGT-3′ and mtR3319, 5′-TGGCCATGGGATTGTTGTTAA-3′. The sequences of primers used for amplification of 18S rRNA gene in nuclear DNA were: 18S1546F, 5′-TAGAGGGACAAG TGGCGTTC-3′ and 18S1650R, 5′-CGCTGAGCCAGTCAGTGT-3′ [8]. The PCR conditions were set up as follows: hot start at 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s, 62 °C for 20 s, and 72 °C for 20 s. The fluorescence intensity was measured at the end of every extension phase at 79 °C.
2.4. Determination of the mtDNA copy number
Standard curves relating both mtDNA and nDNA copies to replication threshold cycles (Ct) were established by using total cellular DNA of 143B osteosarcoma cells (Fig. 1a ) (mtDNA, R2=0.9973; and nDNA, R2=0.9973). For each clinical sample, the Ct values for mtDNA and nDNA were determined and their mtDNA and nDNA amounts relative to the 143B cell were plotted and corrected from the standard curve. Using the 143B cell as the internal standard, whose mtDNA copy number (mtDNA amount/nDNA amount) was defined as one and the relative mtDNA copy numbers of the 29 clinical samples were calculated [8]. Each analysis was duplicated and mean±S.D. of the results was used for data presentation.
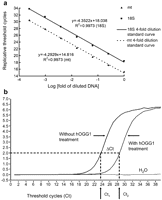
(a) Determination of mtDNA copy number by real-time PCR. Relative mtDNA copy number was determined by the quantitative real-time PCR as described in materials and methods. The efficiency of the real-time PCR amplification was established from 20 ng to 0.4441 pg (4-fold repeated dilutions) cellular DNA from 143B osteosarcoma cells, which were allowed to react with primers specific to mtDNA (ND1 gene) and nDNA (18S rRNA gene), respectively. The correlation coefficient R2 was 0.9973 for nDNA and 0.9973 for mtDNA, respectively. (b) Determination of the degree of oxidative mtDNA damage. The degree of oxidative mtDNA damage is reflected by the abundance of 8-OHdG formation in mtDNA, and it is presented as Ct2–Ct1 (ΔCt). PCR amplification efficiencies of DNA templates containing a single 8-OHdG or two 8-OHdGs at least 13 bp apart were not significantly perturbed (Ct1), while the presence of an abasic site in DNA after treatment of hOGG1 would dramatically reduce the PCR efficiency and thus increase the Ct value (Ct2).
2.5. Determination of the degree of oxidative mtDNA damage (ΔCt)
The content of 8-OHdG in mtDNA was determined by a quantitative real-time PCR method and was presented as ΔCt. If the mtDNA in a sample contains 8-OHdG, the amplification efficiency would decrease after treatment of the DNA sample with hOGG1 to remove the 8-OHdG residue to form an abasic site [9]. The hOGG1 treatment of DNA samples was done as previously described [10]. One μl of each unknown DNA was treated with/or without 1 unit of hOGG1 at 37 °C for 1 h, and the digested DNA were amplified by quantitative real-time PCR using mtF3212 and mtR3319 primers. As illustrated in Fig. 1b, the threshold cycle increased from Ct1 to Ct2 after hOGG1 treatment, if the mtDNA in the sample harbored 8-OHdG. We define the degree of oxidative mtDNA damage as ΔCt, which is Ct2–Ct1. The larger the ΔCt was, the more abundant the 8-OHdG and more oxidative mtDNA damage the sample had. Each analysis was done in duplicate. The mean value of ΔCt was calculated for each sample as an index of oxidative damage to DNA.
3. Statistical analysis
The continuous variables between groups were compared using the Student's t-test/Mann–Whitney U-test, ANOVA/Kruskal–Wallis test, or paired t-test/Wilcoxon signed ranks test when appropriate. Categorical variables were compared using the χ2-test, Fisher exact test, or linear by linear association when appropriate. The relationships between two continuous variables were evaluated by Pearson correlation. Survival probability was calculated and plotted by the Kaplan–Meier method. Log-rank test was used to compare different survival probabilities among different levels within each categorical variable. Possible prognostic factors associated with the survival probability at a significance level of 0.20 or less were considered in a multivariable Cox's proportional hazard regression analysis. The difference between groups is considered significant when a P-value is <0.05.
4. Results
The clinical, pathological and biochemical results of the 29 patients are summarized in Table 1 . The mean and median mtDNA copy numbers of the 29 clinical samples were 0.50 and 0.46, respectively. The mean and median levels of oxidative mtDNA damage (ΔCt) were 3.32 and 3.35, respectively.
Comparison of clinical, pathological and biochemical data of recruited patients in PR, SD and PD groups
| All cases | Treatment response | P-value | |||
| (n=29) | PR (n=11) | SD (n=13) | PD (n=5) | ||
| Primary tumor size (cm) | |||||
| Pre-chemotherapy | 4.8±1.6 | 4.8±1.2 | 5.2±1.7 | 3.4±1.6 | 0.148 |
| Post-chemotherapy | 3.6±2.4 | 2.0±1.4 | 4.7±2.5 | 4.2±1.9 | 0.011 |
| P-value (paired, Wilcoxon signed ranks test) | 0.022 | 0.012 | 0.476 | 0.066 | |
| Median survival (months) | 27.0 | 17.0 | 42.7 | 31.8 | 0.103 |
| Age (years) | 63.5±10.0 | 66.0±5.5 | 60.4±13.2 | 66.2±6.4 | 0.731 |
| Sex (M/F) | 22/7 | 8/3 | 10/3 | 4/1 | 0.945 |
| Smoking history (yes/no) | 20/9 | 7/4 | 10/3 | 3/2 | 0.698 |
| Cell type | |||||
| Squamous/adeno/non-small cell carcinoma | 15/12/2 | 7/3/1 | 7/5/1 | 1/4/0 | 0.396 |
| Location of primary neoplasm | |||||
| RUL/RML/RLL/LUL/LLL | 5/3/8/9/4 | 1/0/4/4/2 | 3/1/3/4/2 | 1/2/1/1/0 | 0.448 |
| Type of operation | |||||
| Lobectomy/bi-lobectomy | 19 | 6/0 | 7/2 | 4/0 | 0.323 |
| Wedge resection | 6 | 4 | 1 | 1 | |
| Explore thoracotomy | 4 | 1 | 3 | 0 | |
| Postoperative adjuvant therapy | |||||
| Yes | 23 | 8 | 10 | 5 | 0.44 |
| No | 6 | 3 | 3 | 0 | |
| Relative mtDNA copy number | |||||
| Median | 0.46 | 0.60 | 0.39 | 0.38 | |
| Mean±S.D. | 0.50±0.22 | 0.58±0.18 | 0.45±0.24 | 0.43±0.25 | 0.213 |
| >0.46 (high) (%) | 13 (45) | 7 (64) | 5 (39) | 1 (20) | 0.089 |
| ≤0.46 (low) (%) | 16 (55) | 4 (36) | 8 (61) | 4 (80) | |
| Degree of oxidative DNA damage (ΔCt) | |||||
| Median | 3.35 | 3.68 | 3.40 | 3.05 | |
| Mean±S.D. | 3.32±0.99 | 3.80±0.74 | 3.14±1.13 | 2.72±0.62 | 0.045 |
| >3.35 (high damage) (%) | 14 (48) | 7 (64) | 7 (54) | 0 (0) | 0.036 |
| ≤3.35 (low damage) (%) | 15 (51) | 4 (36) | 6 (46) | 5 (100) | |
| Operation duration (min) | 224±61 | 233±61 | 211±64 | 236±59 | 0.723 |
| Total hospital stay (days) | 16.5±8.2 | 16.6±8.0 | 17.5±9.7 | 13.4±3.4 | 0.731 |
| Surgical mortality (=30 days) | 2 | 1 | 1 | 0 | 0.792 |
| All cases | Treatment response | P-value | |||
| (n=29) | PR (n=11) | SD (n=13) | PD (n=5) | ||
| Primary tumor size (cm) | |||||
| Pre-chemotherapy | 4.8±1.6 | 4.8±1.2 | 5.2±1.7 | 3.4±1.6 | 0.148 |
| Post-chemotherapy | 3.6±2.4 | 2.0±1.4 | 4.7±2.5 | 4.2±1.9 | 0.011 |
| P-value (paired, Wilcoxon signed ranks test) | 0.022 | 0.012 | 0.476 | 0.066 | |
| Median survival (months) | 27.0 | 17.0 | 42.7 | 31.8 | 0.103 |
| Age (years) | 63.5±10.0 | 66.0±5.5 | 60.4±13.2 | 66.2±6.4 | 0.731 |
| Sex (M/F) | 22/7 | 8/3 | 10/3 | 4/1 | 0.945 |
| Smoking history (yes/no) | 20/9 | 7/4 | 10/3 | 3/2 | 0.698 |
| Cell type | |||||
| Squamous/adeno/non-small cell carcinoma | 15/12/2 | 7/3/1 | 7/5/1 | 1/4/0 | 0.396 |
| Location of primary neoplasm | |||||
| RUL/RML/RLL/LUL/LLL | 5/3/8/9/4 | 1/0/4/4/2 | 3/1/3/4/2 | 1/2/1/1/0 | 0.448 |
| Type of operation | |||||
| Lobectomy/bi-lobectomy | 19 | 6/0 | 7/2 | 4/0 | 0.323 |
| Wedge resection | 6 | 4 | 1 | 1 | |
| Explore thoracotomy | 4 | 1 | 3 | 0 | |
| Postoperative adjuvant therapy | |||||
| Yes | 23 | 8 | 10 | 5 | 0.44 |
| No | 6 | 3 | 3 | 0 | |
| Relative mtDNA copy number | |||||
| Median | 0.46 | 0.60 | 0.39 | 0.38 | |
| Mean±S.D. | 0.50±0.22 | 0.58±0.18 | 0.45±0.24 | 0.43±0.25 | 0.213 |
| >0.46 (high) (%) | 13 (45) | 7 (64) | 5 (39) | 1 (20) | 0.089 |
| ≤0.46 (low) (%) | 16 (55) | 4 (36) | 8 (61) | 4 (80) | |
| Degree of oxidative DNA damage (ΔCt) | |||||
| Median | 3.35 | 3.68 | 3.40 | 3.05 | |
| Mean±S.D. | 3.32±0.99 | 3.80±0.74 | 3.14±1.13 | 2.72±0.62 | 0.045 |
| >3.35 (high damage) (%) | 14 (48) | 7 (64) | 7 (54) | 0 (0) | 0.036 |
| ≤3.35 (low damage) (%) | 15 (51) | 4 (36) | 6 (46) | 5 (100) | |
| Operation duration (min) | 224±61 | 233±61 | 211±64 | 236±59 | 0.723 |
| Total hospital stay (days) | 16.5±8.2 | 16.6±8.0 | 17.5±9.7 | 13.4±3.4 | 0.731 |
| Surgical mortality (=30 days) | 2 | 1 | 1 | 0 | 0.792 |
Comparison of clinical, pathological and biochemical data of recruited patients in PR, SD and PD groups
| All cases | Treatment response | P-value | |||
| (n=29) | PR (n=11) | SD (n=13) | PD (n=5) | ||
| Primary tumor size (cm) | |||||
| Pre-chemotherapy | 4.8±1.6 | 4.8±1.2 | 5.2±1.7 | 3.4±1.6 | 0.148 |
| Post-chemotherapy | 3.6±2.4 | 2.0±1.4 | 4.7±2.5 | 4.2±1.9 | 0.011 |
| P-value (paired, Wilcoxon signed ranks test) | 0.022 | 0.012 | 0.476 | 0.066 | |
| Median survival (months) | 27.0 | 17.0 | 42.7 | 31.8 | 0.103 |
| Age (years) | 63.5±10.0 | 66.0±5.5 | 60.4±13.2 | 66.2±6.4 | 0.731 |
| Sex (M/F) | 22/7 | 8/3 | 10/3 | 4/1 | 0.945 |
| Smoking history (yes/no) | 20/9 | 7/4 | 10/3 | 3/2 | 0.698 |
| Cell type | |||||
| Squamous/adeno/non-small cell carcinoma | 15/12/2 | 7/3/1 | 7/5/1 | 1/4/0 | 0.396 |
| Location of primary neoplasm | |||||
| RUL/RML/RLL/LUL/LLL | 5/3/8/9/4 | 1/0/4/4/2 | 3/1/3/4/2 | 1/2/1/1/0 | 0.448 |
| Type of operation | |||||
| Lobectomy/bi-lobectomy | 19 | 6/0 | 7/2 | 4/0 | 0.323 |
| Wedge resection | 6 | 4 | 1 | 1 | |
| Explore thoracotomy | 4 | 1 | 3 | 0 | |
| Postoperative adjuvant therapy | |||||
| Yes | 23 | 8 | 10 | 5 | 0.44 |
| No | 6 | 3 | 3 | 0 | |
| Relative mtDNA copy number | |||||
| Median | 0.46 | 0.60 | 0.39 | 0.38 | |
| Mean±S.D. | 0.50±0.22 | 0.58±0.18 | 0.45±0.24 | 0.43±0.25 | 0.213 |
| >0.46 (high) (%) | 13 (45) | 7 (64) | 5 (39) | 1 (20) | 0.089 |
| ≤0.46 (low) (%) | 16 (55) | 4 (36) | 8 (61) | 4 (80) | |
| Degree of oxidative DNA damage (ΔCt) | |||||
| Median | 3.35 | 3.68 | 3.40 | 3.05 | |
| Mean±S.D. | 3.32±0.99 | 3.80±0.74 | 3.14±1.13 | 2.72±0.62 | 0.045 |
| >3.35 (high damage) (%) | 14 (48) | 7 (64) | 7 (54) | 0 (0) | 0.036 |
| ≤3.35 (low damage) (%) | 15 (51) | 4 (36) | 6 (46) | 5 (100) | |
| Operation duration (min) | 224±61 | 233±61 | 211±64 | 236±59 | 0.723 |
| Total hospital stay (days) | 16.5±8.2 | 16.6±8.0 | 17.5±9.7 | 13.4±3.4 | 0.731 |
| Surgical mortality (=30 days) | 2 | 1 | 1 | 0 | 0.792 |
| All cases | Treatment response | P-value | |||
| (n=29) | PR (n=11) | SD (n=13) | PD (n=5) | ||
| Primary tumor size (cm) | |||||
| Pre-chemotherapy | 4.8±1.6 | 4.8±1.2 | 5.2±1.7 | 3.4±1.6 | 0.148 |
| Post-chemotherapy | 3.6±2.4 | 2.0±1.4 | 4.7±2.5 | 4.2±1.9 | 0.011 |
| P-value (paired, Wilcoxon signed ranks test) | 0.022 | 0.012 | 0.476 | 0.066 | |
| Median survival (months) | 27.0 | 17.0 | 42.7 | 31.8 | 0.103 |
| Age (years) | 63.5±10.0 | 66.0±5.5 | 60.4±13.2 | 66.2±6.4 | 0.731 |
| Sex (M/F) | 22/7 | 8/3 | 10/3 | 4/1 | 0.945 |
| Smoking history (yes/no) | 20/9 | 7/4 | 10/3 | 3/2 | 0.698 |
| Cell type | |||||
| Squamous/adeno/non-small cell carcinoma | 15/12/2 | 7/3/1 | 7/5/1 | 1/4/0 | 0.396 |
| Location of primary neoplasm | |||||
| RUL/RML/RLL/LUL/LLL | 5/3/8/9/4 | 1/0/4/4/2 | 3/1/3/4/2 | 1/2/1/1/0 | 0.448 |
| Type of operation | |||||
| Lobectomy/bi-lobectomy | 19 | 6/0 | 7/2 | 4/0 | 0.323 |
| Wedge resection | 6 | 4 | 1 | 1 | |
| Explore thoracotomy | 4 | 1 | 3 | 0 | |
| Postoperative adjuvant therapy | |||||
| Yes | 23 | 8 | 10 | 5 | 0.44 |
| No | 6 | 3 | 3 | 0 | |
| Relative mtDNA copy number | |||||
| Median | 0.46 | 0.60 | 0.39 | 0.38 | |
| Mean±S.D. | 0.50±0.22 | 0.58±0.18 | 0.45±0.24 | 0.43±0.25 | 0.213 |
| >0.46 (high) (%) | 13 (45) | 7 (64) | 5 (39) | 1 (20) | 0.089 |
| ≤0.46 (low) (%) | 16 (55) | 4 (36) | 8 (61) | 4 (80) | |
| Degree of oxidative DNA damage (ΔCt) | |||||
| Median | 3.35 | 3.68 | 3.40 | 3.05 | |
| Mean±S.D. | 3.32±0.99 | 3.80±0.74 | 3.14±1.13 | 2.72±0.62 | 0.045 |
| >3.35 (high damage) (%) | 14 (48) | 7 (64) | 7 (54) | 0 (0) | 0.036 |
| ≤3.35 (low damage) (%) | 15 (51) | 4 (36) | 6 (46) | 5 (100) | |
| Operation duration (min) | 224±61 | 233±61 | 211±64 | 236±59 | 0.723 |
| Total hospital stay (days) | 16.5±8.2 | 16.6±8.0 | 17.5±9.7 | 13.4±3.4 | 0.731 |
| Surgical mortality (=30 days) | 2 | 1 | 1 | 0 | 0.792 |
Comparing the treatment response before and after chemotherapy, 11 were PR, 13 were SD and 5 were PD. In the PR group, the tumors became significantly smaller after chemotherapy (P=0.012). On the contrary, the tumors grew larger in the PD group (P=0.066). Twenty-three of the 29 patients received postoperative adjuvant chemo/radiotherapies. Concerning their postoperative overall survivals, the possible prognostic factors are presented in Table 2 . After multivariable analysis, postoperative adjuvant chemo/radiotherapy was found to be the most important and independent prognostic factor. No significant differences in survival were noted among the PR, SD and PD groups (P=0.103; Log Rank), and they might be influenced by the postoperative adjuvant therapies.
Possible prognostic factors affecting the long-term survival after uni-variable and multi-variables analysis
| Median survival (months) | Uni-variable | Multi-variable | |||
| P (log-rank) | Hazard ratio | 95% CI | P (Cox-regression) | ||
| Gender | 0.999 | ||||
| Male (n=22) | 22.1 | ||||
| Female (n=7) | 27.0 | ||||
| Smoking history | 0.652 | ||||
| No | 27.0 | ||||
| Yes | 22.1 | ||||
| Histological type | 0.123 | 0.136 | |||
| Squamous cell carcinoma (n=15) | 37.8 | ||||
| Adenocarcinoma (n=12) | 19.2 | ||||
| Non-small cell carcinoma (n=2) | 8.83 | ||||
| Type of operation | 0.019 | 0.576 | |||
| Lobectomy/bi-lobectomy (n=19) | 31.8 | ||||
| Wedge resection (n=6) | 22.1 | ||||
| Explore thoracotomy (n=4) | 10.0 | ||||
| Postoperative adjuvant chemotherapy | 0.005 | 0.009 | |||
| No (n=6) | 8.8 | 3.677 | 1.394–9.699 | ||
| Yes (n=23) | 31.8 | 1.000 | |||
| Treatment response | 0.103 | 0.207 | |||
| Partial response (PR) (n=11) | 17.0 | ||||
| Stable disease (SD) (n=13) | 42.7 | ||||
| Progressive disease (PD) (n=5) | 31.8 | ||||
| Relative mtDNA copy number | 0.177 | 0.389 | |||
| Low (≤0.46) (n=16) | 30.6 | ||||
| High (>0.46) (n=13) | 17.0 | ||||
| Degree of oxidative mtDNA damage | 0.609 | ||||
| Low (≤3.35) (n=15) | 19.2 | ||||
| High (>3.35) (n=14) | 27.0 | ||||
| Median survival (months) | Uni-variable | Multi-variable | |||
| P (log-rank) | Hazard ratio | 95% CI | P (Cox-regression) | ||
| Gender | 0.999 | ||||
| Male (n=22) | 22.1 | ||||
| Female (n=7) | 27.0 | ||||
| Smoking history | 0.652 | ||||
| No | 27.0 | ||||
| Yes | 22.1 | ||||
| Histological type | 0.123 | 0.136 | |||
| Squamous cell carcinoma (n=15) | 37.8 | ||||
| Adenocarcinoma (n=12) | 19.2 | ||||
| Non-small cell carcinoma (n=2) | 8.83 | ||||
| Type of operation | 0.019 | 0.576 | |||
| Lobectomy/bi-lobectomy (n=19) | 31.8 | ||||
| Wedge resection (n=6) | 22.1 | ||||
| Explore thoracotomy (n=4) | 10.0 | ||||
| Postoperative adjuvant chemotherapy | 0.005 | 0.009 | |||
| No (n=6) | 8.8 | 3.677 | 1.394–9.699 | ||
| Yes (n=23) | 31.8 | 1.000 | |||
| Treatment response | 0.103 | 0.207 | |||
| Partial response (PR) (n=11) | 17.0 | ||||
| Stable disease (SD) (n=13) | 42.7 | ||||
| Progressive disease (PD) (n=5) | 31.8 | ||||
| Relative mtDNA copy number | 0.177 | 0.389 | |||
| Low (≤0.46) (n=16) | 30.6 | ||||
| High (>0.46) (n=13) | 17.0 | ||||
| Degree of oxidative mtDNA damage | 0.609 | ||||
| Low (≤3.35) (n=15) | 19.2 | ||||
| High (>3.35) (n=14) | 27.0 | ||||
Possible prognostic factors affecting the long-term survival after uni-variable and multi-variables analysis
| Median survival (months) | Uni-variable | Multi-variable | |||
| P (log-rank) | Hazard ratio | 95% CI | P (Cox-regression) | ||
| Gender | 0.999 | ||||
| Male (n=22) | 22.1 | ||||
| Female (n=7) | 27.0 | ||||
| Smoking history | 0.652 | ||||
| No | 27.0 | ||||
| Yes | 22.1 | ||||
| Histological type | 0.123 | 0.136 | |||
| Squamous cell carcinoma (n=15) | 37.8 | ||||
| Adenocarcinoma (n=12) | 19.2 | ||||
| Non-small cell carcinoma (n=2) | 8.83 | ||||
| Type of operation | 0.019 | 0.576 | |||
| Lobectomy/bi-lobectomy (n=19) | 31.8 | ||||
| Wedge resection (n=6) | 22.1 | ||||
| Explore thoracotomy (n=4) | 10.0 | ||||
| Postoperative adjuvant chemotherapy | 0.005 | 0.009 | |||
| No (n=6) | 8.8 | 3.677 | 1.394–9.699 | ||
| Yes (n=23) | 31.8 | 1.000 | |||
| Treatment response | 0.103 | 0.207 | |||
| Partial response (PR) (n=11) | 17.0 | ||||
| Stable disease (SD) (n=13) | 42.7 | ||||
| Progressive disease (PD) (n=5) | 31.8 | ||||
| Relative mtDNA copy number | 0.177 | 0.389 | |||
| Low (≤0.46) (n=16) | 30.6 | ||||
| High (>0.46) (n=13) | 17.0 | ||||
| Degree of oxidative mtDNA damage | 0.609 | ||||
| Low (≤3.35) (n=15) | 19.2 | ||||
| High (>3.35) (n=14) | 27.0 | ||||
| Median survival (months) | Uni-variable | Multi-variable | |||
| P (log-rank) | Hazard ratio | 95% CI | P (Cox-regression) | ||
| Gender | 0.999 | ||||
| Male (n=22) | 22.1 | ||||
| Female (n=7) | 27.0 | ||||
| Smoking history | 0.652 | ||||
| No | 27.0 | ||||
| Yes | 22.1 | ||||
| Histological type | 0.123 | 0.136 | |||
| Squamous cell carcinoma (n=15) | 37.8 | ||||
| Adenocarcinoma (n=12) | 19.2 | ||||
| Non-small cell carcinoma (n=2) | 8.83 | ||||
| Type of operation | 0.019 | 0.576 | |||
| Lobectomy/bi-lobectomy (n=19) | 31.8 | ||||
| Wedge resection (n=6) | 22.1 | ||||
| Explore thoracotomy (n=4) | 10.0 | ||||
| Postoperative adjuvant chemotherapy | 0.005 | 0.009 | |||
| No (n=6) | 8.8 | 3.677 | 1.394–9.699 | ||
| Yes (n=23) | 31.8 | 1.000 | |||
| Treatment response | 0.103 | 0.207 | |||
| Partial response (PR) (n=11) | 17.0 | ||||
| Stable disease (SD) (n=13) | 42.7 | ||||
| Progressive disease (PD) (n=5) | 31.8 | ||||
| Relative mtDNA copy number | 0.177 | 0.389 | |||
| Low (≤0.46) (n=16) | 30.6 | ||||
| High (>0.46) (n=13) | 17.0 | ||||
| Degree of oxidative mtDNA damage | 0.609 | ||||
| Low (≤3.35) (n=15) | 19.2 | ||||
| High (>3.35) (n=14) | 27.0 | ||||
From PR, SD to PD groups, both the median (0.60, 0.39 and 0.38) and the mean (0.58, 0.45 and 0.43) relative mtDNA copy numbers were decreased gradually. Using the median relative mtDNA copy number (=0.46) of the 29 patients as the cutoff value, patients with low relative mtDNA copy numbers (≦0.46) were associated with a trend of tumor progression (P=0.089, linear by linear association). Intriguingly, the results showed that the degree of oxidative mtDNA damage (ΔCt) was decreased significantly from PR, SD to PD groups (3.80, 3.14 and 2.72; P=0.045). Using the median ΔCt (=3.35) of the 29 patients as the cutoff value, patients with low oxidative mtDNA damage (≲3.35) were found to display a trend of tumor progression (P=0.036, linear by linear association).
In addition, low levels of mtDNA copy number and oxidative mtDNA damage were found to associate with tumor progression. Using correlation methods, we demonstrate that the mtDNA copy number and ΔCt were significantly associated (Pearson correlation=0.401, P=0.031) (Fig. 2 ).

Correlation between the mtDNA copy number and the degree of oxidative DNA damage in mtDNA. The relative mtDNA copy numbers of the 29 lung cancer tissues with their relationships to oxidative damage in mtDNA are plotted. The results show that the two parameters were significantly correlated (Pearson correlation coefficient=0.401, P=0.031).
5. Discussion
In this study, we demonstrated that: (1) low levels of mtDNA copy number and oxidative mtDNA damage are associated with lung cancer progression after chemotherapy; (2) the degree of oxidative mtDNA damages are significantly associated with the copy number of mtDNA. Although our data were obtained from only 29 consecutive cases, the findings are interesting and clinically relevant. We did not evaluate the cancer specimens before chemotherapy due to the small amount of tissues from bronchoscopic biopsy and prohibition from the Institutional Review Board of our hospital. Thus, we could not determine the mtDNA changes before and after chemotherapy. This might be a limitation in this type of study, although it would offer us additional information.
The decrease in mtDNA copy number has been observed in lung cancer, hepatocellular carcinoma and gastric cancers, especially in the lesions of advanced-stage cancers in the human [11]. A decrease in mtDNA copy number might be an index of disease aggressiveness or progression in these human cancers. Our data might extend this observation to imply that the mtDNA copy number is decreased in lung cancer tissues when the disease is progressed after chemotherapy (P=0.089; Table 1).
Although many investigators suggested that exposure to excess ROS may cause oxidative DNA damage with subsequent carcinogenesis, this claim has not been confirmed by experiments. Thus, the role of ROS and ROS-triggered DNA damages in cancer tissues deserve reappraisal when the disease is progressed [1]. Interestingly, our results revealed that low level of oxidative mtDNA damage is associated with lung cancer progression (P=0.036; Table 1). In human cells, mitochondria are the major organelles responsible for production of endogenous ROS [2]. The amounts of endogenous ROS are proportional to the abundance of mitochondria in tissue cells. The low oxidative mtDNA damage might be a result of decrease in the number of mitochondria with less endogenous ROS formation. Surprisingly, the mtDNA copy number was correlated with oxidative mtDNA damage as revealed in Fig. 2 (P=0.031).
The hallmarks for normal tissues include adequate differentiation with limited cell proliferation, and ability to enter apoptosis. On the contrary, malignant tissues are characterized by de-differentiation with uncontrolled cancer cell proliferation, resistant to apoptosis and prone to immortalization [12]. Based on the viewpoint of life evolution history, from a single anaerobic cell to multiple aerobic mammalian cells, mitochondria play an important role in not only producing ATP to meet cell energy demand but also generating endogenous ROS to support cell differentiation [2,13]. Thus, mitochondria might play a pivotal role in normal cell differentiation and cancer formation (de-differentiation process) depending on the level of endogenous ROS. The results of this study led us to reason that the decrease in mtDNA copy number may imply low abundance of mitochondria, which is related to less endogenous ROS production in progressive lung cancer tissues. Less ROS-triggered cell death occurs concurrently with less ROS-induced cell differentiation, which may favor cancer progression. In line with this notion, some investigators hypothesized that the decrease in ROS may contribute to cancer formation [1,14].
In conclusion, we demonstrated a decrease in the copy number of mtDNA and ROS-triggered damage to mtDNA in lung cancer tissues. The decrease in oxidative DNA damage might be the result of the decrease in the metabolic activity and production of ROS in mitochondria. The low levels of mtDNA copy number and oxidative DNA damage may indicate that cancer cells switch their energy supply from aerobic metabolism to anaerobic glycolysis in the progression of lung cancer tissues. The molecular basis underlying this phenomenon warrants further investigation.
Part of the data were presented at the 107th Annual Congress of Japan Surgical Society (Surgical Forum 90; Lung-Basic Research) by Dr. Chen-Sung Lin on April 13, 2007.
Grant support: Keelung Hospital, Department of Health, Executive Yuan, Keelung 201, Taiwan (No. 96-02) and the ‘Aim for the Top University Plan’ sponsored by the Ministry of Education, Executive Yuan, Taiwan.
Abbreviations: 8-OHdG, 8-hydroxy 2′-deoxyguanosine; hOGG1, human 8-oxoguanine DNA glycosylase 1; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; NSCLC, non-small cell lung cancer; PCR, polymerase chain reaction; ROS, reactive oxygen species.
We would like to express our sincere appreciation to Dr. Teh-Ying Chou for his critical review of the pathological slides and Ms. Chun-Yen Hsian and Mr. Li-Tzu Liu for their technical assistance in the preparation of cancer tissues from pathological paraffin blocks and DNA extraction. The helpful suggestion of Mr. Shi-Bei Wu in the application of the PCR-based method for the determination of 8-OHdG in mtDNA is acknowledged. We also would like to thank Dr. Hui-Chen Lin for her excellent assistance in statistical analysis.




