-
PDF
- Split View
-
Views
-
Cite
Cite
Jingyao Sun, Zhaoyao Hou, Tian Xia, Chang Liu, Qiangwen Huang, Li Wei, Effect of budesonide–formoterol powder inhalation on cough and lung function following thoracoscopic pulmonary surgery: a retrospective cohort study, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 40, Issue 5, May 2025, ivae097, https://doi.org/10.1093/icvts/ivae097
Close - Share Icon Share
Abstract
OBJECTIVES
To explore whether portable budesonide–formoterol powder inhalation can ameliorate cough symptoms and improve pulmonary function recovery in patients who underwent thoracoscopic lung surgery.
METHODS
Clinical data of patients who underwent thoracoscopic pulmonary resection at Henan Provincial People’s Hospital between December 2022 and May 2023 were extracted. To evaluate the impact of continuous postoperative use of portable budesonide–formoterol powder inhalation, patients were divided into two groups: the control group and the case group. Next, we compared the Leicester cough score and pulmonary function indexes of the patients before surgery, 1 month and 6 months after the operation.
RESULTS
A total of 188 cases were included, and the baseline demographic characteristics of both groups were well-balanced. The internal consistency of the Mandarin Chinese version of the Leicester Cough Questionnaire (LCQ-MC) scale, as indicated by Cronbach’s α coefficients, was all greater than 0.8, and there was no significant difference in LCQ-MC scores between the two groups before the operation (Z = −1.173, P = 0.241). Postoperatively, the LCQ-MC score in the case group was significantly higher than that in the control group (18.66 vs 16.79, P < 0.01), with a notable statistically significant difference in the reduction of LCQ-MC scores between the two groups (1.32 vs 3.30, P < 0.01). Analysis of lung function revealed that patients in the case group exhibited significant improvements in FEV1/FVC, FVC, FEV1, PEF, MMEF75/25, MVV, DLCO and the RV/TLC indexes compared to the control group (P < 0.01).
CONCLUSIONS
Portable budesonide–formoterol powder inhalation can alleviate cough symptoms and promote pulmonary function recovery in patients following thoracoscopic lung surgery.
INTRODUCTION
The widespread adoption of the concept of rapid recovery surgery in clinical practice has highlighted the importance of enhancing the quality of life and functional recovery of post-pneumonectomy patients [1, 2]. Research has shown that postoperative coughing is a common symptom among patients undergoing thoracoscopic lung surgery, which increases patient anxiety and decreases their overall quality of life [3]. In addition, the decline in lung function often serves as a significant obstacle to patients unwilling to undergo surgical treatment [4]. Therefore, strategies that mitigate postoperative cough symptoms and promote the recovery of lung function can reduce patient concerns about undergoing surgery and enhance their confidence in the procedure.
The 2020 edition of the Chinese Thoracic Surgery Perioperative Airway Management Guidelines [5] recommends the use of inhaled corticosteroids combined with bronchodilators in patients with chronic obstructive pulmonary disease (COPD) and asthma to effectively improve their respiratory function after surgery. Nevertheless, there is a need for further research to evaluate the effectiveness of this treatment in controlling symptoms of airway hyperreactivity and promoting lung function recovery in the general population following lung surgery. Budesonide–formoterol inhalation powder, a combination medication comprising inhaled corticosteroids and bronchodilators, has demonstrated promising synergistic effects [6].
Inhalation therapy through nebulization offers a direct means of delivering medication to the affected area, making it ideal for the treatment of respiratory system diseases [7]. Studies have shown that nebulized ipratropium bromide can improve lung function and reduce the incidence of postoperative pneumonia in COPD patients undergoing lung surgery [8]. Nevertheless, conventional nebulization therapy relies on aerosol devices and must be administered within a hospital setting. This approach has several drawbacks, including cumbersome operation, extensive time requirements and low drug utilization rate [9]. Therefore, patient compliance is often low, making it difficult for them to actively cooperate with healthcare providers and achieve the desired treatment outcomes. Conversely, portable aerosol inhalation offers several advantages, including small size, easy portability and precise drug administration [10]. Patients can receive treatment anywhere with simple operation, significantly reducing time-related costs and improving patient compliance. This approach can help standardize post-pulmonary surgery treatment beyond the confines of a hospital. Nonetheless, the efficacy of portable powder inhalers for post-pulmonary surgery remains uncertain.
The aim of this study was to investigate the efficacy of portable budesonide–formoterol powder inhalation in ameliorating cough symptoms and promoting pulmonary function recovery among postoperative patients. The findings of this study may provide valuable insights to assist clinicians in making more informed treatment decisions.
MATERIALS AND METHODS
Ethics statement
This retrospective cohort study was approved by the Ethics Committee of Henan Provincial People's Hospital, Zhengzhou, Henan, China (approval No. 2022–65; date of review: 20 June 2022). Formal written consent was obtained from all patients.
Clinical data
Clinical data were extracted from patients who underwent thoracoscopic lung resection surgery at Henan Provincial People's Hospital between December 2022 and May 2023.
Inclusion criteria include patients aged between 18 and 80 years, who underwent thoracoscopic pulmonary lobe, pulmonary segment or pulmonary wedge resection; who received pulmonary function examination at our hospital within 1 week before surgery and 1 month after surgery (4–5 weeks after surgery); and absence of heart, liver, kidney and other important organ diseases.
The following are the exclusion criteria: coexistence of active tuberculosis, bronchial asthma, severe bronchiectasis or COPD before surgery; previous history of radiotherapy or chemotherapy; previous pulmonary or cardiac surgery; tracheal or main bronchial stenosis, severe intraoperative thoracic adhesions, or severe intraoperative or postoperative complications; thoracotomy was performed during the operation or blood loss exceeded 1000 ml [11]; non-compliance with conventional perioperative management; use of antitussive medications within 1 week before surgery or within 2 weeks after surgery; prolonged bed rest; postoperative air leak for >3days; hospitalization lasting > 7 days after surgery; duration of budesonide–formoterol powder inhalation treatment exceeding 1 week but less than 4 weeks; or medication administered within 12 hours before postoperative pulmonary function examination.
Conventional perioperative management
During the surgical operation, a 3–4 cm incision was made in the fourth intercostal space to serve as the operation site, while a 1 cm incision in the seventh intercostal space was used as the observation port. A chest drainage tube was inserted through the observation port. Extubation was considered when the drainage fluid collected within the first 24 hours post-surgery was less than 300 ml and no air leakage was observed. Traditional nebulization (0.5 mg Ipratropium bromide solution + 1 mg budesonide suspension with oxygen inhalation, twice daily) was administered one day before surgery and continued until the day of discharge. Following the surgical procedure, patients were instructed to use budesonide–formoterol powder inhalation twice daily. Each inhalation contained 320 μg of budesonide and 9 μg of formoterol fumarate. After surgery, propacetamol hydrochloride 1.0 g was given twice a day until discharge.
Grouping method
Patients were categorized based on their history of budesonide–formoterol powder inhalation. Patients who discontinued medication spontaneously within 1 week after surgery were assigned to the control group, while patients who continued postoperative medication for 4 weeks or more were assigned to the case group.
Research indicators
Cough score
The Mandarin Chinese version of the Leicester Cough Questionnaire(LCQ-MC) was used to assess the patient's cough condition [12, 13]. LCQ-MC scores were collected for all subjects within 1 week before surgery and 2 months after surgery. Higher scores indicated less severe cough.
Pulmonary function index
Pulmonary function indicators were extracted for all subjects within 1 week before surgery, 1 month 6 six months after surgery. All pulmonary function tests were performed under the guidance of professionals in the Cardiopulmonary Function Department.
Pulmonary function indices included: forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC), forced vital capacity (FVC), forced vital capacity (FEV1), peak expiratory flow (PEF), maximal voluntary ventilation (MVV), maximal mid-expiratory flow (MMEF75/25), residual volume/total lung capacity (RV/TLC), diffusing capacity of the lungs for carbon monoxide (DLCO).
Evaluation methods
Cough scoring index evaluation method
Comparison of the mean difference in LCQ-MC scores between the two groups of subjects after surgery.
Calculation of the postoperative LCQ-MC score drop for the research subjects (drop value = preoperative LCQ-MC score—postoperative LCQ-MC score), followed by a comparison of the difference in the LCQ-MC score drop between the two groups.
LCQ-MC scale reliability evaluation method
Cronbach's alpha coefficient was utilized to assess the reliability of the LCQ-MC scale. A Cronbach's α coefficient ranging from 0.7 to 0.8 indicates acceptable scale reliability, while a coefficient above 0.8 indicates excellent reliability.
Pulmonary function index evaluation method
Calculation of the decline in lung function index for the research subjects (decrease = preoperative index—postoperative index) and determination of the decline rate (decline rate = decline ÷ preoperative index). Subsequently, we compared the decline rate between the two groups.
Statistical methods
Data were processed using the WPS Office software, and statistical analysis was conducted using the SPSS 25.0 software. The reliability of the LCQ-MC scale was verified by calculating Cronbach’s α coefficient. Quantitative data at baseline were compared using the t-test, categorical data were analysed using the chi-square test and graded data were compared using the rank-sum test. Comparison between groups was performed using the paired rank-sum test and rank-sum test. Differences and change rates of pulmonary function indicators between groups were compared using the independent sample t–test. All tests were two-sided, and P < 0.05 was considered statistically significant [14, 15].
RESULTS
Baseline characteristics
Table 1 presents a summary of the baseline characteristics of patients. After applying the inclusion and exclusion criteria, a total of 188 cases were included in the study, with 92 cases in the control group and 96 cases in the case group. There was no significant difference in the baseline characteristics of the two groups, indicating that the patients were comparable.
| Case group . | Control group . | P . | |
|---|---|---|---|
| n = 96 . | n = 92 . | ||
| Age (years), median (Q1, Q3) | 60.0 (54.0,66.0) | 58.0 (51.0,63.7) | 0.073 |
| Female, n (%) | 55 (57.29%) | 55 (59.78%) | 0.729 |
| Body mass index (kg/m2), median (Q1, Q3) | 24.86 (22.36,27.09) | 25.05 (23.23,26.70) | 0.403 |
| Body surface area (m2), median (Q1, Q3) | 1.66 (1.55,1.75) | 1.67 (1.55,1.80) | 0.279 |
| Smoking history, n (%) | 0.289 | ||
| Never smoker | 69 (71.88%) | 75 (81.52%) | |
| Ex-smokera | 15 (15.62%) | 10 (10.87%) | |
| Current smokerb | 12 (12.50%) | 7 (7.61%) | |
| Smoking indexc, median (Q1, Q3) | 551.9 (300.0,840.0) | 378.9 (125.0,600.0) | 0.082 |
| Type of surgical resection., n (%) | 0.291 | ||
| Lobectomy | 45 (46.87%) | 40 (43.48%) | |
| Segmentectomy | 28 (29.17%) | 36 (39.13%) | |
| Wedge resection | 23 (23.96%) | 16 (17.39%) | |
| Operation time, median (Q1, Q3) | 135.4 (95.0,173.7) | 144.3 (105.0,185.0) | 0.207 |
| Postoperative physical activity leveld, n (%) | 0.743 | ||
| Low level | 92 (95.83%) | 89 (96.74%) | |
| Intermediate level | 4 (4.17%) | 3 (3.26%) | |
| High level | 0(0%) | 0(0%) |
| Case group . | Control group . | P . | |
|---|---|---|---|
| n = 96 . | n = 92 . | ||
| Age (years), median (Q1, Q3) | 60.0 (54.0,66.0) | 58.0 (51.0,63.7) | 0.073 |
| Female, n (%) | 55 (57.29%) | 55 (59.78%) | 0.729 |
| Body mass index (kg/m2), median (Q1, Q3) | 24.86 (22.36,27.09) | 25.05 (23.23,26.70) | 0.403 |
| Body surface area (m2), median (Q1, Q3) | 1.66 (1.55,1.75) | 1.67 (1.55,1.80) | 0.279 |
| Smoking history, n (%) | 0.289 | ||
| Never smoker | 69 (71.88%) | 75 (81.52%) | |
| Ex-smokera | 15 (15.62%) | 10 (10.87%) | |
| Current smokerb | 12 (12.50%) | 7 (7.61%) | |
| Smoking indexc, median (Q1, Q3) | 551.9 (300.0,840.0) | 378.9 (125.0,600.0) | 0.082 |
| Type of surgical resection., n (%) | 0.291 | ||
| Lobectomy | 45 (46.87%) | 40 (43.48%) | |
| Segmentectomy | 28 (29.17%) | 36 (39.13%) | |
| Wedge resection | 23 (23.96%) | 16 (17.39%) | |
| Operation time, median (Q1, Q3) | 135.4 (95.0,173.7) | 144.3 (105.0,185.0) | 0.207 |
| Postoperative physical activity leveld, n (%) | 0.743 | ||
| Low level | 92 (95.83%) | 89 (96.74%) | |
| Intermediate level | 4 (4.17%) | 3 (3.26%) | |
| High level | 0(0%) | 0(0%) |
Quit smoking over 4 weeks.
Have not quit smoking or quit smoking for less than 4 weeks.
Only subjects with a history of smoking were counted.
Classification according to the International Physical Activity Scale IPAQ.
| Case group . | Control group . | P . | |
|---|---|---|---|
| n = 96 . | n = 92 . | ||
| Age (years), median (Q1, Q3) | 60.0 (54.0,66.0) | 58.0 (51.0,63.7) | 0.073 |
| Female, n (%) | 55 (57.29%) | 55 (59.78%) | 0.729 |
| Body mass index (kg/m2), median (Q1, Q3) | 24.86 (22.36,27.09) | 25.05 (23.23,26.70) | 0.403 |
| Body surface area (m2), median (Q1, Q3) | 1.66 (1.55,1.75) | 1.67 (1.55,1.80) | 0.279 |
| Smoking history, n (%) | 0.289 | ||
| Never smoker | 69 (71.88%) | 75 (81.52%) | |
| Ex-smokera | 15 (15.62%) | 10 (10.87%) | |
| Current smokerb | 12 (12.50%) | 7 (7.61%) | |
| Smoking indexc, median (Q1, Q3) | 551.9 (300.0,840.0) | 378.9 (125.0,600.0) | 0.082 |
| Type of surgical resection., n (%) | 0.291 | ||
| Lobectomy | 45 (46.87%) | 40 (43.48%) | |
| Segmentectomy | 28 (29.17%) | 36 (39.13%) | |
| Wedge resection | 23 (23.96%) | 16 (17.39%) | |
| Operation time, median (Q1, Q3) | 135.4 (95.0,173.7) | 144.3 (105.0,185.0) | 0.207 |
| Postoperative physical activity leveld, n (%) | 0.743 | ||
| Low level | 92 (95.83%) | 89 (96.74%) | |
| Intermediate level | 4 (4.17%) | 3 (3.26%) | |
| High level | 0(0%) | 0(0%) |
| Case group . | Control group . | P . | |
|---|---|---|---|
| n = 96 . | n = 92 . | ||
| Age (years), median (Q1, Q3) | 60.0 (54.0,66.0) | 58.0 (51.0,63.7) | 0.073 |
| Female, n (%) | 55 (57.29%) | 55 (59.78%) | 0.729 |
| Body mass index (kg/m2), median (Q1, Q3) | 24.86 (22.36,27.09) | 25.05 (23.23,26.70) | 0.403 |
| Body surface area (m2), median (Q1, Q3) | 1.66 (1.55,1.75) | 1.67 (1.55,1.80) | 0.279 |
| Smoking history, n (%) | 0.289 | ||
| Never smoker | 69 (71.88%) | 75 (81.52%) | |
| Ex-smokera | 15 (15.62%) | 10 (10.87%) | |
| Current smokerb | 12 (12.50%) | 7 (7.61%) | |
| Smoking indexc, median (Q1, Q3) | 551.9 (300.0,840.0) | 378.9 (125.0,600.0) | 0.082 |
| Type of surgical resection., n (%) | 0.291 | ||
| Lobectomy | 45 (46.87%) | 40 (43.48%) | |
| Segmentectomy | 28 (29.17%) | 36 (39.13%) | |
| Wedge resection | 23 (23.96%) | 16 (17.39%) | |
| Operation time, median (Q1, Q3) | 135.4 (95.0,173.7) | 144.3 (105.0,185.0) | 0.207 |
| Postoperative physical activity leveld, n (%) | 0.743 | ||
| Low level | 92 (95.83%) | 89 (96.74%) | |
| Intermediate level | 4 (4.17%) | 3 (3.26%) | |
| High level | 0(0%) | 0(0%) |
Quit smoking over 4 weeks.
Have not quit smoking or quit smoking for less than 4 weeks.
Only subjects with a history of smoking were counted.
Classification according to the International Physical Activity Scale IPAQ.
Cough score results
LCQ-MC scale reliability evaluation
The Cronbach’s α coefficients for both groups were greater than 0.8 both before and 1 month after the operation (Table 2). This suggests that the LCQ-MC score data obtained in this study exhibit good reliability.
| Case group (n = 96) . | Control group (n = 92) . | Whole sample (n = 188) . | |
|---|---|---|---|
| Before surgery | 0.863 | 0.813 | 0.837 |
| After surgery | 0.821 | 0.811 | 0.878 |
| Case group (n = 96) . | Control group (n = 92) . | Whole sample (n = 188) . | |
|---|---|---|---|
| Before surgery | 0.863 | 0.813 | 0.837 |
| After surgery | 0.821 | 0.811 | 0.878 |
| Case group (n = 96) . | Control group (n = 92) . | Whole sample (n = 188) . | |
|---|---|---|---|
| Before surgery | 0.863 | 0.813 | 0.837 |
| After surgery | 0.821 | 0.811 | 0.878 |
| Case group (n = 96) . | Control group (n = 92) . | Whole sample (n = 188) . | |
|---|---|---|---|
| Before surgery | 0.863 | 0.813 | 0.837 |
| After surgery | 0.821 | 0.811 | 0.878 |
Comparison of LCQ-MC scores before and after operation
There were no significant differences in preoperative LCQ-MC scores between the two groups (20.03 vs 18.66, Z=−1.173, P = 0.241). However, significant differences were observed in LCQ-MC scores before and after operation in both the case group (Z=−8.16, P < 0.01) and the control group (Z=−8.33, P < 0.01). Furthermore, there were significant differences in the postoperative LCQ-MC score between the two groups (Z=−11.082, P < 0.01) (Table 3).
Comparison of LCQ-MC scores between the two groups before and after surgery
| Before surgery median (Q1, Q3) . | After surgery median (Q1, Q3) . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 20.03 (19.15,20.48) | 18.66 (18.25,19.21) | −8.16 | <0.01 |
| Control group (n = 92) | 20.17 (19.85,20.46) | 16.79 (16.22,17.15) | −8.33 | <0.01 |
| Z | −1.173 | −11.082 | ||
| P | 0.241 | <0.01 | ||
| Before surgery median (Q1, Q3) . | After surgery median (Q1, Q3) . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 20.03 (19.15,20.48) | 18.66 (18.25,19.21) | −8.16 | <0.01 |
| Control group (n = 92) | 20.17 (19.85,20.46) | 16.79 (16.22,17.15) | −8.33 | <0.01 |
| Z | −1.173 | −11.082 | ||
| P | 0.241 | <0.01 | ||
Comparison of LCQ-MC scores between the two groups before and after surgery
| Before surgery median (Q1, Q3) . | After surgery median (Q1, Q3) . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 20.03 (19.15,20.48) | 18.66 (18.25,19.21) | −8.16 | <0.01 |
| Control group (n = 92) | 20.17 (19.85,20.46) | 16.79 (16.22,17.15) | −8.33 | <0.01 |
| Z | −1.173 | −11.082 | ||
| P | 0.241 | <0.01 | ||
| Before surgery median (Q1, Q3) . | After surgery median (Q1, Q3) . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 20.03 (19.15,20.48) | 18.66 (18.25,19.21) | −8.16 | <0.01 |
| Control group (n = 92) | 20.17 (19.85,20.46) | 16.79 (16.22,17.15) | −8.33 | <0.01 |
| Z | −1.173 | −11.082 | ||
| P | 0.241 | <0.01 | ||
Comparison of cough score differences
The postoperative LCQ-MC score drop in the case group was 1.32 (1.03, 1.66) points while that in the control group was 3.30 (2.98, 3.73) points. These values (difference 1.99, 95% CI: 1.83, 2.16) were significantly different between the two groups (Z=−11.57, P < 0.01) (Table 4). Analysis revealed that the decrease in the LCQ-MC score in the case group was significantly lower compared with that of the control group.
| Score drop median (Q1, Q3) . | Difference and 95%CI . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 1.32 (1.03,1.66) | 1.99 (1.83,2.16) | −11.57 | <0.01 |
| Control group (n = 92) | 3.30 (2.98,3.73) | |||
| Score drop median (Q1, Q3) . | Difference and 95%CI . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 1.32 (1.03,1.66) | 1.99 (1.83,2.16) | −11.57 | <0.01 |
| Control group (n = 92) | 3.30 (2.98,3.73) | |||
| Score drop median (Q1, Q3) . | Difference and 95%CI . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 1.32 (1.03,1.66) | 1.99 (1.83,2.16) | −11.57 | <0.01 |
| Control group (n = 92) | 3.30 (2.98,3.73) | |||
| Score drop median (Q1, Q3) . | Difference and 95%CI . | Wilcoxon signed rank-sum test . | ||
|---|---|---|---|---|
| Z . | P . | |||
| Case group (n = 96) | 1.32 (1.03,1.66) | 1.99 (1.83,2.16) | −11.57 | <0.01 |
| Control group (n = 92) | 3.30 (2.98,3.73) | |||
Lung function index results
Compared to the control group, 1 month after surgery, the case group had significantly lower decrease rates in FEV1/FVC (difference: −5.76%), FVC (difference: −13.54%), FEV1 (difference: −17.28%), PEF (difference: −12.20%), MMEF75/25 (difference −23.77%) and MVV (difference: −14.33%) (P < 0.01) as shown in Table 5.
Comparison of pulmonary function indicator decline rates 1 month after surgery between the two groups of research subjects
| Decline rate (%), mean ± SD . | Difference and 95% CI . | Independent sample t-test . | |||
|---|---|---|---|---|---|
| Case group . | Control group . | t . | P . | ||
| n = 96 . | n = 92 . | ||||
| FEV1/FVC | −2.37±5.77 | 3.39±6.51 | −5.76 (−7.54, −4.00) | −6.43 | <0.01 |
| FVC | 8.70±9.02 | 22.24±9.84 | −13.54 (−16.25, −10.82) | −9.84 | <0.01 |
| FEV1 | 7.45±9.03 | 24.73±8.98 | −17.28 (−19.87, −14.69) | −13.16 | <0.01 |
| PEF | 4.48±11.46 | 16.68±11.63 | −12.20 (−15.52, −8.88) | −7.24 | <0.01 |
| MMEF75/25 | 6.72±21.88 | 30.49±18.15 | −23.77 (−29.57, −17.98) | −8.09 | <0.01 |
| MVV | −0.16±18.39 | 14.17±14.79 | −14.33 (−19.15, −9.52) | −5.87 | <0.01 |
| RV/TLC | 1.64±17.12 | −9.81±14.41 | 11.45 (6.88, 16.01) | 4.95 | <0.01 |
| DLCO | 10.87±14.39 | 16.68±12.31 | −5.51 (−9.66, −1.96) | −2.97 | <0.01 |
| Decline rate (%), mean ± SD . | Difference and 95% CI . | Independent sample t-test . | |||
|---|---|---|---|---|---|
| Case group . | Control group . | t . | P . | ||
| n = 96 . | n = 92 . | ||||
| FEV1/FVC | −2.37±5.77 | 3.39±6.51 | −5.76 (−7.54, −4.00) | −6.43 | <0.01 |
| FVC | 8.70±9.02 | 22.24±9.84 | −13.54 (−16.25, −10.82) | −9.84 | <0.01 |
| FEV1 | 7.45±9.03 | 24.73±8.98 | −17.28 (−19.87, −14.69) | −13.16 | <0.01 |
| PEF | 4.48±11.46 | 16.68±11.63 | −12.20 (−15.52, −8.88) | −7.24 | <0.01 |
| MMEF75/25 | 6.72±21.88 | 30.49±18.15 | −23.77 (−29.57, −17.98) | −8.09 | <0.01 |
| MVV | −0.16±18.39 | 14.17±14.79 | −14.33 (−19.15, −9.52) | −5.87 | <0.01 |
| RV/TLC | 1.64±17.12 | −9.81±14.41 | 11.45 (6.88, 16.01) | 4.95 | <0.01 |
| DLCO | 10.87±14.39 | 16.68±12.31 | −5.51 (−9.66, −1.96) | −2.97 | <0.01 |
Comparison of pulmonary function indicator decline rates 1 month after surgery between the two groups of research subjects
| Decline rate (%), mean ± SD . | Difference and 95% CI . | Independent sample t-test . | |||
|---|---|---|---|---|---|
| Case group . | Control group . | t . | P . | ||
| n = 96 . | n = 92 . | ||||
| FEV1/FVC | −2.37±5.77 | 3.39±6.51 | −5.76 (−7.54, −4.00) | −6.43 | <0.01 |
| FVC | 8.70±9.02 | 22.24±9.84 | −13.54 (−16.25, −10.82) | −9.84 | <0.01 |
| FEV1 | 7.45±9.03 | 24.73±8.98 | −17.28 (−19.87, −14.69) | −13.16 | <0.01 |
| PEF | 4.48±11.46 | 16.68±11.63 | −12.20 (−15.52, −8.88) | −7.24 | <0.01 |
| MMEF75/25 | 6.72±21.88 | 30.49±18.15 | −23.77 (−29.57, −17.98) | −8.09 | <0.01 |
| MVV | −0.16±18.39 | 14.17±14.79 | −14.33 (−19.15, −9.52) | −5.87 | <0.01 |
| RV/TLC | 1.64±17.12 | −9.81±14.41 | 11.45 (6.88, 16.01) | 4.95 | <0.01 |
| DLCO | 10.87±14.39 | 16.68±12.31 | −5.51 (−9.66, −1.96) | −2.97 | <0.01 |
| Decline rate (%), mean ± SD . | Difference and 95% CI . | Independent sample t-test . | |||
|---|---|---|---|---|---|
| Case group . | Control group . | t . | P . | ||
| n = 96 . | n = 92 . | ||||
| FEV1/FVC | −2.37±5.77 | 3.39±6.51 | −5.76 (−7.54, −4.00) | −6.43 | <0.01 |
| FVC | 8.70±9.02 | 22.24±9.84 | −13.54 (−16.25, −10.82) | −9.84 | <0.01 |
| FEV1 | 7.45±9.03 | 24.73±8.98 | −17.28 (−19.87, −14.69) | −13.16 | <0.01 |
| PEF | 4.48±11.46 | 16.68±11.63 | −12.20 (−15.52, −8.88) | −7.24 | <0.01 |
| MMEF75/25 | 6.72±21.88 | 30.49±18.15 | −23.77 (−29.57, −17.98) | −8.09 | <0.01 |
| MVV | −0.16±18.39 | 14.17±14.79 | −14.33 (−19.15, −9.52) | −5.87 | <0.01 |
| RV/TLC | 1.64±17.12 | −9.81±14.41 | 11.45 (6.88, 16.01) | 4.95 | <0.01 |
| DLCO | 10.87±14.39 | 16.68±12.31 | −5.51 (−9.66, −1.96) | −2.97 | <0.01 |
The decrease in DLCO rate in the case group was significantly lower compared with that of the control group (difference: −2.35%, P < 0.01), while the decline in RV/TLC rate was significantly higher than that of the control group (difference: 11.45%, P < 0.01) as shown in Table 5.
There are still notable differences in the above indicators 6 months after surgery (all P < 0.01). The rate of decline in lung function in the case group is lower compared to the control group, as shown in Table 6.
Comparison of pulmonary function indicator decline rates 6 months after surgery between the two groups of research subjects
| Decline rate (%), median (Q1, Q3) . | Wilcoxon rank-sum test . | |||
|---|---|---|---|---|
| Case group . | Control group . | Z . | P . | |
| n = 96 . | n = 92 . | |||
| FEV1/FVC | 0.85 (−1.90,5.25) | 5.58 (3.96,9.06) | −6.22 | <0.01 |
| FVC | 0.83 (−7.20,6.19) | 11.65 (8.45,24.41) | −9.08 | <0.01 |
| FEV1 | 4.41 (−0.41,8.58) | 14.73 (8.83,29.61) | −8.13 | <0.01 |
| PEF | 0.80 (−4.78,8.37) | 10.65 (6.97,15.19) | −7.40 | <0.01 |
| MMEF75/25 | 0.92 (−15.47,18.90) | 27.71 (16.69,41.83) | −7.78 | <0.01 |
| MVV | 6.44 (−3.88,14.28) | 19.80 (11.77,28.17) | −6.71 | <0.01 |
| RV/TLC | 4.65 (−0.92,10.80) | −7.61 (−14.56,−0.21) | −10.89 | <0.01 |
| DLCO | 4.94 (−1.15,11.19) | 13.62 (7.58,21.00) | −6.36 | <0.01 |
| Decline rate (%), median (Q1, Q3) . | Wilcoxon rank-sum test . | |||
|---|---|---|---|---|
| Case group . | Control group . | Z . | P . | |
| n = 96 . | n = 92 . | |||
| FEV1/FVC | 0.85 (−1.90,5.25) | 5.58 (3.96,9.06) | −6.22 | <0.01 |
| FVC | 0.83 (−7.20,6.19) | 11.65 (8.45,24.41) | −9.08 | <0.01 |
| FEV1 | 4.41 (−0.41,8.58) | 14.73 (8.83,29.61) | −8.13 | <0.01 |
| PEF | 0.80 (−4.78,8.37) | 10.65 (6.97,15.19) | −7.40 | <0.01 |
| MMEF75/25 | 0.92 (−15.47,18.90) | 27.71 (16.69,41.83) | −7.78 | <0.01 |
| MVV | 6.44 (−3.88,14.28) | 19.80 (11.77,28.17) | −6.71 | <0.01 |
| RV/TLC | 4.65 (−0.92,10.80) | −7.61 (−14.56,−0.21) | −10.89 | <0.01 |
| DLCO | 4.94 (−1.15,11.19) | 13.62 (7.58,21.00) | −6.36 | <0.01 |
Comparison of pulmonary function indicator decline rates 6 months after surgery between the two groups of research subjects
| Decline rate (%), median (Q1, Q3) . | Wilcoxon rank-sum test . | |||
|---|---|---|---|---|
| Case group . | Control group . | Z . | P . | |
| n = 96 . | n = 92 . | |||
| FEV1/FVC | 0.85 (−1.90,5.25) | 5.58 (3.96,9.06) | −6.22 | <0.01 |
| FVC | 0.83 (−7.20,6.19) | 11.65 (8.45,24.41) | −9.08 | <0.01 |
| FEV1 | 4.41 (−0.41,8.58) | 14.73 (8.83,29.61) | −8.13 | <0.01 |
| PEF | 0.80 (−4.78,8.37) | 10.65 (6.97,15.19) | −7.40 | <0.01 |
| MMEF75/25 | 0.92 (−15.47,18.90) | 27.71 (16.69,41.83) | −7.78 | <0.01 |
| MVV | 6.44 (−3.88,14.28) | 19.80 (11.77,28.17) | −6.71 | <0.01 |
| RV/TLC | 4.65 (−0.92,10.80) | −7.61 (−14.56,−0.21) | −10.89 | <0.01 |
| DLCO | 4.94 (−1.15,11.19) | 13.62 (7.58,21.00) | −6.36 | <0.01 |
| Decline rate (%), median (Q1, Q3) . | Wilcoxon rank-sum test . | |||
|---|---|---|---|---|
| Case group . | Control group . | Z . | P . | |
| n = 96 . | n = 92 . | |||
| FEV1/FVC | 0.85 (−1.90,5.25) | 5.58 (3.96,9.06) | −6.22 | <0.01 |
| FVC | 0.83 (−7.20,6.19) | 11.65 (8.45,24.41) | −9.08 | <0.01 |
| FEV1 | 4.41 (−0.41,8.58) | 14.73 (8.83,29.61) | −8.13 | <0.01 |
| PEF | 0.80 (−4.78,8.37) | 10.65 (6.97,15.19) | −7.40 | <0.01 |
| MMEF75/25 | 0.92 (−15.47,18.90) | 27.71 (16.69,41.83) | −7.78 | <0.01 |
| MVV | 6.44 (−3.88,14.28) | 19.80 (11.77,28.17) | −6.71 | <0.01 |
| RV/TLC | 4.65 (−0.92,10.80) | −7.61 (−14.56,−0.21) | −10.89 | <0.01 |
| DLCO | 4.94 (−1.15,11.19) | 13.62 (7.58,21.00) | −6.36 | <0.01 |
DISCUSSION
Cough improvement effect
The reliability of the LCQ-MC score was assessed based on Cronbach’s α coefficient. We found that the Cronbach’s α coefficient for each group, both before and after the operation, was greater than 0.8. This indicated that the LCQ-MC score used in this study was highly reliable and suitable for evaluating post-lung surgery cough symptoms.
Furthermore, the results demonstrated that the preoperative LCQ-MC scores in both groups were comparable, but postoperative LCQ-MC scores were significantly difference. This suggests that budesonide–formoterol powder inhalation effectively mitigates postoperative cough symptoms.
The difference in postoperative LCQ-MC scores between the two groups was 1.99 (1.83, 2.16), which was greater than the minimum clinically significant difference for chronic cough (1.3) but falling just short of the minimum for acute cough (2.0) [16, 17]. This indicates that budesonide–formoterol powder inhalation not only effectively alleviates postoperative cough but also prevents the development of chronic cough within 1-month post-surgery.
Lung function recovery effect
Significant differences were observed in the decline rates of ventilatory function indexes between the two groups (P < 0.01).
One month after surgery, the case group exhibited decline rates of FEV1/FVC and MVV below 0, indicating improvements in FEV1/FVC and MVV compared to preoperative values. The decline rates of other various ventilatory function indicators were consistently greater than 0, indicating that both groups showed reductions in FVC, FEV1, PEF and MMEF75/25. Despite this, the case group demonstrated a significantly lower decline rate in FEV1/FVC, falling 5.76% below that of the control group. Similarly, the decline rate in other ventilatory function indicators was at least 12.20% lower in the case group compared to the control group. These findings suggest that budesonide–formoterol powder inhalation holds promise for enhancing postoperative pulmonary surgery. The improvement in ventilation function is indeed remarkable. Regarding pulmonary diffusion function, the case group experienced a 1.64% decrease in RV/TLC postoperatively compared to preoperative levels, while the control group showed a 9.81% increase (P < 0.01). Furthermore, the decline rate of DLCO in the case group was significantly lower compared with that of the control group (10.87% vs 16.68%) (P < 0.01).
As in 6 months after surgery, although the patients in the case group having ceased inhalant use for 5 months, the decrease in lung function indicators remained less pronounced compared to the control group.
Gu's team conducted a study comparing lung function in patients who underwent segmentectomy versus lobectomy before and 6 months after surgery. The research differentiated between the two surgical methods based on the number of lung segments removed. The findings indicated that in the control group, FEV1 decreased by 14.73 (8.83, 29.61) % and DLCO decreased by 13.62 (7.58, 21.00) % after 6 months [18]. The study in this article, which included various surgical resection methods, aligns with the findings of Gu et al. It was observed that the rate of decrease in FEV1 in the control group was significantly higher at 1 month post-surgery compared to 6 months post-surgery. This difference could be attributed to factors such as postoperative incision pain restricting chest wall movement and inadequate compensatory expansion of the remaining lung. The decline in DLCO post-surgery may be linked to the mechanical trauma inflicted on the residual lung during the surgical procedure.
These findings suggest that budesonide–formoterol powder inhalation can improve pulmonary diffusing function in postoperative patients.
Application prospect of portable budesonide–formoterol powder inhalation
Persistent cough and impaired lung function have remained common complications following lung resection. In recent years, clinical practice has turned to glucocorticoids combined with bronchodilators and oxygen aerosol inhalation, following the guidelines for perioperative airway management in thoracic surgery [5], in an effort to address these issues. However, as postoperative hospital stays have shortened, the frequency of postoperative nebulization has significantly decreased, leading to a great reduction in alleviating cough symptoms and promoting lung function recovery. Portable inhalers offer a pragmatic approach, eliminating the reliance on sophisticated hospital-based aerosol devices and recurrent hospital visits, thus addressing the constraints of inhaled drug administration following surgery.
This study has demonstrated that patients who continued to use portable budesonide–formoterol powder inhalation experienced significantly improved cough symptoms 1 month after surgery compared to patients who did not continue the treatment. This improvement has the potential to reduce the incidence of postoperative chronic cough. Moreover, patients who continued medication exhibited lower loss of lung function compared to those who did not, aligning with the requirements of the surgical accelerated recovery theory and perioperative airway management guidelines. These results underscore the feasibility of using portable budesonide–formoterol powder inhalation to improve cough symptoms and promote lung function recovery following lung surgery.
Therefore, the results of this study can serve as a foundation for future randomized controlled trial (RCT) studies and further investigation into the potential of portable budesonide–formoterol powder inhalation as a substitute for traditional postoperative nebulization.
Impacts related to COVID-19 infection
Following the outbreak of novel coronavirus pneumonia in 2019, numerous studies have indicated a significantly elevated risk associated with surgery for COVID-19-positive patients. These patients generally experience poorer prognosis, with even worse outcomes in the context of lung surgery. Regarding the timing of surgery following COVID-19 infection, most experts recommend that thoracic surgery should be postponed until the patient has fully recovered [19]. The subjects included in this study underwent surgery between December 2022 and May 2023, a period marked by lifting control measures on COVID-19 infection. Consequently, the study included subjects who had been infected with COVID-19 for less than 2 weeks or during the perioperative period. However, upon analysing the timing of COVID-19 infection in the research subjects, no significant difference was observed between the two groups regarding the duration between COVID-19 diagnosis and the day of surgery (Z=−0.14, P = 0.889) (Figure 1). Therefore, this study does not affect the analysis of the impact of COVID-19 infection.
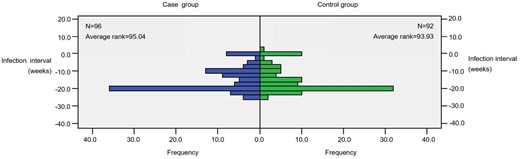
Furthermore, as a result of the infection's impact, individuals have become more cautious about venturing outdoors. Consequently, there has been a significant decrease in the number of people coming for follow-up examinations after undergoing surgery at our hospital. As a result, this study collected a small sample size. This increases the chance of Type II error, i.e., the likelihood of detecting actual differences decreases. RCT studies with larger sample sizes are expected to further explore related issues.
Grouping bias of included patients
The subjects included in this study were patients undergoing thoracoscopic surgery. All patients were prescribed portable budesonide formoterol powder inhalation after surgery. The standardized medication administration times were used to categorize the study participants. The control group comprised patients who discontinued medication for various reasons within 1 week of surgery. This indicates that the control group subjects had relatively low medication adherence. The impact of compliance on this study could not be determined, and more rigorous RCT studies are needed to verify the results of this study.
CONCLUSION
In summary, this study provides preliminary confirmation that portable budesonide–formoterol powder inhalation can effectively ameliorate cough symptoms and promote pulmonary function recovery in patients following thoracoscopic pulmonary resection.
FUNDING
This work was supported by the Science and Technology Research Project of Henan Province (grant number 222102310511) from the Ministry of Science and Technology of Henan Province.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY
Due to the sensitive nature of patient privacy, the data used in this study cannot be publicly shared.
Author contributions
Jingyao Sun: Conceptualization; Data curation; Formal analysis; Writing—original draft. Zhaoyao Hou: Data curation; Formal analysis. Tian Xia: Conceptualization; Methodology; Writing—review & editing. Chang Liu: Visualization. Qiangwen Huang: Conceptualization; Software. Li Wei: Conceptualization; Methodology; Supervision; Validation; Writing—review & editing
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Giuseppe Cardillo and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- DLCO
Diffusing capacity of the lungs for carbon monoxide
- FEV1
Forced expiratory volume in one second
- FEV1/FVC
Forced expiratory volume in one second/Forced vital capacity
- FVC
Forced vital capacity
- LCQ-MC
Mandarin Chinese version of the Leicester Cough Questionnaire
- MMEF75/25
Maximal mid-expiratory flow
- MVV
Maximal voluntary ventilation
- PEF
Peak expiratory flow
- RV/TLC
Residual volume/Total lung capacity





