-
PDF
- Split View
-
Views
-
Cite
Cite
Beatrix Hoksch, Renée Fahrner, Ralph Alexander Schmid, Procalcitonin and brain natriuretic peptide as parameters in the postoperative course of patients with major pulmonary resection, Interactive CardioVascular and Thoracic Surgery, Volume 6, Issue 2, April 2007, Pages 155–159, https://doi.org/10.1510/icvts.2006.143073
Close - Share Icon Share
Abstract
Postoperative infections and cardiac events are the major morbidity factors after thoracic surgery and dominating causes of death. Therefore, a sensitive blood marker is needed for an early diagnosis of complications. Twenty-two patients admitted with lung cancer were enrolled in this study. Procalcitonin, brain natriuretic peptide, C-reactive peptide and interleukin-6 levels were recorded preoperatively and postoperatively on days 1–5. Laboratory values of patients with cardiac or infectious complications were compared to patients without complications. During postoperative course procalcitonin and brain natriuretic peptide levels elevated in all patients, but both had higher peak levels in patients with infectious or cardiac complication than without these complications. Interleukin-6 levels were increased on day one and showed a slower decrease in case of complications than without complications. In general, brain natriuretic peptide and procalcitonin levels are increased in the postoperative course after major pulmonary resection, but cardiac and infectious complications are associated with higher levels and a slower decrease than without complications. Interleukin-6 levels showed a slower decrease in patients with complications in the postoperative course than without complications. So the combination of procalcitonin, brain natriuretic peptide, and interleukin-6 seems to be useful for an optimized postoperative monitoring.
1. Introduction
Although the mortality and morbidity after lung surgery have decreased over the past decade, lung resection can be a high risk procedure. Postoperative infections and cardiac events are two of the major morbidity factors after thoracic surgery and dominating causes of death. Incidences of postoperative infections such as pneumonia are reported up to 25% [1]. Postoperative arrhythmia and particular atrial fibrillation is seen frequently after lung resections with reported percentages from 12–22% [2,3]. Older age, male gender, history of heart failure and right pneumonectomy were reported as risk factors for postoperative arrhythmia [2,3].
So for a sufficient therapy a marker, which would be highly specific, highly sensitive, easy to measure, rapid, inexpensive, is needed to detect these complications as early as possible. Brain natriuretic peptide (BNP) is an accepted laboratory value for left ventricular dysfunction [4–6]. It is reported that patients with severe sepsis or septic shock show higher levels of BNP because of poor left ventricular function [7,8].
Procalcitonin (PCT) is a suitable parameter for bacterial infections, and its accuracy is higher than C-reactive peptide (CRP) levels [9,10]. The release of PCT is quick and thus allows earlier diagnosis of sepsis [11]. In the postoperative course the differentiation between surgical trauma and infection can be hard, but several studies showed that PCT is superior to the conventional parameters such as CRP and leukocytes to diagnose postoperative infection [12–15]. But to date, little is known about the value of PCT after lung resection [12]. In the presented investigation, a new measurement of PCT was used with a lower limit of detection of 0.06 ng/ml and a higher precision compared to former studies [12]. However, only anatomical lung resections were counted in our investigation.
The aim of this clinical pilot study was to assess the importance and usefulness of PCT, BNP, and IL-6 in comparison to conventional parameters (leukocytes, CRP) in patients undergoing major pulmonary resections as diagnostic parameters in the postoperative course.
2. Materials and methods
2.1. Patients
From October 2005 to January 2006, 22 patients admitted with lung cancer were enrolled in this prospective clinical investigation. Pulmonary resections were done because of non small-cell lung cancer (n=18), and other carcinoma originated of the lung (n=4). All patients obtained a preoperative cardiac diagnostic by echocardiography or stress-ECG. In 14 patients lobectomies were performed (lower lobe resection n=7, upper lobe resection n=7), in two patients bilobectomies and in six patients pneumonectomies. All surgical procedures were carried out with intraoperative single shot antibiotic prophylaxis. Postoperative course including survival and complications (such as infections or cardiac complications) were recorded. Pulmonary infections which were diagnosed by radiological findings and non-pulmonary infections such as urinary tract infection, catheter associated infection or severe wound infections were counted as infectious complications. The occurrence of arrhythmia or cardiac insufficiency were assessed as cardiac complications. Blood samples were taken preoperatively and during the postoperative days 1–5 including blood count, CRP, PCT, BNP, and IL-6. Patients with preoperative steroid therapy, severe infections, heart failure or myocardial infarction in the last 30 days were excluded from this trial. On the basis of cardiac and infectious complications two groups were formed to compare laboratory values separatly. The two groups showed no differences between age, gender or type of operation (Table 1
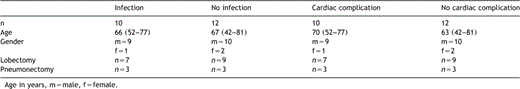
Approval for the investigation was given by all patients in written consent.
2.2. Assays
PCT was measured using the quantitative Kryptor-Test PCT (Brahms Diagnostica AG, Germany) with a lower detection limit of 0.06 ng/ml. Plasma samples were analyzed for IL-6 by enzyme immunoassay (EIA). PCT and IL-6 samples were frozen by −20 °C and measurements were performed all together after one month. Fluoroimmunoassay (Triage BNP-Test, Biosite Diagnostic) was used for BNP measurements using EDTA-blood which was analyzed within 4 h. CRP plasma levels were examined by a standard immunological test (Roche, Hitachi 911).
3. Results
The median age of the 22 enrolled patients was 67 years (range: 43–81 years). The median operation time was 175 min (80–275 min) with a median postoperative hospitalization of 10 days (5–33 days). The postoperative follow-up was 30–270 days (median 217 days). After a follow-up of six months overall survival was 86% (n=19). Three patients died after 30, 36, and 68 days. In two cases the patients died after discharge and the cause of death was unknown. The third patient died of right heart failure caused by prolonged septical course and ARDS.
3.1. Complications
In the postoperative course cardiac complications occurred in ten patients. Nine of them developed arrhythmia such as atrial fibrillation or ventricular arrhythmia. Pulmonary edema was detected in five patients. In ten patients infections were identified (pulmonary infections n=5, non-pulmonary infections n=5), consequently a calculated antibiotic therapy was started.
3.2. Comparison of laboratory values of patients with vs. without complications
Table 2 shows the median results and ranges of analyzed laboratory values in the perioperative course in patients with (cardiac or infectious) and without complications. Patients with complications had higher CRP levels compared to the patients without complications (202 mg/l [113–231 mg/l] vs. 171 mg/l [89–270 mg/l]). On day two the maximum level of PCT was reached in both groups, and showed a higher peak level in case of complications (0.36 ng/ml [0.12–7.3 ng/ml] vs. 0.21 ng/ml [0.12–0.84 ng/ml]). Analogous BNP levels increased in the postoperative course, had a higher peak level in case of complications and showed only a minimal decrease in comparison to patients without complications (153 pg/ml [17–721 pg/ml] vs. 78 pg/ml [25–237 pg/ml]). Similar peak levels of IL-6 were seen on day one in both groups (84 pg/ml [28–249 pg/ml] vs. 90 pg/ml [23–179 pg/ml]), but the decrease of IL-6 in patients with complications was slower than in patients without.
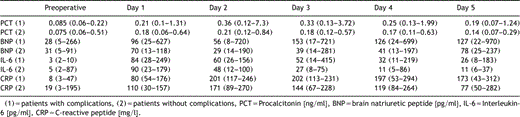
Comparison of median laboratory values of patients with (n=12) vs. without complications (n=10) (results given as median and range)
3.3. Comparison of laboratory values of patients with vs. without cardiac complications
In Table 3 median laboratory values and ranges of the two groups with cardiac versus without cardiac complications are given. Patients with cardiac complications showed increased CRP levels over four days beginning on day two and in comparison to patients without cardiac complications CRP levels were higher. In the group with cardiac complications, BNP levels showed earlier and higher peak levels than in patients without cardiac complications (186 pg/ml [17–721 pg/ml] vs. 78 pg/ml [22–237 pg/ml]). On day three, BNP level had almost fivefold account in patients with cardiac complications than in patients without. On day two, cardiac complications were associated with higher peak levels of PCT than in the comparison group (0.33 ng/ml [0.12–0.77] vs. 0.23 ng/ml [0.12–7.3 ng/ml]). There was almost no difference in peak levels of IL-6 in both groups (84 pg/ml [28–143 pg/ml] vs. 90 pg/ml [23–249 pg/ml]), but the decrease of IL-6 was slower in patients with cardiac complications than in patients without.
Comparison of laboratory values of patients with cardiac (n=10) vs. without cardiac complications (n=12) (results given as median and range)
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.085 (0.06–0.22) | 0.23 (0.1–1.31) | 0.33 (0.12–0.77) | 0.32 (0.13–0.61) | 0.24 (0.13–0.79) | 0.18 (0.07–0.71) |
| PCT (2) | 0.075 (0.06–0.51) | 0.18 (0.06–0.46) | 0.23 (0.12–7.3) | 0.2 (0.12–3.72) | 0.18 (0.11–1.99) | 0.14 (0.07–1.24) |
| BNP (1) | 28 (14–266) | 96 (25–627) | 81 (9–720) | 186 (17–721) | 136 (33–699) | 163 (22–970) |
| BNP (2) | 31 (5–95) | 70 (13–118) | 29 (8–190) | 39 (14–281) | 40 (13–197) | 78 (22–237) |
| IL-6 (1) | 3 (2–10) | 84 (28–143) | 69 (26–156) | 66 (14–415) | 32 (11–107) | 35 (9–183) |
| IL-6 (2) | 5 (2–87) | 90 (23–249) | 48 (12–106) | 26 (8–75) | 13 (5–219) | 11 (6–37) |
| CRP (1) | 11 (3–47) | 80 (54–176) | 201 (117–246) | 202 (113–231) | 211 (53–294) | 173 (53–312) |
| CRP (2) | 8 (3–195) | 85 (30–157) | 176 (89–270) | 143 (67–228) | 118 (66–264) | 77 (43–282) |
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.085 (0.06–0.22) | 0.23 (0.1–1.31) | 0.33 (0.12–0.77) | 0.32 (0.13–0.61) | 0.24 (0.13–0.79) | 0.18 (0.07–0.71) |
| PCT (2) | 0.075 (0.06–0.51) | 0.18 (0.06–0.46) | 0.23 (0.12–7.3) | 0.2 (0.12–3.72) | 0.18 (0.11–1.99) | 0.14 (0.07–1.24) |
| BNP (1) | 28 (14–266) | 96 (25–627) | 81 (9–720) | 186 (17–721) | 136 (33–699) | 163 (22–970) |
| BNP (2) | 31 (5–95) | 70 (13–118) | 29 (8–190) | 39 (14–281) | 40 (13–197) | 78 (22–237) |
| IL-6 (1) | 3 (2–10) | 84 (28–143) | 69 (26–156) | 66 (14–415) | 32 (11–107) | 35 (9–183) |
| IL-6 (2) | 5 (2–87) | 90 (23–249) | 48 (12–106) | 26 (8–75) | 13 (5–219) | 11 (6–37) |
| CRP (1) | 11 (3–47) | 80 (54–176) | 201 (117–246) | 202 (113–231) | 211 (53–294) | 173 (53–312) |
| CRP (2) | 8 (3–195) | 85 (30–157) | 176 (89–270) | 143 (67–228) | 118 (66–264) | 77 (43–282) |
(1)=patients with complications, (2)=patients without complications, PCT=Procalcitonin [ng/ml], BNP=brain natriuretic peptide [pg/ml], IL-6=Interleukin-6 [pg/ml], CRP=C-reactive peptide [mg/l].
Comparison of laboratory values of patients with cardiac (n=10) vs. without cardiac complications (n=12) (results given as median and range)
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.085 (0.06–0.22) | 0.23 (0.1–1.31) | 0.33 (0.12–0.77) | 0.32 (0.13–0.61) | 0.24 (0.13–0.79) | 0.18 (0.07–0.71) |
| PCT (2) | 0.075 (0.06–0.51) | 0.18 (0.06–0.46) | 0.23 (0.12–7.3) | 0.2 (0.12–3.72) | 0.18 (0.11–1.99) | 0.14 (0.07–1.24) |
| BNP (1) | 28 (14–266) | 96 (25–627) | 81 (9–720) | 186 (17–721) | 136 (33–699) | 163 (22–970) |
| BNP (2) | 31 (5–95) | 70 (13–118) | 29 (8–190) | 39 (14–281) | 40 (13–197) | 78 (22–237) |
| IL-6 (1) | 3 (2–10) | 84 (28–143) | 69 (26–156) | 66 (14–415) | 32 (11–107) | 35 (9–183) |
| IL-6 (2) | 5 (2–87) | 90 (23–249) | 48 (12–106) | 26 (8–75) | 13 (5–219) | 11 (6–37) |
| CRP (1) | 11 (3–47) | 80 (54–176) | 201 (117–246) | 202 (113–231) | 211 (53–294) | 173 (53–312) |
| CRP (2) | 8 (3–195) | 85 (30–157) | 176 (89–270) | 143 (67–228) | 118 (66–264) | 77 (43–282) |
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.085 (0.06–0.22) | 0.23 (0.1–1.31) | 0.33 (0.12–0.77) | 0.32 (0.13–0.61) | 0.24 (0.13–0.79) | 0.18 (0.07–0.71) |
| PCT (2) | 0.075 (0.06–0.51) | 0.18 (0.06–0.46) | 0.23 (0.12–7.3) | 0.2 (0.12–3.72) | 0.18 (0.11–1.99) | 0.14 (0.07–1.24) |
| BNP (1) | 28 (14–266) | 96 (25–627) | 81 (9–720) | 186 (17–721) | 136 (33–699) | 163 (22–970) |
| BNP (2) | 31 (5–95) | 70 (13–118) | 29 (8–190) | 39 (14–281) | 40 (13–197) | 78 (22–237) |
| IL-6 (1) | 3 (2–10) | 84 (28–143) | 69 (26–156) | 66 (14–415) | 32 (11–107) | 35 (9–183) |
| IL-6 (2) | 5 (2–87) | 90 (23–249) | 48 (12–106) | 26 (8–75) | 13 (5–219) | 11 (6–37) |
| CRP (1) | 11 (3–47) | 80 (54–176) | 201 (117–246) | 202 (113–231) | 211 (53–294) | 173 (53–312) |
| CRP (2) | 8 (3–195) | 85 (30–157) | 176 (89–270) | 143 (67–228) | 118 (66–264) | 77 (43–282) |
(1)=patients with complications, (2)=patients without complications, PCT=Procalcitonin [ng/ml], BNP=brain natriuretic peptide [pg/ml], IL-6=Interleukin-6 [pg/ml], CRP=C-reactive peptide [mg/l].
3.4. Comparison of laboratory values of patients with infections vs. no infections
In Table 4 median laboratory values of the two groups with infections versus no infections are given. CRP levels were only slightly increased (198 mg/l [113–232 mg/l] vs. 173 mg/l [89–270 mg/l]) but showed a slower decrease in the further course in patients with proven infections than in patients of the comparison group. BNP levels in patients with infections were higher and the peak level was earlier than in patients without infectious complications (123 pg/ml [24–699 pg/ml] vs. 82 pg/ml [25–237 pg/ml]). PCT levels showed high values in the group with proven infections whereas the group without infections did not. Both groups showed peak levels on day two (0.36 ng/ml [0.12– 7.3 ng/ml] vs. 0.23 ng/ml [0.12–0.84 ng/ml]). Peak levels of IL-6 were in both groups on day one (77 pg/ml [28–249 pg/ml] vs. 95 pg/ml [23–179 pg/ml]), with a higher level in patients without infectious complications. In the further postoperative course, IL-6 values showed a slower decrease in the case of infectious complications than in the comparison group. On day five the value of infected patients was threefold higher than in patients without infections (44 pg/ml [8–183 pg/ml] vs. 13 pg/ml [6–37 pg/ml]).
Comparison of laboratory values of patients with infections (n=10) vs. without infections (n=12) (results given as median and range)
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.075 (0.06–0.2) | 0.21 (0.1–1.31) | 0.36 (0.12–0.73) | 0.32 (0.13–3.72) | 0.23 (0.13–1.99) | 0.18 (0.07–1.24) |
| PCT (2) | 0.095 (0.06–0.51) | 0.185 (0.06–0.46) | 0.23 (0.12–0.84) | 0.2 (0.12–0.61) | 0.18 (0.11–0.61) | 0.14 (0.07–0.65) |
| BNP (1) | 29 (5–91) | 80 (25–627) | 51 (8–720) | 113 (17–721) | 123 (24–699) | 111 (22–970) |
| BNP (2) | 28 (5–91) | 80 (13–162) | 50 (14–190) | 54 (14–348) | 57 (13–300) | 82 (25–237) |
| IL-6 (1) | 4 (2–10) | 77 (28–249) | 54 (26–156) | 40 (14–415) | 32 (11–219) | 44 (8–183) |
| IL-6 (2) | 4 (2–87) | 95 (23–179) | 55 (12–100) | 29 (8–78) | 13 (5–86) | 13 (6–37) |
| CRP (1) | 10 (3–47) | 80 (54–176) | 191 (117–246) | 198 (113–231) | 178 (53–294) | 187 (43–312) |
| CRP (2) | 9 (3–195) | 99 (30–157) | 173 (89–270) | 165 (67–228) | 126 (84–264) | 94 (50–282) |
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.075 (0.06–0.2) | 0.21 (0.1–1.31) | 0.36 (0.12–0.73) | 0.32 (0.13–3.72) | 0.23 (0.13–1.99) | 0.18 (0.07–1.24) |
| PCT (2) | 0.095 (0.06–0.51) | 0.185 (0.06–0.46) | 0.23 (0.12–0.84) | 0.2 (0.12–0.61) | 0.18 (0.11–0.61) | 0.14 (0.07–0.65) |
| BNP (1) | 29 (5–91) | 80 (25–627) | 51 (8–720) | 113 (17–721) | 123 (24–699) | 111 (22–970) |
| BNP (2) | 28 (5–91) | 80 (13–162) | 50 (14–190) | 54 (14–348) | 57 (13–300) | 82 (25–237) |
| IL-6 (1) | 4 (2–10) | 77 (28–249) | 54 (26–156) | 40 (14–415) | 32 (11–219) | 44 (8–183) |
| IL-6 (2) | 4 (2–87) | 95 (23–179) | 55 (12–100) | 29 (8–78) | 13 (5–86) | 13 (6–37) |
| CRP (1) | 10 (3–47) | 80 (54–176) | 191 (117–246) | 198 (113–231) | 178 (53–294) | 187 (43–312) |
| CRP (2) | 9 (3–195) | 99 (30–157) | 173 (89–270) | 165 (67–228) | 126 (84–264) | 94 (50–282) |
(1)=patients with complications, (2)=patients without complications, PCT=Procalcitonin [ng/ml], BNP=brain natriuretic peptide [pg/ml], IL-6=Interleukin-6 [pg/ml], CRP=C-reactive peptide [mg/l].
Comparison of laboratory values of patients with infections (n=10) vs. without infections (n=12) (results given as median and range)
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.075 (0.06–0.2) | 0.21 (0.1–1.31) | 0.36 (0.12–0.73) | 0.32 (0.13–3.72) | 0.23 (0.13–1.99) | 0.18 (0.07–1.24) |
| PCT (2) | 0.095 (0.06–0.51) | 0.185 (0.06–0.46) | 0.23 (0.12–0.84) | 0.2 (0.12–0.61) | 0.18 (0.11–0.61) | 0.14 (0.07–0.65) |
| BNP (1) | 29 (5–91) | 80 (25–627) | 51 (8–720) | 113 (17–721) | 123 (24–699) | 111 (22–970) |
| BNP (2) | 28 (5–91) | 80 (13–162) | 50 (14–190) | 54 (14–348) | 57 (13–300) | 82 (25–237) |
| IL-6 (1) | 4 (2–10) | 77 (28–249) | 54 (26–156) | 40 (14–415) | 32 (11–219) | 44 (8–183) |
| IL-6 (2) | 4 (2–87) | 95 (23–179) | 55 (12–100) | 29 (8–78) | 13 (5–86) | 13 (6–37) |
| CRP (1) | 10 (3–47) | 80 (54–176) | 191 (117–246) | 198 (113–231) | 178 (53–294) | 187 (43–312) |
| CRP (2) | 9 (3–195) | 99 (30–157) | 173 (89–270) | 165 (67–228) | 126 (84–264) | 94 (50–282) |
| Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| PCT (1) | 0.075 (0.06–0.2) | 0.21 (0.1–1.31) | 0.36 (0.12–0.73) | 0.32 (0.13–3.72) | 0.23 (0.13–1.99) | 0.18 (0.07–1.24) |
| PCT (2) | 0.095 (0.06–0.51) | 0.185 (0.06–0.46) | 0.23 (0.12–0.84) | 0.2 (0.12–0.61) | 0.18 (0.11–0.61) | 0.14 (0.07–0.65) |
| BNP (1) | 29 (5–91) | 80 (25–627) | 51 (8–720) | 113 (17–721) | 123 (24–699) | 111 (22–970) |
| BNP (2) | 28 (5–91) | 80 (13–162) | 50 (14–190) | 54 (14–348) | 57 (13–300) | 82 (25–237) |
| IL-6 (1) | 4 (2–10) | 77 (28–249) | 54 (26–156) | 40 (14–415) | 32 (11–219) | 44 (8–183) |
| IL-6 (2) | 4 (2–87) | 95 (23–179) | 55 (12–100) | 29 (8–78) | 13 (5–86) | 13 (6–37) |
| CRP (1) | 10 (3–47) | 80 (54–176) | 191 (117–246) | 198 (113–231) | 178 (53–294) | 187 (43–312) |
| CRP (2) | 9 (3–195) | 99 (30–157) | 173 (89–270) | 165 (67–228) | 126 (84–264) | 94 (50–282) |
(1)=patients with complications, (2)=patients without complications, PCT=Procalcitonin [ng/ml], BNP=brain natriuretic peptide [pg/ml], IL-6=Interleukin-6 [pg/ml], CRP=C-reactive peptide [mg/l].
4. Discussion
Until now no human study has evaluated the time course, relationship, and clinical relevance of changes in PCT, CRP, BNP and IL-6 in patients undergoing a major lung resection for lung cancer. Therefore, the aim of this pilot study was to assess the accuracy of one parameter or of a combination of these parameters as marker(s) of postoperative cardiac or infectious complications after major thoracic surgery.
In general, postoperatively, PCT and BNP levels were increasing in all patients. Especially in patients with complications during the first five postoperative days PCT and BNP levels were higher than in patients without complications. IL-6 showed in patients with complications a slower decrease than the comparison group.
In the case of cardiac complications, BNP release was higher and the peak level was earlier than in patients without cardiac complications. Arrhythmia or pulmonary edema caused increased wall stretch and led to the release of BNP [5,6]. Without cardiac complications BNP levels showed only a slight increase in the first five postoperative days, probably as a sign of adaptation to the new vascular resistance in the pulmonary circulation [4,5]. PCT levels also increased in the case of cardiac complications, but this pathophysiological pathway is still unknown.
The increase of PCT and a quick normalization as an effect of surgical trauma without any infectious complication is reported [14,15]. As reported before [10,12,13], in the presented investigation PCT showed a higher increase and a slower decrease in the postoperative course in case of infection than without infection. Despite the small number of patients in this investigation it was not possible to form a cut-off value, but a level of 1 ng/ml was seen as cut-off value for diagnosis of infection in former studies [12].
In the case of postoperative infection, BNP levels were higher during the observation period than in patients without infectious complications. One reason for this fact could be that in case of infection the cardiac stress is higher which leads to an increased wall stretch and finally to a higher release of BNP. An interesting point for further studies is that in this investigation the increase of PCT and BNP levels emerged before the clinical appearance of infectious complications and simultaneous to occurrence of arrhythmia.
Postoperative IL-6, as a pro-inflammatoric parameter, showed an increase and a slower decrease in patients with infections or cardiac complications than without. The underlying hypothesis is that cardiac as well as infectious complications are associated with an increased activity of the immune system which leads to a higher release of IL-6.
The CRP showed in the presented investigation a very unspecific course with delayed reaction and prolonged increase of serum concentration. Cardiac or infectious complications did not seem to have significant influence compared to the group without complications.
Altogether the patients in this investigation had a high rate of cardiac or infectious complications. The main reason may be that this is the typical high-risk patient collective of a university hospital. Another reason might be that particularly in the case of infectious complications not only severe infections such as pneumonia, empyema or sepsis were assessed as complications but also cases like urinary tract infections.
In conclusion, cardiac and infectious complications are associated with higher BNP and PCT levels and earlier peak levels than without these complications. Patients with complications showed a slower decrease of IL-6 than patients without. Because of our small caseload the differences between the compared groups did not reach statistical significance. But the combination of BNP, PCT, and IL-6 seems to be an appropiate diagnostic tool to optimize the postoperative course regarding cardiac or infectious complications in patients undergoing major pulmonary resection. We postulate that high BNP and PCT levels with a slow decrease in the further course, as well as a slow decrease of IL-6, are associated with complications which seem to occur with a certain delay after the ascent of BNP and PCT. Concluding, further investigations are necessary to verify the postulated correlations and trends.
Presented at the joint 20th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 14th Annual Meeting of the European Society of Thoracic Surgeons, Stockholm, Sweden, September 10–13, 2006.
References
Conference discussion
Dr. M. Lanuti (Boston, Massachusetts, USA): Were these the only acute-phase reactants or cytokines that you measured, or was there a panel that you measured and these were the only ones that were significant?
Dr. Fahrner: Although the differences we have seen were not significant we think to have in procalcitonin, brain natriuretic peptide and Interleukin-6 three useful parameters to detect pulmonary infections or cardiac complications.
Dr. Van Schil: But the question was did you measure other parameters in the blood.
Dr. Fahrner: We also measured interleukin-10, which showed no significant values, and, of course, white blood count was also measured in the routine laboratory.
Dr. Van Schil: And was there a correlation with the white blood cell count?
Dr. Fahrner: The white blood cell count was very unspecific. We had patients with infections in which they were very low and patients without infections which had very high leukocytes.
Dr. A. End (Vienna, Austria): Could you please specify the complications? What is a complication? Define it, please.
Dr. Fahrner: Arryhthmia which were detected by ECG and cardiac insufficiency were counted as cardiac complications. Infectious complications were determined through positive microbiological results which were found in cases of clinical suspicion like postoperative fever and through radiological findings in case of pneumonia. But in our investigation we have not seen any severe infectious complications such as empyema or sepsis.
Dr. T. Szoke (Szeged, Hungary): When did you observe the elevation of these markers, before the signs of infection, during the signs, or after the signs of infection?
Dr. Fahrner: The complications occurred during the first postoperative days in which we measured our parameters. But until now we have no definite correlation whether these parameters increase earlier than the signs of infections.
Dr. A. Turna (Istanbul, Turkey): What would you recommend exactly for the patients with higher BNP, PCT, and interleukin-6 in terms of postoperative monitoring? Would you suggest one more ICU unit stay or anything else?
Dr. Fahrner: Can you repeat your question?
Dr. Turna: You concluded that it is useful for optimized postoperative monitoring. What do you mean by that?
Dr. Fahrner: In case of high values of BNP or Procalcitonin we hope that we can react earlier or are more sensitized for cardiac or infectious complications. And they might help us to monitor our therapy.
Dr. Van Schil: Yes, but more practically, would you keep the patient on medium care, intensive care? Would you start antibiotics earlier than usual?
Dr. Fahrner: I think this depends on the clinical situation. Actually I would say you should start earlier with antibiotics in case of high values of BNP or procalcitonin. But I think we should wait until we have further informations before we can give a definite suggestion.
- brain natriuretic peptide
- cardiac event
- cause of death
- peptides
- reference values
- infections
- heart
- infection as complication of medical care
- interleukin-6
- morbidity
- thoracic surgery procedures
- lung volume reduction
- lung cancer
- postoperative infections
- procalcitonin
- cardiac complications
- brain natriuretic peptide measurement




