-
PDF
- Split View
-
Views
-
Cite
Cite
Angelina Olbrich, Arash Motekallemi, Heinz Deschka, Heinrich Rotering, Jürgen Sindermann, Nana-Maria Wagner, Henryk Welp, Angelo M Dell’Aquila, Prospective evaluation of the Moleculight i:X™ in the early detection of driveline infections, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 40, Issue 4, April 2025, ivae215, https://doi.org/10.1093/icvts/ivae215
Close - Share Icon Share
Abstract
Driveline infection (DLI) is a common complication in patients with left ventricular assist devices. This complication can seriously undermine quality of life while on left ventricular assist devices. Current diagnosis of a DLI in the outpatient setting is based on clinical examination and later bacteria isolation. The Moleculight i:XTM is a handheld fluorescence imaging device capable to visualize bacterial colonization in real-time. We here evaluated the performance of the Moleculight i:XTM for diagnosis of DLIs as this device may have the potential advantage to rapidly identify infection and therefore promptly influence therapy.
A total of 107 examinations in patients with suspected DLIs were prospectively included in this study. All examinations took place in the outpatient setting. In addition to the standard treatment, Moleculight fluorescence images were captured and swabs were taken at the area of maximal luminosity. Wounds and pictures were reviewed and classified as positive or negative by a wound specialist and two heart surgeons independently from microbiological results.
The Moleculight i:XTM showed positive results (red fluorescence) in 19 cases (17.76%), whereas microbiological examination was positive for microorganisms in 74 cases (69.16%). The most common bacteria was Staphylococcus aureus. The findings resulted in a sensitivity of 13.51% and a specificity of 72.73%. The positive predictive value was 52.63% and the negative predictive value was 27.27%. Sub-analyses of different wound dressings or previous antibiotic treatment did not show any relevant difference.
The results of the Moleculight i:X show a low sensitivity and specificity when being used to detect DLIs in the outpatient setting. Clinical examination and swabs should remain the gold standard despite the delay for bacteria isolation and consequent antibiotic treatment. Sensitivity and specificity of the Moleculight i:X in open wounds after surgical revision of the driveline remain to be clarified.
INTRODUCTION
Driveline infection (DLI) is a frequent complication undermining quality of life of patients on left ventricular assist devices (LVADs). Latest literature reports an incidence of DLIs from 14% to 59% in the first 2 years after implantation [1–4]. Current classification of VAD specific infections differentiates deep from superficial DLIs according to the depth of infection [5]. While Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) [6] is progressively becoming an additional tool for assessing the extent of infection to the deep layers, the standard diagnosis of superficial infection is based on clinical inspection of the driveline (DL) and late bacteria isolation [5, 7]. In this setting, if there is the suspicion of infection at the piercing site of the DL, swabs are generally taken. In case of bacteria isolation, specific antibiotic treatment can be started [8]. The Moleculight i:XTM (Moleculight Inc, Toronto, Canada) is a fluorescence real-time camera capable of identifying bacterial colonization (Fig. 1). It may provide the potential advantage to rapidly identify infection and therefore promptly influence therapy. The potential use of this tool for the diagnosis of DLI has been suggested in a case report [9] and its usefulness has been postulated in various other medical and surgical fields [10–17]. We here aimed to assess the sensitivity and specificity of Moleculight i:XTM for DLI in our outpatient collective of LVAD patients recruited between March 2020 and May 2022.
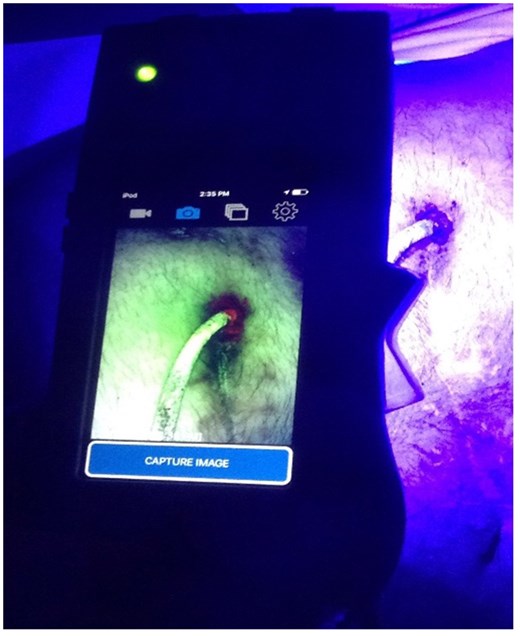
The Moleculight i:X in the outpatient setting. The fluorescence light around the entry side of the driveline is a sign for infection.
DEVICE, PATIENTS AND METHODS
Ethics statement
The study was approved by the ethical committee (Approval Number: 2020–530-f-S), and patients gave their verbal and written consent prior to the examination.
The Moleculight i:XTM
The Moleculight i:XTM uses an iPod Touch® by Apple® (Apple Inc, Cupertino CA, United States) as a camera and for image storage. The iPod is embedded in a casing with a built-in safe ultraviolet light (405 nm) that can be turned on and off by a toggle switch. Two lights indicate whether the distance to take a picture is enough (approximately 10 cm) and whether the room is dark enough to take a fluorescence image [18]. The device is able to detect bacterial colonization in real-time at a minimum bacterial load of 104 CFU/g. The ultraviolet light triggers the emission of red fluorescence from porphyrines as they are generally produced by bacteria (Fig. 1). Tissues such as collagen appear green whereas Pseudomonas species appear in a cyan shade [18, 19]. In vitro, the Moleculight is supposed to be able to detect 28/32 bacteria that are common in wounds, including one of the most common bacteria found in wounds, Staphylococcus aureus, and one of four yeasts [20]. Bacterial microorganisms that could not be identified by the Moleculight in vitro are all non-porphyrin producing and include Enterococcus faecalis, Finegoldia magna, Streptococcus agalactiae and Streptococcus mitis. Species seems to be more essential for the intensity of red fluorescence than the bacterial load. The red fluorescence could not be detected on biofilms when ALA was not used whereas all samples that were supplemented with ALA showed red fluorescence [20].
Study population
This prospective study was conducted in the Department of Heart Surgery of the University Hospital of Muenster (Germany).
The sample size was calculated by the Clinical Institute of Biometrics and Clinical Research of the University of Muenster using the point estimator for sensitivity and specificity. All patients with suspected infections of the DL were included. An infection was suspected when the piercing site was reddened or pus was visible. Exclusion criteria was the presence of psoriasis around the piercing site of the DL as skin conditions can per se alter the colour and luminosity of the pictures.
Standard treatment and study design
Our standard treatment consists of routine visits every 6 weeks for bridge-to-transplantation and up to 3 months for definitive therapy (DT) patients. Wound dressing change is recommended once to thrice a week under sterile conditions [21]. After removing the dressing and inspecting the entry site during outpatient care, swabs are taken if an infection is suspected [7]. The exit site of the DL is then cleaned with hypochlorite rinsing solutions (ActiMaris; AG, Buchs, Switzerland), and if an infection was suspected or proven, oral antibiotics were started [8, 21–23]. In case of a systemic infection, the patient was hospitalized [23–25].
Dressing material includes Metalline® drainage pads or Kerrasol™ Gel along with sterile wound dressing.
Since March 2020, we additionally integrated the Moleculight i:X for the diagnosis of DLI in LVAD outpatient care. In case of a suspected infection [5, 26, 27], images were captured using the Moleculight (Fig. 1) before the wound was cleaned. First, a regular picture of the wound was taken. In addition, the room was darkened and the ultraviolet light of the Moleculight i:X was turned on. As the lamp on the device indicated the room was dark enough, a fluorescence image was captured. Swabs of the wound were taken in the area of maximal luminosity. The pictures were then evaluated by two heart surgeons and a wound specialist unaware of late microbiological results. In a second step, the results of the Moleculight i:X were coupled to the results of the microbiological examinations. Additional sub-analyses of possible influences such as material of the dressing as well as the bacterial species were performed.
Statistical analysis
The caseload planning was done by the Clinical Institute of Biometrics and Clinical Research of the University of Muenster using the point estimator for sensitivity and specificity. Continuous variables are presented as median and interquartile differences. Categorical variables are shown as counts and percentages. Data analysis for the assessment of sensitivity and specificity was performed using SPSS for Windows (Version 23/SPSS Inc., 25 Chicago, IL, USA) and Excel 2010 (Microsoft, Redmond, WA, USA).
RESULTS
A total of 107 examinations in 46 LVAD patients (see Table 1, 40 males and 6 females, mean age 55.87 years, SD 12.09) with suspected DLI were prospectively included. Mean supporting time was 34.82 months (SD 25.06). A total of 27 patients were supported with the Heartmate 3 (Thoratec Corporation, Pleasanton, CA), 2 with a Heartmate II (Thoratec Corporation, Pleasanton, CA) and 17 patients with the HeartWare HVAD (HeartWare International, Framingham, MA). Also, 1 patient was examined 9 times, 3 patients were examined six times, 1 patient was examined five times, 4 patients were examined four times, 5 patients were examined 3 times, 12 patients were examined twice and 20 patients were seen only once. According to the images taken by the Moleculight i:X, 19 examinations (17.76%) were evaluated as positive and 88 cases (82.24%) as negative. Microbiological examinations detected microorganisms in 74 cases (69.16%) including S. aureus in 30 cases; Serratia marcescens in 7 cases; Staphylococcus epidermidis in 6 cases; Corynebacterium amycolatum in 3 cases; Staphylococcus caprae in 3 cases; Staphylococcus lugdunensis in 2 cases; and Corynebacterium striatum, Dermabacter hominis, Escherichia coli, Staphylococcus simulans and S. agalactiae were each found in 1 case. Colonization with more than one microorganism occurred in 18 cases. A total of 23 swabs resulted negative, and 10 swabs showed a physiological flora. Coupling the results of the Moleculight i:X with those of the microbiological cultures, there were 10 true positive and 9 false positive (Fig. 2). Also, 64 false-negative (Fig. 3) and 24 true-negative cases (see Tables 2 and 3). This resulted in a sensitivity of 13.51% and a specificity of 72.73% with a positive predictive value (PPV) and negative predictive value (NPV) of 52.63% and 27.27%, respectively (see Table 4).
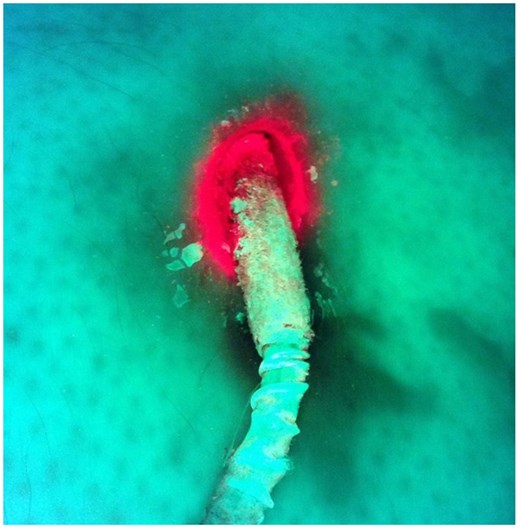
False-positive result. Microbiological examination found bacteria of the physiological flora only.
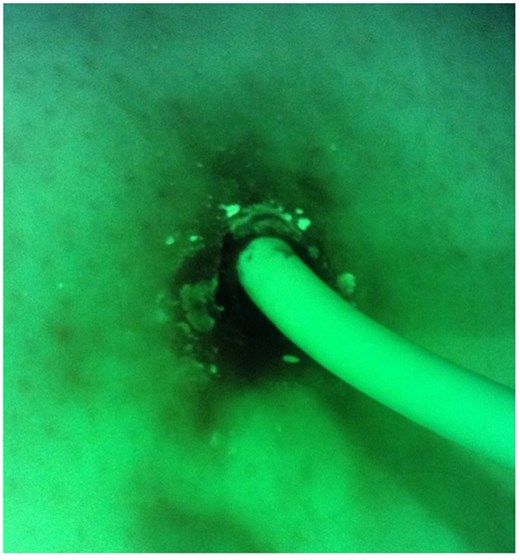
False-negative result. Microbiological examination confirmed colonization with Staphylococcus aureus.
| Patient . | Sex . | Age . | Pump type . | (Months after LVAD implantation) . |
|---|---|---|---|---|
| 1 | M | 49 | HM 3 | 28 |
| 2 | M | 42 | HM 3 | 8 |
| 3 | M | 75 | HM 3 | 10 |
| 4 | M | 69 | HM 2 | 41 |
| 5 | M | 39 | HM 3 | 22 |
| 6 | M | 46 | HM 3 | 20 |
| 7 | F | 67 | HM 3 | 20 |
| 8 | M | 53 | HW | 94 |
| 9 | F | 44 | HM 3 | 11 |
| 10 | M | 64 | HM 3 | 34 |
| 11 | M | 65 | HW | 51 |
| 12 | M | 64 | HM 3 | 25 |
| 13 | M | 64 | HM 3 | 23 |
| 14 | M | 57 | HW | 64 |
| 15 | M | 67 | HW | 96 |
| 16 | M | 60 | HW | 42 |
| 17 | M | 56 | HW | 48 |
| 18 | F | 40 | HW | 51 |
| 19 | M | 49 | HM 3 | 29 |
| 20 | M | 49 | HW | 48 |
| 21 | M | 65 | HM 3 | 29 |
| 22 | M | 54 | HM 3 | 22 |
| 23 | M | 47 | HW | 48 |
| 24 | M | 58 | HM 2 | 15 |
| 25 | M | 47 | HW | 50 |
| 26 | F | 65 | HW | 100 |
| 27 | M | 21 | HW | 23 |
| 28 | M | 69 | HM 3 | 30 |
| 29 | F | 56 | HW | 16 |
| 30 | M | 67 | HM 3 | 20 |
| 31 | M | 49 | HM 3 | 20 |
| 32 | M | 64 | HW | 90 |
| 33 | M | 48 | HW | 21 |
| 34 | M | 58 | HM 3 | 28 |
| 35 | M | 70 | HM 3 | 6 |
| 36 | M | 60 | HM 3 | 8 |
| 37 | M | 59 | HM 3 | 7 |
| 38 | M | 57 | HW | 63 |
| 39 | F | 61 | HM 3 | 19 |
| 40 | M | 25 | HW | 71 |
| 41 | M | 62 | HM 3 | 19 |
| 42 | M | 29 | HM 3 | 7 |
| 43 | M | 73 | HM 3 | 8 |
| 44 | M | 69 | HM 3 | 56 |
| 45 | M | 54 | HM 3 | 13 |
| 46 | M | 64 | HM 3 | 48 |
| Patient . | Sex . | Age . | Pump type . | (Months after LVAD implantation) . |
|---|---|---|---|---|
| 1 | M | 49 | HM 3 | 28 |
| 2 | M | 42 | HM 3 | 8 |
| 3 | M | 75 | HM 3 | 10 |
| 4 | M | 69 | HM 2 | 41 |
| 5 | M | 39 | HM 3 | 22 |
| 6 | M | 46 | HM 3 | 20 |
| 7 | F | 67 | HM 3 | 20 |
| 8 | M | 53 | HW | 94 |
| 9 | F | 44 | HM 3 | 11 |
| 10 | M | 64 | HM 3 | 34 |
| 11 | M | 65 | HW | 51 |
| 12 | M | 64 | HM 3 | 25 |
| 13 | M | 64 | HM 3 | 23 |
| 14 | M | 57 | HW | 64 |
| 15 | M | 67 | HW | 96 |
| 16 | M | 60 | HW | 42 |
| 17 | M | 56 | HW | 48 |
| 18 | F | 40 | HW | 51 |
| 19 | M | 49 | HM 3 | 29 |
| 20 | M | 49 | HW | 48 |
| 21 | M | 65 | HM 3 | 29 |
| 22 | M | 54 | HM 3 | 22 |
| 23 | M | 47 | HW | 48 |
| 24 | M | 58 | HM 2 | 15 |
| 25 | M | 47 | HW | 50 |
| 26 | F | 65 | HW | 100 |
| 27 | M | 21 | HW | 23 |
| 28 | M | 69 | HM 3 | 30 |
| 29 | F | 56 | HW | 16 |
| 30 | M | 67 | HM 3 | 20 |
| 31 | M | 49 | HM 3 | 20 |
| 32 | M | 64 | HW | 90 |
| 33 | M | 48 | HW | 21 |
| 34 | M | 58 | HM 3 | 28 |
| 35 | M | 70 | HM 3 | 6 |
| 36 | M | 60 | HM 3 | 8 |
| 37 | M | 59 | HM 3 | 7 |
| 38 | M | 57 | HW | 63 |
| 39 | F | 61 | HM 3 | 19 |
| 40 | M | 25 | HW | 71 |
| 41 | M | 62 | HM 3 | 19 |
| 42 | M | 29 | HM 3 | 7 |
| 43 | M | 73 | HM 3 | 8 |
| 44 | M | 69 | HM 3 | 56 |
| 45 | M | 54 | HM 3 | 13 |
| 46 | M | 64 | HM 3 | 48 |
| Patient . | Sex . | Age . | Pump type . | (Months after LVAD implantation) . |
|---|---|---|---|---|
| 1 | M | 49 | HM 3 | 28 |
| 2 | M | 42 | HM 3 | 8 |
| 3 | M | 75 | HM 3 | 10 |
| 4 | M | 69 | HM 2 | 41 |
| 5 | M | 39 | HM 3 | 22 |
| 6 | M | 46 | HM 3 | 20 |
| 7 | F | 67 | HM 3 | 20 |
| 8 | M | 53 | HW | 94 |
| 9 | F | 44 | HM 3 | 11 |
| 10 | M | 64 | HM 3 | 34 |
| 11 | M | 65 | HW | 51 |
| 12 | M | 64 | HM 3 | 25 |
| 13 | M | 64 | HM 3 | 23 |
| 14 | M | 57 | HW | 64 |
| 15 | M | 67 | HW | 96 |
| 16 | M | 60 | HW | 42 |
| 17 | M | 56 | HW | 48 |
| 18 | F | 40 | HW | 51 |
| 19 | M | 49 | HM 3 | 29 |
| 20 | M | 49 | HW | 48 |
| 21 | M | 65 | HM 3 | 29 |
| 22 | M | 54 | HM 3 | 22 |
| 23 | M | 47 | HW | 48 |
| 24 | M | 58 | HM 2 | 15 |
| 25 | M | 47 | HW | 50 |
| 26 | F | 65 | HW | 100 |
| 27 | M | 21 | HW | 23 |
| 28 | M | 69 | HM 3 | 30 |
| 29 | F | 56 | HW | 16 |
| 30 | M | 67 | HM 3 | 20 |
| 31 | M | 49 | HM 3 | 20 |
| 32 | M | 64 | HW | 90 |
| 33 | M | 48 | HW | 21 |
| 34 | M | 58 | HM 3 | 28 |
| 35 | M | 70 | HM 3 | 6 |
| 36 | M | 60 | HM 3 | 8 |
| 37 | M | 59 | HM 3 | 7 |
| 38 | M | 57 | HW | 63 |
| 39 | F | 61 | HM 3 | 19 |
| 40 | M | 25 | HW | 71 |
| 41 | M | 62 | HM 3 | 19 |
| 42 | M | 29 | HM 3 | 7 |
| 43 | M | 73 | HM 3 | 8 |
| 44 | M | 69 | HM 3 | 56 |
| 45 | M | 54 | HM 3 | 13 |
| 46 | M | 64 | HM 3 | 48 |
| Patient . | Sex . | Age . | Pump type . | (Months after LVAD implantation) . |
|---|---|---|---|---|
| 1 | M | 49 | HM 3 | 28 |
| 2 | M | 42 | HM 3 | 8 |
| 3 | M | 75 | HM 3 | 10 |
| 4 | M | 69 | HM 2 | 41 |
| 5 | M | 39 | HM 3 | 22 |
| 6 | M | 46 | HM 3 | 20 |
| 7 | F | 67 | HM 3 | 20 |
| 8 | M | 53 | HW | 94 |
| 9 | F | 44 | HM 3 | 11 |
| 10 | M | 64 | HM 3 | 34 |
| 11 | M | 65 | HW | 51 |
| 12 | M | 64 | HM 3 | 25 |
| 13 | M | 64 | HM 3 | 23 |
| 14 | M | 57 | HW | 64 |
| 15 | M | 67 | HW | 96 |
| 16 | M | 60 | HW | 42 |
| 17 | M | 56 | HW | 48 |
| 18 | F | 40 | HW | 51 |
| 19 | M | 49 | HM 3 | 29 |
| 20 | M | 49 | HW | 48 |
| 21 | M | 65 | HM 3 | 29 |
| 22 | M | 54 | HM 3 | 22 |
| 23 | M | 47 | HW | 48 |
| 24 | M | 58 | HM 2 | 15 |
| 25 | M | 47 | HW | 50 |
| 26 | F | 65 | HW | 100 |
| 27 | M | 21 | HW | 23 |
| 28 | M | 69 | HM 3 | 30 |
| 29 | F | 56 | HW | 16 |
| 30 | M | 67 | HM 3 | 20 |
| 31 | M | 49 | HM 3 | 20 |
| 32 | M | 64 | HW | 90 |
| 33 | M | 48 | HW | 21 |
| 34 | M | 58 | HM 3 | 28 |
| 35 | M | 70 | HM 3 | 6 |
| 36 | M | 60 | HM 3 | 8 |
| 37 | M | 59 | HM 3 | 7 |
| 38 | M | 57 | HW | 63 |
| 39 | F | 61 | HM 3 | 19 |
| 40 | M | 25 | HW | 71 |
| 41 | M | 62 | HM 3 | 19 |
| 42 | M | 29 | HM 3 | 7 |
| 43 | M | 73 | HM 3 | 8 |
| 44 | M | 69 | HM 3 | 56 |
| 45 | M | 54 | HM 3 | 13 |
| 46 | M | 64 | HM 3 | 48 |
Comparison of the results of fluorescence imaging and microbiological examination
| Patient . | Moleculight . | Microbiological examination . | Dressing . |
|---|---|---|---|
| 1 | Positive | Corynebacterium amycolatum | Gel |
| 2 | Negative | Physiological flora | Metalline |
| Negative | Staphylococcus simulans | Metalline | |
| 3 | Negative | Staphylococcus caprae | Gel |
| Negative | Physiological flora | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae, Physiological flora | Gel | |
| Negative | Negative | Gel | |
| Negative | Staphylococcus caprae, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus epidermidis | Honey | |
| Negative | Staphylococcus epidermidis | Honey | |
| 4 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Escherichia coli | Gel | |
| Negative | Escherichia coli, Staphylococcus epidermidis | Gel | |
| 5 | Negative | Staphylococcus aureus | Metalline |
| 6 | Positive | Corynebacterium amycolatum | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 7 | Negative | Escherichia coli, Enterococcus faecalis | Gel |
| Negative | Escherichia coli, Klebsiella oxytoca | Metalline | |
| 8 | Positive | Negative | Metalline |
| 9 | Negative | Negative | Gel |
| Negative | Serratia marcescens, Staphylococcus aureus | Gel | |
| Negative | Serratia marcescens | Gel | |
| 10 | Positive | Negative | Metalline |
| Positive | Negative | Metalline | |
| 11 | Positive | Physiological flora | Metalline |
| 12 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| 13 | Negative | Negative | Metalline |
| 14 | Positive | Physiological flora | Gel |
| Positive | Physiological flora | Metalline | |
| 15 | Negative | Negative | Metalline |
| 16 | Negative | Physiological flora | Metalline |
| 17 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 18 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Negative | Metalline | |
| 19 | Negative | Staphylococcus aureus | Gel |
| 20 | Negative | Staphylococcus simulans, Corynebacterium amycolatum | Metalline |
| Positive | Physiological flora | Gel | |
| 21 | Negative | Staphylococcus aureus | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 22 | Positive | Corynebacterium amycolatum, Staphylococcus epidermidis | Metalline |
| Positive | Physiological flora | Metalline | |
| 23 | Positive | Corynebacterium amycolatum | Metalline |
| Negative | Physiological flora | Metalline | |
| 24 | Negative | Staphylococcus capitis, Staphylococcus epidermidis | Metalline |
| 25 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus lugdunensis, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus hominis ssp. hominis | Gel | |
| 26 | Negative | Staphylococcus simulans, Corynebacterium amycolatum, Staphylococcus haemolyticus | Gel |
| 27 | Positive | Corynebacterium amycolatum | Metalline |
| 28 | Positive | Serratia marcescens | Gel |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| 29 | Positive | Staphylococcus aureus | Metalline |
| 30 | Positive | Staphylococcus aureus | Metalline |
| 31 | Negative | Physiological flora | Gel |
| Negative | Streptococcus agalactiae | Gel | |
| Negative | Dermabacter hominis | Gel | |
| Negative | Staphylococcus aureus | Honey | |
| 32 | Negative | Negative | Gel |
| 33 | Negative | Negative | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 34 | Negative | Staphylococcus epidermidis | Metalline |
| 35 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| 36 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Honey | |
| Negative | Streptococcus agalactiae, Staphylococcus aureus | Honey | |
| 37 | Negative | Negative | Gel |
| Positive | Negative | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 38 | Negative | Negative | Gel |
| Negative | Negative | Metalline | |
| Negative | Corynebacterium striatum | Gel | |
| 39 | Negative | Staphylococcus epidermidis, Corynebacterium amycolatum | Gel |
| 40 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus aureus | Honey | |
| 41 | Negative | Staphylococcus epidermidis | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 42 | Negative | Staphylococcus epidermidis | Metalline |
| 43 | Negative | Staphylococcus epidermidis | Metalline |
| Negative | Staphylococcus lugdunensis | Metalline | |
| 44 | Negative | Staphylococcus lugdunensis | Metalline |
| 45 | Negative | Enterococcus cloacae, Klebsiella oxytoca, Staphylococcus lugdunensis | Metalline |
| 46 | Negative | Staphylococcus caprae, Staphylococcus lugdunensis | Metalline |
| Patient . | Moleculight . | Microbiological examination . | Dressing . |
|---|---|---|---|
| 1 | Positive | Corynebacterium amycolatum | Gel |
| 2 | Negative | Physiological flora | Metalline |
| Negative | Staphylococcus simulans | Metalline | |
| 3 | Negative | Staphylococcus caprae | Gel |
| Negative | Physiological flora | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae, Physiological flora | Gel | |
| Negative | Negative | Gel | |
| Negative | Staphylococcus caprae, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus epidermidis | Honey | |
| Negative | Staphylococcus epidermidis | Honey | |
| 4 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Escherichia coli | Gel | |
| Negative | Escherichia coli, Staphylococcus epidermidis | Gel | |
| 5 | Negative | Staphylococcus aureus | Metalline |
| 6 | Positive | Corynebacterium amycolatum | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 7 | Negative | Escherichia coli, Enterococcus faecalis | Gel |
| Negative | Escherichia coli, Klebsiella oxytoca | Metalline | |
| 8 | Positive | Negative | Metalline |
| 9 | Negative | Negative | Gel |
| Negative | Serratia marcescens, Staphylococcus aureus | Gel | |
| Negative | Serratia marcescens | Gel | |
| 10 | Positive | Negative | Metalline |
| Positive | Negative | Metalline | |
| 11 | Positive | Physiological flora | Metalline |
| 12 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| 13 | Negative | Negative | Metalline |
| 14 | Positive | Physiological flora | Gel |
| Positive | Physiological flora | Metalline | |
| 15 | Negative | Negative | Metalline |
| 16 | Negative | Physiological flora | Metalline |
| 17 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 18 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Negative | Metalline | |
| 19 | Negative | Staphylococcus aureus | Gel |
| 20 | Negative | Staphylococcus simulans, Corynebacterium amycolatum | Metalline |
| Positive | Physiological flora | Gel | |
| 21 | Negative | Staphylococcus aureus | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 22 | Positive | Corynebacterium amycolatum, Staphylococcus epidermidis | Metalline |
| Positive | Physiological flora | Metalline | |
| 23 | Positive | Corynebacterium amycolatum | Metalline |
| Negative | Physiological flora | Metalline | |
| 24 | Negative | Staphylococcus capitis, Staphylococcus epidermidis | Metalline |
| 25 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus lugdunensis, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus hominis ssp. hominis | Gel | |
| 26 | Negative | Staphylococcus simulans, Corynebacterium amycolatum, Staphylococcus haemolyticus | Gel |
| 27 | Positive | Corynebacterium amycolatum | Metalline |
| 28 | Positive | Serratia marcescens | Gel |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| 29 | Positive | Staphylococcus aureus | Metalline |
| 30 | Positive | Staphylococcus aureus | Metalline |
| 31 | Negative | Physiological flora | Gel |
| Negative | Streptococcus agalactiae | Gel | |
| Negative | Dermabacter hominis | Gel | |
| Negative | Staphylococcus aureus | Honey | |
| 32 | Negative | Negative | Gel |
| 33 | Negative | Negative | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 34 | Negative | Staphylococcus epidermidis | Metalline |
| 35 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| 36 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Honey | |
| Negative | Streptococcus agalactiae, Staphylococcus aureus | Honey | |
| 37 | Negative | Negative | Gel |
| Positive | Negative | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 38 | Negative | Negative | Gel |
| Negative | Negative | Metalline | |
| Negative | Corynebacterium striatum | Gel | |
| 39 | Negative | Staphylococcus epidermidis, Corynebacterium amycolatum | Gel |
| 40 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus aureus | Honey | |
| 41 | Negative | Staphylococcus epidermidis | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 42 | Negative | Staphylococcus epidermidis | Metalline |
| 43 | Negative | Staphylococcus epidermidis | Metalline |
| Negative | Staphylococcus lugdunensis | Metalline | |
| 44 | Negative | Staphylococcus lugdunensis | Metalline |
| 45 | Negative | Enterococcus cloacae, Klebsiella oxytoca, Staphylococcus lugdunensis | Metalline |
| 46 | Negative | Staphylococcus caprae, Staphylococcus lugdunensis | Metalline |
Comparison of the results of fluorescence imaging and microbiological examination
| Patient . | Moleculight . | Microbiological examination . | Dressing . |
|---|---|---|---|
| 1 | Positive | Corynebacterium amycolatum | Gel |
| 2 | Negative | Physiological flora | Metalline |
| Negative | Staphylococcus simulans | Metalline | |
| 3 | Negative | Staphylococcus caprae | Gel |
| Negative | Physiological flora | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae, Physiological flora | Gel | |
| Negative | Negative | Gel | |
| Negative | Staphylococcus caprae, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus epidermidis | Honey | |
| Negative | Staphylococcus epidermidis | Honey | |
| 4 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Escherichia coli | Gel | |
| Negative | Escherichia coli, Staphylococcus epidermidis | Gel | |
| 5 | Negative | Staphylococcus aureus | Metalline |
| 6 | Positive | Corynebacterium amycolatum | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 7 | Negative | Escherichia coli, Enterococcus faecalis | Gel |
| Negative | Escherichia coli, Klebsiella oxytoca | Metalline | |
| 8 | Positive | Negative | Metalline |
| 9 | Negative | Negative | Gel |
| Negative | Serratia marcescens, Staphylococcus aureus | Gel | |
| Negative | Serratia marcescens | Gel | |
| 10 | Positive | Negative | Metalline |
| Positive | Negative | Metalline | |
| 11 | Positive | Physiological flora | Metalline |
| 12 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| 13 | Negative | Negative | Metalline |
| 14 | Positive | Physiological flora | Gel |
| Positive | Physiological flora | Metalline | |
| 15 | Negative | Negative | Metalline |
| 16 | Negative | Physiological flora | Metalline |
| 17 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 18 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Negative | Metalline | |
| 19 | Negative | Staphylococcus aureus | Gel |
| 20 | Negative | Staphylococcus simulans, Corynebacterium amycolatum | Metalline |
| Positive | Physiological flora | Gel | |
| 21 | Negative | Staphylococcus aureus | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 22 | Positive | Corynebacterium amycolatum, Staphylococcus epidermidis | Metalline |
| Positive | Physiological flora | Metalline | |
| 23 | Positive | Corynebacterium amycolatum | Metalline |
| Negative | Physiological flora | Metalline | |
| 24 | Negative | Staphylococcus capitis, Staphylococcus epidermidis | Metalline |
| 25 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus lugdunensis, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus hominis ssp. hominis | Gel | |
| 26 | Negative | Staphylococcus simulans, Corynebacterium amycolatum, Staphylococcus haemolyticus | Gel |
| 27 | Positive | Corynebacterium amycolatum | Metalline |
| 28 | Positive | Serratia marcescens | Gel |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| 29 | Positive | Staphylococcus aureus | Metalline |
| 30 | Positive | Staphylococcus aureus | Metalline |
| 31 | Negative | Physiological flora | Gel |
| Negative | Streptococcus agalactiae | Gel | |
| Negative | Dermabacter hominis | Gel | |
| Negative | Staphylococcus aureus | Honey | |
| 32 | Negative | Negative | Gel |
| 33 | Negative | Negative | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 34 | Negative | Staphylococcus epidermidis | Metalline |
| 35 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| 36 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Honey | |
| Negative | Streptococcus agalactiae, Staphylococcus aureus | Honey | |
| 37 | Negative | Negative | Gel |
| Positive | Negative | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 38 | Negative | Negative | Gel |
| Negative | Negative | Metalline | |
| Negative | Corynebacterium striatum | Gel | |
| 39 | Negative | Staphylococcus epidermidis, Corynebacterium amycolatum | Gel |
| 40 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus aureus | Honey | |
| 41 | Negative | Staphylococcus epidermidis | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 42 | Negative | Staphylococcus epidermidis | Metalline |
| 43 | Negative | Staphylococcus epidermidis | Metalline |
| Negative | Staphylococcus lugdunensis | Metalline | |
| 44 | Negative | Staphylococcus lugdunensis | Metalline |
| 45 | Negative | Enterococcus cloacae, Klebsiella oxytoca, Staphylococcus lugdunensis | Metalline |
| 46 | Negative | Staphylococcus caprae, Staphylococcus lugdunensis | Metalline |
| Patient . | Moleculight . | Microbiological examination . | Dressing . |
|---|---|---|---|
| 1 | Positive | Corynebacterium amycolatum | Gel |
| 2 | Negative | Physiological flora | Metalline |
| Negative | Staphylococcus simulans | Metalline | |
| 3 | Negative | Staphylococcus caprae | Gel |
| Negative | Physiological flora | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae | Gel | |
| Negative | Staphylococcus caprae, Physiological flora | Gel | |
| Negative | Negative | Gel | |
| Negative | Staphylococcus caprae, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus epidermidis | Honey | |
| Negative | Staphylococcus epidermidis | Honey | |
| 4 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Escherichia coli | Gel | |
| Negative | Escherichia coli, Staphylococcus epidermidis | Gel | |
| 5 | Negative | Staphylococcus aureus | Metalline |
| 6 | Positive | Corynebacterium amycolatum | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 7 | Negative | Escherichia coli, Enterococcus faecalis | Gel |
| Negative | Escherichia coli, Klebsiella oxytoca | Metalline | |
| 8 | Positive | Negative | Metalline |
| 9 | Negative | Negative | Gel |
| Negative | Serratia marcescens, Staphylococcus aureus | Gel | |
| Negative | Serratia marcescens | Gel | |
| 10 | Positive | Negative | Metalline |
| Positive | Negative | Metalline | |
| 11 | Positive | Physiological flora | Metalline |
| 12 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Metalline | |
| Negative | Staphylococcus aureus | Gel | |
| 13 | Negative | Negative | Metalline |
| 14 | Positive | Physiological flora | Gel |
| Positive | Physiological flora | Metalline | |
| 15 | Negative | Negative | Metalline |
| 16 | Negative | Physiological flora | Metalline |
| 17 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 18 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Negative | Metalline | |
| 19 | Negative | Staphylococcus aureus | Gel |
| 20 | Negative | Staphylococcus simulans, Corynebacterium amycolatum | Metalline |
| Positive | Physiological flora | Gel | |
| 21 | Negative | Staphylococcus aureus | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 22 | Positive | Corynebacterium amycolatum, Staphylococcus epidermidis | Metalline |
| Positive | Physiological flora | Metalline | |
| 23 | Positive | Corynebacterium amycolatum | Metalline |
| Negative | Physiological flora | Metalline | |
| 24 | Negative | Staphylococcus capitis, Staphylococcus epidermidis | Metalline |
| 25 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus lugdunensis, Staphylococcus aureus | Gel | |
| Negative | Staphylococcus hominis ssp. hominis | Gel | |
| 26 | Negative | Staphylococcus simulans, Corynebacterium amycolatum, Staphylococcus haemolyticus | Gel |
| 27 | Positive | Corynebacterium amycolatum | Metalline |
| 28 | Positive | Serratia marcescens | Gel |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| Negative | Serratia marcescens | Gel | |
| 29 | Positive | Staphylococcus aureus | Metalline |
| 30 | Positive | Staphylococcus aureus | Metalline |
| 31 | Negative | Physiological flora | Gel |
| Negative | Streptococcus agalactiae | Gel | |
| Negative | Dermabacter hominis | Gel | |
| Negative | Staphylococcus aureus | Honey | |
| 32 | Negative | Negative | Gel |
| 33 | Negative | Negative | Metalline |
| Negative | Staphylococcus aureus | Gel | |
| 34 | Negative | Staphylococcus epidermidis | Metalline |
| 35 | Negative | Negative | Gel |
| Negative | Negative | Gel | |
| Negative | Staphylococcus aureus | Gel | |
| 36 | Negative | Staphylococcus aureus | Gel |
| Negative | Staphylococcus aureus | Gel | |
| Negative | Negative | Honey | |
| Negative | Streptococcus agalactiae, Staphylococcus aureus | Honey | |
| 37 | Negative | Negative | Gel |
| Positive | Negative | Gel | |
| Negative | Negative | Gel | |
| Positive | Staphylococcus aureus | Gel | |
| 38 | Negative | Negative | Gel |
| Negative | Negative | Metalline | |
| Negative | Corynebacterium striatum | Gel | |
| 39 | Negative | Staphylococcus epidermidis, Corynebacterium amycolatum | Gel |
| 40 | Negative | Staphylococcus aureus | Metalline |
| Negative | Negative | Metalline | |
| Negative | Staphylococcus aureus | Honey | |
| 41 | Negative | Staphylococcus epidermidis | Gel |
| Negative | Staphylococcus aureus | Gel | |
| 42 | Negative | Staphylococcus epidermidis | Metalline |
| 43 | Negative | Staphylococcus epidermidis | Metalline |
| Negative | Staphylococcus lugdunensis | Metalline | |
| 44 | Negative | Staphylococcus lugdunensis | Metalline |
| 45 | Negative | Enterococcus cloacae, Klebsiella oxytoca, Staphylococcus lugdunensis | Metalline |
| 46 | Negative | Staphylococcus caprae, Staphylococcus lugdunensis | Metalline |
Overview of the positive and negative results using the Moleculight and microbiological swabs
| Moleculight . | Microbiology . | |
|---|---|---|
| Positive | 19 (17.76%) | 74 (69.16%) |
| 10 (Physiological flora) | ||
| Negative | 88 (82.24%) | 23 |
| Moleculight . | Microbiology . | |
|---|---|---|
| Positive | 19 (17.76%) | 74 (69.16%) |
| 10 (Physiological flora) | ||
| Negative | 88 (82.24%) | 23 |
Bold values indicate microbiological and bedside results in cases with a suspected driveline infection.
Overview of the positive and negative results using the Moleculight and microbiological swabs
| Moleculight . | Microbiology . | |
|---|---|---|
| Positive | 19 (17.76%) | 74 (69.16%) |
| 10 (Physiological flora) | ||
| Negative | 88 (82.24%) | 23 |
| Moleculight . | Microbiology . | |
|---|---|---|
| Positive | 19 (17.76%) | 74 (69.16%) |
| 10 (Physiological flora) | ||
| Negative | 88 (82.24%) | 23 |
Bold values indicate microbiological and bedside results in cases with a suspected driveline infection.
| Positive microbiological swap . | Negative microbiological swap/Physiological flora . | ||
|---|---|---|---|
| Positive result of the Moleculight | 10 (True positive) | 9 (False positive) | PPV 52.63% |
| Negative result of the Moleculight | 64 (False negative) | 24 (True negative) | NPV 27.27% |
| Sensitivity 13.51% | Specificity 72.73% |
| Positive microbiological swap . | Negative microbiological swap/Physiological flora . | ||
|---|---|---|---|
| Positive result of the Moleculight | 10 (True positive) | 9 (False positive) | PPV 52.63% |
| Negative result of the Moleculight | 64 (False negative) | 24 (True negative) | NPV 27.27% |
| Sensitivity 13.51% | Specificity 72.73% |
Bold values indicate sensitivity and specificity of the moleculight i:X.
| Positive microbiological swap . | Negative microbiological swap/Physiological flora . | ||
|---|---|---|---|
| Positive result of the Moleculight | 10 (True positive) | 9 (False positive) | PPV 52.63% |
| Negative result of the Moleculight | 64 (False negative) | 24 (True negative) | NPV 27.27% |
| Sensitivity 13.51% | Specificity 72.73% |
| Positive microbiological swap . | Negative microbiological swap/Physiological flora . | ||
|---|---|---|---|
| Positive result of the Moleculight | 10 (True positive) | 9 (False positive) | PPV 52.63% |
| Negative result of the Moleculight | 64 (False negative) | 24 (True negative) | NPV 27.27% |
| Sensitivity 13.51% | Specificity 72.73% |
Bold values indicate sensitivity and specificity of the moleculight i:X.
In 10 cases, microbiological examination resulted in microorganisms of the physiological flora that could be found in the area of the entry site of the DL. In five cases (50%), the Moleculight has shown a positive result (Fig. 2).
Sub-analyses of different wound dressings yielded the following results. Of the 38 DLs covered with Metalline drainage pads, the Moleculight detected 11 wounds as positive, of which 5 cases were true positive and 10 cases were true negative. There were 6 false-positive and 17 false-negative cases resulting in a sensitivity and specificity of 22.73% and 62.5% with PPV of 45.45% and NPV of 37.04%, respectively. Of the 62 cases in which the DL was covered with Kerrasol Gel and sterile wound dressing, the Moleculight detected eight wounds as positive while the microbiological examination was positive in 46 cases. There were 5 true-positive and 13 true-negative cases, 3 false-positive and 41 false-negative cases. The sensitivity and specificity of DLIs treated with Kerrasol gel were 10.87% and 81.25% with a PPV and NPV of 62.5% and 24.07%, respectively. In patients who were treated with medical honey, we could see that the Moleculight i:X was not able to create a proper fluorescence image in cases where a thick layer of honey was surrounding the entry site of the DL. In seven patients, the layer of honey was thin or absorbed already, and therefore, the images could be used for our study. Yet, in all seven cases, the Moleculight has shown no positive signal, whereas swab results showed that microorganisms could be found in six cases (see Fig. 2). Using the Moleculight, it was not possible to differentiate between colonization and infection, whereas microbiological results showed the difference between these two.
DISCUSSION
The present study showed that the Moleculight is not suitable for the detection of DLIs as the sensitivity and specificity were 13.51% and 72.73% with PPV and NPVs of 52.63% and 27.27%, respectively.
In contrast with the aforementioned results, recent literature has shown a PPV of up to 100% for wound infections in vivo [10]. Blumenthal et al. reported promising results in the diagnosis of infection for burn [15] and military trauma wounds [14]. Burn wounds were also examined by Farhan et al. who found the use of fluorescence imaging in paediatric burn wounds promising [16] despite its limitations such as the need of complete darkness and difficulties while evaluating fluorescence images [17]. A larger study on diabetic foot ulcers shows a sensitivity and specificity of 78% but a lower PPV of 64% [28]. Another study including wounds with moderate to high bacterial load only (106–1010 CFU/g) reported low sensitivity (55%) on high colonization but a high PPV (up to 100%) [11]. In the outpatient setting of patients who underwent plastic surgeries, the device performed with a sensitivity of 100% and a specificity of 78% at a PPV of 95.4% [12].
For the VAD-specific infections, Keenan et al. presented one case of DLI diagnosed through the Moleculight. Of note, using the Moleculight, they found a margin of red fluorescence around the DL entry side and through this guidance they could extend the area of debridement [9]. Similarly, Moelleken et al. examined chronic leg ulcers and demonstrated that the Moleculight was particularly useful in guiding the extend of surgical debridement [13]. Those findings were supported by Raizman et al. who performed wound debridement and afterwards acquired fluorescence images of the wounds. In cases of remaining red fluorescence signals, the Moleculight was used to target the areas of bacterial burden and enlarge the debridement area [29]. Despite the encouraging results in literature and the promising case study of Keenan et al., we were not able to confirm those results for the diagnosis of DLI.
Possible explanations for this performance failure can reside in many reasons. First, our series deals with suspicion of DLIs and no wound underwent surgical debridement. Thus, lower bacterial concentration could have played an important role. Second, interactions with dressing material could have reduced the fluorescence signal. In fact, silver, which is a component of Metalline drainage pads, makes the signal darker. The element absorbs or completely blocks the fluorescence light [18]. The pads were removed before taking the images in our study but as we did not clean the wounds before acquiring an image, it cannot be ruled out that parts of the silver were still on the entry site. Yet, the difference between the different types of dressings was not relevant.
Finally, the physiological flora could have altered the interpretation of the results as well, as those bacteria also produce porphyrins and may result in positive results using the Moleculight (Fig. 2). This phenomenon can explain the fact that the Moleculight has shown a positive signal in 50% of those cases. In this regard, most DLIs in our study resulted to be colonized by Staphylococcal species, which represents a common bacteria of microflora of the skin [30].
In summary, the Moleculight shows a low sensitivity and specificity when being used to detect DLIs in the outpatient setting. Therefore, according to our study, the Moleculight i:X cannot help physicians decide whether to start a broad antibiotical treatment before swab results. Given the low prediction performance the tool is not even recommended for a first screening in an outpatient setting due to very high proportion of false negatives. Clinical examination and swabs should remain the gold standard despite delay of microbiological workup. Sensitivity and specificity of the Moleculight in open wounds after surgical revision of DLs remains to be clarified.
FUNDING
No funding was required.
Conflict of interest: None declared.
DATA AVAILABILITY
Raw data were generated at the Department of Cardiac- and Thoracic Surgery at the University Hospital Münster, Germany. Derived data supporting the findings of this study are available from the corresponding author on request.
Author contributions
Angelina Olbrich: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing. Arash Motekallemi: Conceptualization; Writing—review & editing. Heinz Deschka: Conceptualization; Writing—review & editing. Heinrich Rotering: Conceptualization; Investigation; Methodology; Resources; Writing—review & editing. Jürgen Sindermann: Conceptualization; Investigation; Writing—review & editing. Nana-Maria Wagner: Visualization; Writing—review & editing. Henryk Welp: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing—review & editing. Angelo Maria Dell’Aquila: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Giovanni Alfonso Chiariello, Suvitesh Luthra and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- DL
Driveline
- DLI
Driveline infection
- DT
Definitive therapy
- HM 2
Heart Mate 2
- HM 3
Heart Mate 3
- HW
Heartware
- ISHLT
International Society for Heart and Lung Transplantation
- LVAD
Left ventricular assist device
- VAC
Vacuum-assisted closure
Author notes
Henryk Welp and Angelo M. Dell’Aquila contributed equally to this work.





