-
PDF
- Split View
-
Views
-
Cite
Cite
Luca Aerts, Dennis W den Uijl, Justin G L Luermans, Bart Maesen, Temporary sinus node dysfunction following ablation of atrial fibrillation in patients with aberrancy of the sinus node artery: a case series, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 39, Issue 1, July 2024, ivae135, https://doi.org/10.1093/icvts/ivae135
Close - Share Icon Share
Abstract
Our goal was to investigate sinus node dysfunction (SND) as a rare complication following surgical and catheter atrial fibrillation (AF) ablation in patients with an aberrant sinus node artery (SNA).
We used a retrospective analysis of 3 patients with an aberrant SNA who underwent different AF ablation procedures: 1 concomitant to aortic valve replacement, 1 thoracoscopic hybrid AF ablation and 1 catheter AF ablation.
All patients experienced temporary SND perioperatively. In the first patient, sinus rhythm (SR) recovered by postoperative day 6. In the second patient, SR returned by postoperative day 14. The third patient had a sinus arrest during ablation that restored to SR immediately post-procedure. All patients had normal SR at the 3-month follow-up.
Awareness of SNA anatomy can help to prevent iatrogenic SND during AF ablation. If SND occurs, a wait-and-see approach is recommended, given that sinus node function seems to recover. Because SND recovers, the benefits of posterior wall isolation could outweigh the disadvantages of temporary SND.
INTRODUCTION
Sinus node dysfunction (SND) is a rare complication of atrial fibrillation (AF) ablation and might be attributed to thermal injury of the sinus node artery (SNA) [1, 2]. If the SNA originates from the left coronary artery, its aberrant trajectory runs along the base of the left atrial appendage (LAA), close to the ridge, continuing over the left atrial roof and is therefore more prone to thermal injury during ablation procedures [3].
CASE SERIES
The first patient was a 72-year-old man diagnosed with severe aortic valve stenosis and paroxysmal AF, without any preoperative medication. Due to severe atrial dilatation (left atrial volume index 78 ml/m2), it was decided to perform posterior wall and superior caval vein isolation in addition to pulmonary vein isolation (PVI), concomitant with aortic valve replacement. All ablation lesions were conducted on-pump with beating heart using a bipolar radiofrequency (RF) clamp (EMT1, AtriCure, Mason, OH, USA), and the LAA was excluded (AtriClip ACH 35 mm). During weaning, absent atrial activity necessitated placement of temporary atrial pacemaker wires. The first postoperative electrocardiogram (ECG) showed atrial-paced rhythm at 80 beats per minute (bpm). On postoperative day (POD) 4, sinus rhythm (SR) recurred, atrial pacing was stopped and sotalol 40 mg b.i.d. was initiated. On POD 5, AF recurred at 130 bpm, and sotalol was increased to 80 mg b.i.d. On POD 6, sotalol was discontinued because the patient converted to sinus bradycardia with chronotropic incompetence. Retrospective analysis revealed an aberrant SNA on the coronary angiogram (Fig. 1A). Despite chronotropic incompetent sinus bradycardia, there were no symptoms or haemodynamic instability related to SND. Thus, the patient was safely discharged on POD 10 with 40 mg of sotalol daily. Holter monitoring at 3 months and ECG at 6 months showed SR (87 bpm) without sinus arrest/pauses. There were no symptoms of SND.
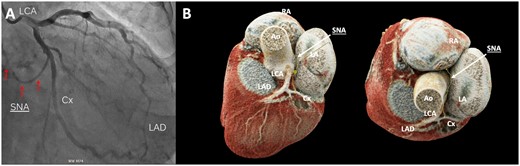
(A) Left coronary angiography: the sinus node artery (arrows) originates from the circumflex artery. (B) Coronary computed tomography angiography of the sinus node artery (yellow line and white arrows) originating from the proximal part of the circumflex artery and running over the left atrial roof towards the sinus node. Ao: aorta; Cx: circumflex artery; LA: left atrium; LAD: left anterior descending artery; LCA: left coronary artery; RA: right atrium; SNA: sinus node artery.
The second patient was a 59-year-old man with symptomatic paroxysmal AF who previously underwent cryo-PVI and redo RF-PVI. Preoperatively, the patient used sotalol 40 mg daily and no anticoagulants. He underwent a one-stage hybrid thoracoscopic AF ablation. The procedure consisted of epicardial PVI and a box lesion in 1 continuous lesion in combination with epicardial superior caval vein isolation using the Gemini-S Clamp (Medtronic, Minneapolis, MN, USA) and epicardial LAA exclusion (Atriclip Pro 2 35 mm, Atricure), followed by transcatheter endocardial validation. During positioning of the coronary sinus catheter, the patient spontaneously converted from AF to atrioventricular (AV) nodal escape rhythm, necessitating temporary atrial pacing. Preoperative coronary computed tomography angiography (CCTA) revealed an aberrant SNA, branching from the proximal region of the left circumflex artery (Cx) (Fig. 1B). Junctional rhythm persisted until POD 3. Because the junctional rhythm was adequate, atrial pacing was stopped. During the postoperative period, atrial flutter and continuous AF occurred, and electrical cardioversion was performed with conversion to junctional rhythm. Until discharge from the hospital on POD 14, the patient showed SR and junctional rhythm in competition. At the 3-month follow-up, 48-hour Holter monitoring showed chronotropic competent SR with a mean frequency of 70 bpm, ranging from 56 to 142 bpm, with no symptoms of bradycardia and without the use of antiarrhythmic drugs.
The third patient was a 57-year-old man who underwent RF-PVI catheter ablation for paroxysmal AF. Preoperative CCTA revealed an uncommon trajectory of the SNA between the LAA and the left superior pulmonary vein (LSPV) (Fig. 2). During ablation at this region (3.5 mm irrigated tip, 30 W, 43 gr. C), sinus arrest occurred, necessitating atrial pacing. After termination of thermal application, SR recovered instantly. Additional applications in this region consistently resulted in a similar response, which was assumed to be a sign of thermal injury to the SNA. To avoid permanent damage, only short applications were delivered until bidirectional isolation of LSPVs was achieved. After ablation, and during follow-up at 3 and 6 months, there were no signs or symptoms of SND. At the latest follow-up visit, no bradycardia-related symptoms or sinus pauses were observed on ECGs. No antiarrhythmic drugs were used either pre- or postoperatively. None of the patients had a history of SND preoperatively.
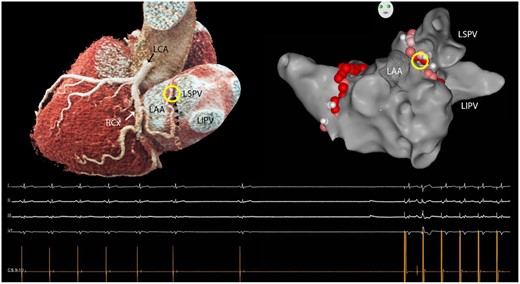
Three-dimensional volume rendered reconstruction of the coronary anatomy on cardiac computed tomography (left). The sinus node artery (small arrows) originates from the circumflex artery and courses underneath the left atrial appendage before ascending between the left atrial appendage and the left superior pulmonary vein towards the left atrial roof and cavo-atrial junction. Ablation in the superior part of this region (circle) repeatedly resulted in sinus arrest necessitating atrial pacing as shown on the electrocardiogram underneath the 3-dimensional images. LAA: left atrial appendage; LCA: left coronary artery; LSPV/LIPV: left superior/inferior pulmonary vein; RCx: circumflex artery.
Written informed consent was obtained from all patients.
DISCUSSION
This case series supports the hypothesis that an aberrant trajectory of the SNA is a potential risk for developing SND due to thermal SNA injury during ablation. However, in all patients, sinus node function fully recovered. These findings suggest that SND after ablation is temporary and that a wait-and-see period of approximately 14 days allowing for sinus node recovery may be considered before implanting a pacemaker.
As part of an ablation procedure workup, CCTA is commonly performed to evaluate PV anatomy, concomitant coronary artery disease and other anomalies. However, the course of the SNA is rarely taken into consideration when planning the procedure. This artery, which supplies the sinoatrial node area, is one of the largest and most commonly found atrial arteries [4]. In case of an aberrant course of the SNA, patients undergoing ablation are more prone to thermal injury of the sinus node complex by interruption of its blood supply along its course. The case report of Kitamura et al. supports the theory that SND is caused by thermal injury of the SNA with angiographic evidence. Radiofrequency catheter ablation was performed, and temporary sinus arrest and junctional rhythm occurred; direct perioperative coronary angiography showed interrupted blood flow in the SNA branching off the proximal part of the Cx [1].
Barra et al. reported 6 patients with SND after AF ablation, hypothesizing that thermal energy plays an important role in causing injury to the SNA during catheter ablation. They described an SNA originating from the Cx as a potential risk factor for SND after ablation [2].
Still, the underlying mechanisms of SND after AF ablation are a matter of debate. First, SND can be the consequence of thermal injury to the SNA. Second, SND can occur by injury to the autonomic ganglia (near the sinus node) following ablation or atriotomy. The atriotomy triggers local inflammation and oedema around the sinus node or its blood supply, leading to slower conduction and fibrosis with scar formation [5].
Third, in AF ablation, sick sinus syndrome can present as SND. Left atrial lesions can temporarily suppress the vagal innervation of the AV node, causing faster spontaneous AV node rates than the SA node rate. This situation results in junctional rhythm pre- or preoperative, and can be misinterpreted as SND.
Given that an aberrant SNA is potentially at risk for SND, the question arises concerning whether one should perform an additional roof line to complete the box lesion in patients with AF with preoperatively diagnosed aberrant SNA. The posterior wall of the left atrium plays an important role in the perpetuation of AF to maintain SR [6]. Although patients with an aberrant SNA suffered pre- and postoperative temporary SND in our series, they all had full recovery of sinus node function after 3 months of follow-up. Therefore, the benefits of posterior wall isolation could outweigh the disadvantages of temporary SND.
FUNDING
None declared.
Conflict of interest: Bart Maesen is a consultant for Atricure and Medtronic.
DATA AVAILABILITY
All relevant data are within the manuscript and its Supporting Information files.
Author contributions
All authors contributed following the criteria recommended by the International Committee of Medical Journal Editors (ICMJE).
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Marco Gemelli and Tomislav Kopjar for their contributions to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- AF
Atrial fibrillation
- AV
Atrioventricular
- bpm
Beats per minute
- CCTA
Coronary computed tomography angiography
- Cx
Circumflex artery
- ECG
Electrocardiogram
- LAA
Left atrial appendage
- POD
Postoperative day
- PVI
Pulmonary vein isolation
- RF
Radiofrequency
- SNA
Sinus node artery
- SND
Sinus node dysfunction
- SR
Sinus rhythm




