-
PDF
- Split View
-
Views
-
Cite
Cite
Tohru Sakuragi, Yukio Okazaki, Masahiro Mitsuoka, Tsuyoshi Itoh, Dramatic hemostasis of the transected pulmonary artery model using SOFT COAG electrosurgical output, Interactive CardioVascular and Thoracic Surgery, Volume 7, Issue 5, October 2008, Pages 764–766, https://doi.org/10.1510/icvts.2008.177923
Close - Share Icon Share
Abstract
We report the use of low-voltage, automatically regulated, electrosurgical coagulation to seal the bleeding from pulmonary arteries inadvertently during surgical intervention. Conventional electrosurgical coagulation uses high voltage, which generates intensive heat in the tissue. The heat results in carbonized eschar formation that can be easily broken by mechanical stress and lead to postoperative bleeding. SOFT COAG output automatically regulates the output voltage to a maximum of 200 Volts, preventing the generation of sparking. Thus, there is no formation of carbonized eschar. The instrument generates Joule heat alone in the tissue and the temperature rises to below boiling point, which effectively coagulates protein. Initial experiments, using a beagle model, clearly demonstrated the effectiveness and reliability of sealing both macroscopically and histopathologically. SOFT CAOG can be easily adopted both in open thoracotomy as well as in thoracoscopic procedures.
1. Introduction
In anatomical pulmonary resection, such as lobectomy, we occasionally encounter accidental injury to the pulmonary artery. While the occurrence is rare, it can be life threatening [1]. If encountered during the thoracoscopic procedure, we must convert swiftly to a thoracotomy [2].
To be prepared for such a serious situation, we used the transected pulmonary artery model in beagle dogs to establish the validity of SOFT COAG output of a modern electrosurgical system (such as VIO300D, ERBE Elektromedizin GmbH, Germany) in closing.
Conventional electrosurgical units involve both Joule heating generated by the passing of electrical current through the electrical resistance of human tissue, which results in tissue temperatures well above the boiling point, and intensive heat generated by high voltage components, which forms carbonized eschar. Modern electrosurgical generators, such as the VIO300D (ERBE Elektromedizin GmbH, Germany) has a unique coagulation output mode called ‘SOFT COAG’, that automatically regulates its output voltage to stay below 200 Volts (V), causing the generation of Joule heat alone, and no tissue carbonization to result. Protein within the target tissue is effectively coagulated at temperatures between 70 and 80 °C due to the Joule heat generated in the tissue [3].
2. Material and methods
The SOFT COAG output of the VIO300D was evaluated to determine if it can seal transected pulmonary arteries in a beagle dog model. All of the animals involved in this study received humane care in compliance with the European Convention on Animal Care. The Animal Research Committee of the Saga University of Medicine approved all procedures. Beagle dogs (n=4) were anesthetized with an intravenous injection of pentobarbital sodium (15 mg/kg body weight) then endotracheally intubated. General anesthesia was maintained by inhalation of isoflurane under mechanical ventilation. The animal was placed in the left lateral position. The right chest was entered through the fifth intercostal space.
After the upper lobectomy, a pulmonary artery of the upper lobe was pinched up with forceps and transected at the root, which caused active bleeding (Videos 1 , 2 ). After the temporary control of the bleeding with a finger, a ball electrode with a tip diameter of 3 mm (Fig. 1b ) was placed against the bleeding point and moved in a circular motion. SOFT COAG was set at Effect 3 on the VIO300D. VIO's Effect represents the setting of power voltage. There are 8 levels of effects on the SOFT COAGV; Effect 1 is 55 V, and Effect 8, which is the max setting, is 190 V. It will not exceed over 200 V, even on max setting. Effect 3 is 88 V.


The electrosurgical system. (a) VIO300D generator (ERBE Electromedizin GmbH, Tuebingen, Germany). (b) Ball electrode with 3 mm tip diameter.
3. Results
Hemostasis was achieved almost immediately in one case (Video 1) while it took longer in the other three cases (Video 2). The sealed bleeding points did not bleed again even if rubbed vigorously by surgical instruments (Videos 1, 2). Histological examination showed that the transected lesion, identified in the defect of the elastic endothelial layer, was plugged with coagulated fibrin and surrounding connective tissue of the adventitia are coagulated showing fused collagen in the absence of tissue carbonization (Fig. 2 ). This phenomenon was estimated to be established by the momentary coagulation of the plasma fibrinogen into the fibrin and protein in the adventitia.

Histological examination showed that the transected lesion, identified in the defect of the elastic endothelial layer (triangles), was plugged with coagulated fibrin and surrounding connective tissue of the adventitia are coagulated showing fused collagen in the absence of tissue carbonization (Fig. 2). This phenomenon was estimated to be established by the momentary coagulation of the plasma fibrinogen into the fibrin and protein in the adventitia.
4. Discussion
This is the first report of the hemostasis of active bleeding from the pulmonary artery using the SOFT COAG in an animal model. We were able to easily stop the bleeding from the root of the transected pulmonary artery by simply applying the tip of the ball-shaped electrode in a circular motion.
Transmitting low-frequency current through the human body causes severe injuries, such as electrochemical reactions and cardiac and respiratory malfunction [4]. Electric Surgical Unit, however, adopts high-frequency current (300 KHz ∼ 1 MHz) to prevent these fatal accidents. The mechanism of conventional electrical coagulation is that electric current by set output flows into tissues, resulting in the rapid rise of the temperature within the tissues of the contact point. When cell fluid inside tissues reaches the boiling point and then turns dehydrated and dry, the flow of electric current is hindered, leading to voltage rise based on the principle (output=current×voltage). Above 200 Vp voltage produces sparks which incinerate the target tissues (voltage of conventional electrical coagulation is more than 1000 Vp) (Fig. 3a ). The electrical coagulation with the high-temperature sparks is effective only for capillaries but not larger blood vessels due to the risk of their burst. Additionally, the intensive heat generated by the sparks form carbonized eschar. Tissue carbonization has a negative influence on the healing progress and leads to the risk of rebleeding by tissue peel-off. Modern electrosurgical generators, such as the VIO300D (ERBE Elektromedizin GmbH, Germany) has a unique coagulation output mode called ‘SOFT COAG’, that automatically regulates its output voltage to stay below 200 V (Fig. 3b), causing the generation of Joule heat alone, and no tissue carbonization to result. Protein within the target tissue is effectively coagulated at temperatures between 70 and 80 °C due to the Joule heat generated in the tissue [3].
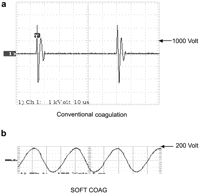
(a) Wave form of conventional coagulation: pulse-modulated sinusoidal alternating voltage Max. peak voltage: >1,000 Vp, (b) Wave form of SOFT COAG: unmodulated sinusoidal alternating voltage Max. peak voltage: <200 Vp.
Intense abrasion did not induce re-bleeding from the area sealed by this method. Subsequent histology of the treated area clearly demonstrated that coagulated fibrin and fused collagen led to the hemostasis. Carbonized eschar (not seen with this technique but, typical of the conventional electrosurgical coagulation), can be easily broken by such mechanical forces.
Bleeding from pulmonary artery during surgical procedures can be life threatening. While we have not yet used the SOFT COAG in a clinical setting, we believe that it will be useful in such cases. Even if bleeding could not be controlled completely, this method could buy precious time for a more permanent corrective action, such as suturing, to control the bleeding.
We thank Dr Edmund J. Miller, Director of Cardiopulmonary Research, The Feinstein Institute for Medical Research, for critical reviews.
References
- pulmonary artery
- hemorrhage
- hemostatic function
- blood coagulation
- blood coagulation tests
- heat (physical force)
- postoperative hemorrhage
- stress, mechanical
- surgical procedures, operative
- thoracoscopy
- thoracotomy
- hemostasis procedures
- temperature
- coagulation process
- eschar
- volt
- boiling point temperature
- joule, unit of energy




