-
PDF
- Split View
-
Views
-
Cite
Cite
Thibault Schaeffer, Paul Philipp Heinisch, Helena Staehler, Stanimir Georgiev, Christoph Röhlig, Alfred Hager, Peter Ewert, Jürgen Hörer, Masamichi Ono, Morphology of the native ascending aorta after the Norwood procedure for aortic atresia: impact on survival and right ventricular dysfunction, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 39, Issue 1, July 2024, ivae101, https://doi.org/10.1093/icvts/ivae101
Close - Share Icon Share
Abstract
Our goal was to evaluate the impact of variable morphology of the native ascending aorta after the Norwood I procedure in patients with hypoplastic left heart syndrome/aortic atresia on long-term survival and systemic right ventricular dysfunction.
Of 151 survivors of the Norwood procedure for hypoplastic left heart syndrome/aortic atresia at our institution between January 2001 and December 2020, we included patients with available and measurable aortograms prior to stage II palliation. The diameter of the native ascending aorta, the length of the native ascending aorta and the angle between the native ascending aorta and the proximal pulmonary artery were measured. We investigated the impact of these morphologic parameters on mortality and on right ventricular dysfunction (defined as at least moderate).
Angiograms were available for 78 patients. The median diameter of the native ascending aorta was 3.2 mm (2.6–3.7), the median length of the native ascending aorta was 15.4 mm (13.3–17.9) and the median angle between the native ascending aorta and the proximal pulmonary artery was 44° (35°–51°). During the median follow-up of 6.5 years, 8 (10%) patients died and systemic right ventricular dysfunction occurred in 19 patients (24%). No significant association between aortic morphology and mortality could be detected. Right ventricular function was negatively affected by a larger angle between the native ascending aorta and the proximal pulmonary artery [odds ratio 1.07 (1.01–1.14), P = 0.02].
In survivors of the Norwood procedure for hypoplastic left heart syndrome/aortic atresia with available angiograms, no significant association between native aortic morphology and mortality could be demonstrated after stage II palliation, within the scope of this limited study. A larger anastomosis angle between the native ascending aorta and the proximal pulmonary artery emerged as a risk factor for right ventricular dysfunction.
INTRODUCTION
Staged palliation for hypoplastic left heart syndrome (HLHS) is associated with substantial mortality [1]. A major predictor of mortality in these patients is the deterioration of the systemic right ventricle (RV) function, the mechanisms of which are not completely understood [2, 3]. Histologic analysis of the failing RV in hypoplastic left hearts reveals chronic ischaemia, suggesting that impaired coronary perfusion may be involved [4]. Impaired coronary perfusion also directly affects survival and was proven to be the main cause of early death after the Norwood procedure [5]. Due to an exclusively retrograde flow into the aortic root, coronary perfusion in patients with HLHS and aortic atresia (HLHS/AA) is particularly vulnerable. Thus, minor morphologic changes of the proximal aorta, before and after the Norwood procedure/stage I palliation, may impair coronary perfusion with obvious prognostic implications in these patients. The diameter of the native ascending aorta (nAAo) has been reported as a risk factor for death after stage I palliation for patients with HLHS by some authors but not by others [6–9]. The literature on the long-term impact of aortic morphology after the first stage of palliation is scarce. Increased stiffness and size discrepancy of the neo-aorta have been associated with reduced coronary flow and adverse clinical events in patients with HLHS after having the Norwood procedure [10]. To our knowledge, the morphologic parameters of the nAAo, such as its length and its angle at the level of the amalgamation with the proximal pulmonary artery (PPA) (i.e. nAAo–PPA anastomosis) have not been studied as prognostic factors in this population.
With this study, we sought to investigate the impact of the comprehensive morphology of the nAAo after the Norwood I procedure on mortality and RV dysfunction in patients undergoing staged palliation for HLHS/AA.
MATERIALS AND METHODS
Ethical statement
This retrospective study was approved by the institutional review board of the Technical University of Munich (approval number: 305/20 S-KH on 2 June 2020). Due to the retrospective nature of the study, the need for individual patient consent was waived.
Patients and data collection
We selected patients from the medical records after having the Norwood for HLHS/AA at our institution between January 2001 and December 2020. A total of 151 patients were identified from the institution’s echocardiography and cardiovascular databases. Retrospective chart review was performed, with data collected including demographic, preoperative, operative, postoperative and follow-up data. The most current vital status and follow-up data were obtained from our institutional single-ventricle database, which is regularly tracked. Because only selected patients underwent cardiac catheterization in the interstage period, we chose to exclude patients who died before stage II to avoid a selection bias. Re-coarctation was reported for patients who had required either surgical or percutaneous intervention on the isthmus during the interstage.
Surgical procedures
The operative techniques of stage I (Norwood procedure), stage II (bidirectional cavopulmonary shunt) and stage III palliation (total cavopulmonary connection) as performed in our centre have been described in previous reports [11–13]. The nAAo–PPA anastomosis in the Norwood procedure was performed by all our surgeons in a standard fashion with the double-barrel technique (side-to-side), without a V-shaped incision in the proximal PA. A polydioxanone running suture was used for the anastomosis between the great vessels, and a polypropylene running suture was used for the aortic arch augmentation and the anastomosis of the great vessels with the patch. The pulmonary circulation was provided by implanting either a right ventricle-to-pulmonary artery shunt, a central aorto-pulmonary shunt or a modified Blalock-Taussig shunt.
Angiographic measurement
Aortic morphology was assessed on angiograms from the cardiac catheterization routinely performed prior to stage II palliation. The choice of whether or not to inject the proximal ascending aorta was left to the discretion of the cardiologist in charge and was not related to the patient's haemodynamic status. The diameter of the nAAo, the length of the nAAo, defined as the distance between the aortic root and the nAAo–PPA anastomosis and the angle of the native great vessels at the level of the nAAo–PPA anastomosis were measured on the ascending aortogram in the left lateral view. For measurement of the nAAo–PPA anastomosis angle, a reference line was drawn from the centre of the neo-aortic valve to the centre of the anastomosis segment between the pulmonary artery and the patch. The angle of the nAAo–PPA anastomosis was measured at the intersection of this line with another line running from the centre of the native aortic root along the nAAo (Fig. 1).
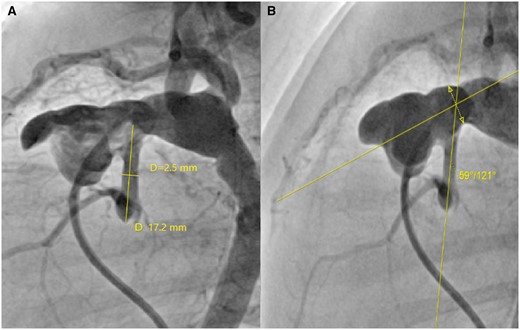
Measurement of aortic morphology in a preoperative angiogram, left lateral view, before stage II palliation (bidirectional cavopulmonary shunt): (A) Diameter and length of the native ascending aorta; (B) angle of the native ascending aorta–proximal pulmonary artery anastomosis. The interrupted line with the double arrow in the panel (B) represents the segment of the anastomosis between the pulmonary artery and the patch, through the centre of which the angle with the native ascending aorta is measured.
Estimation of right ventricular function
An experienced cardiologist reviewed echocardiograms from pre-, post-stage II palliation and post-stage III palliation. The RV function was assessed by an eyeball estimation of the ejection fraction (EF) as well as with fractional area change. The RV function was then classified semi-quantitatively according to the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging: normal (EF ≥ 52%), mildly abnormal (EF 41–51%), moderately abnormal (30–40%) and severely abnormal (EF < 30%) [14].
Statistical analysis
Categorical variables were expressed as absolute number (percentages), and continuous variables were expressed as median (interquartile range). For practical reasons, due to the substantial heterogeneity in patient follow-up and the disparity in RV function assessment over time, we selected the minimal estimation of RV function reported for each patient after stage II palliation. The RV dysfunction was defined as an at least moderately abnormal function (estimated EF ≤ 40%). Preoperative characteristics, angiographic findings and postoperative outcomes were compared between patients with and without RV dysfunction. The Shapiro-Wilk test was used to assess the normal distribution of continuous variables. Normally distributed variables were compared with a sample t-test, and non-normal distributed variables were compared with a Wilcoxon rank sum test. A χ2 test was used to compare categorical variables. The impact of selected parameters on mortality was analysed using multivariable Cox regression, the proportional hazard assumption being tested using Schoenfeld residuals. The impact of selected parameters on the occurrence of RV dysfunction was analysed using multivariable logistic regression. Results were expressed with either an odds ratio or a hazard ratio with the 95% confidence interval, accordingly. A P-value of <0.05 was considered significant. A modified Blalock-Taussig shunt and a central aorto-pulmonary shunt were reported as a single entity, i.e. a systemic-to-pulmonary shunt, as opposed to a right ventricle–pulmonary artery shunt (RV-PA shunt). Atrioventricular (AV) valve regurgitation was considered clinically significant when it was at least moderate and was included as such in the comparative and regression models. The median follow-up duration was estimated with the so-called ‘reverse Kaplan–Meier method’, whereby patients were censored for death and end of follow-up counted as an event. Data analysis and graphs were performed with R version 4.0.4 for Unix (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
Due to the lack of aortography in the angiograms of 73 patients, we included 78 patients with available and measurable aortography prior to stage II palliation. Patient characteristics at stage II palliation are listed in Table 1. Mitral morphology included 37 cases of mitral atresia (47%) and 41 cases of mitral stenosis (53%). Shunt types at the Norwood procedure were predominantly of the RV-PA type (75%). At the time of stage II palliation, 11 patients (14%) presented with RV dysfunction, and 9 patients (12%) had an intervention for re-coarctation during the interstage. Thirteen patients (17%) had coronary fistulas diagnosed with either echocardiography or angiography; 9 of these patients had, morphologically speaking, mitral type ‘stenosis’. The AV valve was at least moderately regurgitant in 19 patients (25%). From the first patient in 2001 until the last one in 2020, the patients were evenly distributed with a median year of inclusion in 2010 (2007–2016).
Patient characteristics prior to stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | n (%)/median (IQR) . |
|---|---|
| Number of patients | 78 |
| Sex (female) | 19 (24) |
| Age at angiography (months) | 3.1 (2.5–4.0) |
| Weight (kg) | 5.0 (4.6–5.5) |
| Cardiac diagnosis | |
| Mitral atresia | 37 (47) |
| Mitral stenosis | 41 (53) |
| Norwood procedure | |
| RV-PA conduit | 59 (76) |
| Sytemic-to-pulmonary shunt | 19 (24) |
| CPB time (min) | 138 (112–161) |
| Coronary fistulas | 13 (17) |
| Re-coarctation | 11 (14) |
| Atrioventricular valve regurgitation | |
| None | 28 (36) |
| Trivial | 31 (39) |
| Moderate | 13 (17) |
| Severe | 6 (8) |
| RV dysfunction ≥ moderate | 9 (12) |
| Morphology of the nAAo | |
| Diameter (mm) | 3.2 (2.6–3.7) |
| Length (mm) | 15.4 (13.3–17.9) |
| nAAo–PPA anastomosis angle | 44° (35°–51°) |
| Variable . | n (%)/median (IQR) . |
|---|---|
| Number of patients | 78 |
| Sex (female) | 19 (24) |
| Age at angiography (months) | 3.1 (2.5–4.0) |
| Weight (kg) | 5.0 (4.6–5.5) |
| Cardiac diagnosis | |
| Mitral atresia | 37 (47) |
| Mitral stenosis | 41 (53) |
| Norwood procedure | |
| RV-PA conduit | 59 (76) |
| Sytemic-to-pulmonary shunt | 19 (24) |
| CPB time (min) | 138 (112–161) |
| Coronary fistulas | 13 (17) |
| Re-coarctation | 11 (14) |
| Atrioventricular valve regurgitation | |
| None | 28 (36) |
| Trivial | 31 (39) |
| Moderate | 13 (17) |
| Severe | 6 (8) |
| RV dysfunction ≥ moderate | 9 (12) |
| Morphology of the nAAo | |
| Diameter (mm) | 3.2 (2.6–3.7) |
| Length (mm) | 15.4 (13.3–17.9) |
| nAAo–PPA anastomosis angle | 44° (35°–51°) |
CPB: cardiopulmonary bypass; IQR: interquartile range; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV: right ventricle; RV-PA: right ventricle-to-pulmonary artery shunt.
Patient characteristics prior to stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | n (%)/median (IQR) . |
|---|---|
| Number of patients | 78 |
| Sex (female) | 19 (24) |
| Age at angiography (months) | 3.1 (2.5–4.0) |
| Weight (kg) | 5.0 (4.6–5.5) |
| Cardiac diagnosis | |
| Mitral atresia | 37 (47) |
| Mitral stenosis | 41 (53) |
| Norwood procedure | |
| RV-PA conduit | 59 (76) |
| Sytemic-to-pulmonary shunt | 19 (24) |
| CPB time (min) | 138 (112–161) |
| Coronary fistulas | 13 (17) |
| Re-coarctation | 11 (14) |
| Atrioventricular valve regurgitation | |
| None | 28 (36) |
| Trivial | 31 (39) |
| Moderate | 13 (17) |
| Severe | 6 (8) |
| RV dysfunction ≥ moderate | 9 (12) |
| Morphology of the nAAo | |
| Diameter (mm) | 3.2 (2.6–3.7) |
| Length (mm) | 15.4 (13.3–17.9) |
| nAAo–PPA anastomosis angle | 44° (35°–51°) |
| Variable . | n (%)/median (IQR) . |
|---|---|
| Number of patients | 78 |
| Sex (female) | 19 (24) |
| Age at angiography (months) | 3.1 (2.5–4.0) |
| Weight (kg) | 5.0 (4.6–5.5) |
| Cardiac diagnosis | |
| Mitral atresia | 37 (47) |
| Mitral stenosis | 41 (53) |
| Norwood procedure | |
| RV-PA conduit | 59 (76) |
| Sytemic-to-pulmonary shunt | 19 (24) |
| CPB time (min) | 138 (112–161) |
| Coronary fistulas | 13 (17) |
| Re-coarctation | 11 (14) |
| Atrioventricular valve regurgitation | |
| None | 28 (36) |
| Trivial | 31 (39) |
| Moderate | 13 (17) |
| Severe | 6 (8) |
| RV dysfunction ≥ moderate | 9 (12) |
| Morphology of the nAAo | |
| Diameter (mm) | 3.2 (2.6–3.7) |
| Length (mm) | 15.4 (13.3–17.9) |
| nAAo–PPA anastomosis angle | 44° (35°–51°) |
CPB: cardiopulmonary bypass; IQR: interquartile range; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV: right ventricle; RV-PA: right ventricle-to-pulmonary artery shunt.
Angiographic findings
The median age at angiography was 3.1 (2.4–3.9) months. As measured prior to stage II palliation, the median nAAo diameter was 3.2 (2.6–3.7) mm, the median nAAo length was 15.4 (13.1–17.9) mm and the median nAAo–PPA anastomosis angle was 44° (35°–51°). Figure 2 illustrates the variability of the measured nAAo–PPA anastomosis angles in the angiograms.
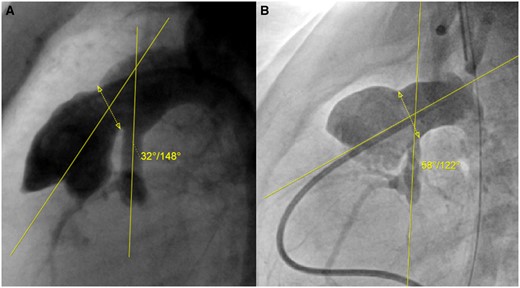
Various angles of native ascending aorta–proximal pulmonary artery anastomosis after the Norwood procedure: (A) Narrow angle (15th percentile); (B) large angle (86th percentile). The interrupted line with the double arrow represents the segment of the anastomosis between the proximal pulmonary artery and the patch, through the centre of which the angle with the native ascending aorta is measured.
Outcomes after stage II palliation
Stage II palliation was performed in all patients, and stage III palliation, in 53 patients (63%). During a median follow-up of 6.5 years (1.9–9.4), 8 patients died (10%), and RV dysfunction was reported in 19 patients (24%).
Comparison of patients with and without right ventricle dysfunction after stage II palliation
Comparisons are detailed in Table 2. The statistical comparison of the 2 groups revealed a higher rate of RV dysfunction before stage II (P < 0.001) and a higher rate of death (P = 0.027) in patients with reported RV dysfunction after stage II. It also highlighted a significantly larger nAAo–PPA anastomosis angle in patients with RV dysfunction (P = 0.04).
Comparison of patients with and without right ventricular dysfunction after stage II palliation.
| Variable . | Normal RV function (n = 59) . | RV dysfunction (n = 19) . | P-value . |
|---|---|---|---|
| Sex (female) | 14 (24) | 5 (26) | 1 |
| Age at angiography (months) | 3.2 (2.6–4.0) | 2.9 (2.4–3.7) | 0.44 |
| Weight (kg) | 5.1 (4.8–5.6) | 4.6 (4.5–5.1) | 0.017 |
| Mitral atresia | 31 (53) | 6 (32) | 0.18 |
| Norwood procedure | |||
| RV-PA conduit | 42 (71) | 17 (89) | 0.19 |
| Systemic-to-pulmonary shunt | 17 (29) | 2 (11) | 0.19 |
| CPB time (min) | 131 (105–160) | 146 (134–141) | 0.17 |
| Coronary fistulas | 10 (17) | 3 (16) | 1 |
| Re-coarctation | 10 (17) | 1 (5) | 0.37 |
| Atrioventricular valve regurgitation ≥ moderate | 15 (25) | 4 (21) | 0.93 |
| RV dysfunction ≥ moderate prior to stage II | 2 (3) | 7 (37) | <0.001 |
| Morphology of the nAAo | |||
| Diameter (mm) | 3.2 (2.6–3.7) | 3.1 (2.5–4.0) | 0.91 |
| Length (mm) | 15.7 (13.7–18.1) | 15.1 (12.7–17.7) | 0.50 |
| nAAo–PPA anastomosis angle | 42° (34°–50 °) | 49° (44°–52°) | 0.04 |
| Stage III (total cavopulmonary anastomosis) | 44 (75) | 11 (58) | 0.27 |
| Age at stage III (years) | 1.8 (1.6–2.1) | 1.8 (1.5–2.1) | 0.37 |
| Death | 3 (5) | 5 (26) | 0.03 |
| Variable . | Normal RV function (n = 59) . | RV dysfunction (n = 19) . | P-value . |
|---|---|---|---|
| Sex (female) | 14 (24) | 5 (26) | 1 |
| Age at angiography (months) | 3.2 (2.6–4.0) | 2.9 (2.4–3.7) | 0.44 |
| Weight (kg) | 5.1 (4.8–5.6) | 4.6 (4.5–5.1) | 0.017 |
| Mitral atresia | 31 (53) | 6 (32) | 0.18 |
| Norwood procedure | |||
| RV-PA conduit | 42 (71) | 17 (89) | 0.19 |
| Systemic-to-pulmonary shunt | 17 (29) | 2 (11) | 0.19 |
| CPB time (min) | 131 (105–160) | 146 (134–141) | 0.17 |
| Coronary fistulas | 10 (17) | 3 (16) | 1 |
| Re-coarctation | 10 (17) | 1 (5) | 0.37 |
| Atrioventricular valve regurgitation ≥ moderate | 15 (25) | 4 (21) | 0.93 |
| RV dysfunction ≥ moderate prior to stage II | 2 (3) | 7 (37) | <0.001 |
| Morphology of the nAAo | |||
| Diameter (mm) | 3.2 (2.6–3.7) | 3.1 (2.5–4.0) | 0.91 |
| Length (mm) | 15.7 (13.7–18.1) | 15.1 (12.7–17.7) | 0.50 |
| nAAo–PPA anastomosis angle | 42° (34°–50 °) | 49° (44°–52°) | 0.04 |
| Stage III (total cavopulmonary anastomosis) | 44 (75) | 11 (58) | 0.27 |
| Age at stage III (years) | 1.8 (1.6–2.1) | 1.8 (1.5–2.1) | 0.37 |
| Death | 3 (5) | 5 (26) | 0.03 |
CPB: cardiopulmonary bypass; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV: right ventricle; RV-PA: right ventricle-to-pulmonary artery.
Bold values indicate statistical significance.
Comparison of patients with and without right ventricular dysfunction after stage II palliation.
| Variable . | Normal RV function (n = 59) . | RV dysfunction (n = 19) . | P-value . |
|---|---|---|---|
| Sex (female) | 14 (24) | 5 (26) | 1 |
| Age at angiography (months) | 3.2 (2.6–4.0) | 2.9 (2.4–3.7) | 0.44 |
| Weight (kg) | 5.1 (4.8–5.6) | 4.6 (4.5–5.1) | 0.017 |
| Mitral atresia | 31 (53) | 6 (32) | 0.18 |
| Norwood procedure | |||
| RV-PA conduit | 42 (71) | 17 (89) | 0.19 |
| Systemic-to-pulmonary shunt | 17 (29) | 2 (11) | 0.19 |
| CPB time (min) | 131 (105–160) | 146 (134–141) | 0.17 |
| Coronary fistulas | 10 (17) | 3 (16) | 1 |
| Re-coarctation | 10 (17) | 1 (5) | 0.37 |
| Atrioventricular valve regurgitation ≥ moderate | 15 (25) | 4 (21) | 0.93 |
| RV dysfunction ≥ moderate prior to stage II | 2 (3) | 7 (37) | <0.001 |
| Morphology of the nAAo | |||
| Diameter (mm) | 3.2 (2.6–3.7) | 3.1 (2.5–4.0) | 0.91 |
| Length (mm) | 15.7 (13.7–18.1) | 15.1 (12.7–17.7) | 0.50 |
| nAAo–PPA anastomosis angle | 42° (34°–50 °) | 49° (44°–52°) | 0.04 |
| Stage III (total cavopulmonary anastomosis) | 44 (75) | 11 (58) | 0.27 |
| Age at stage III (years) | 1.8 (1.6–2.1) | 1.8 (1.5–2.1) | 0.37 |
| Death | 3 (5) | 5 (26) | 0.03 |
| Variable . | Normal RV function (n = 59) . | RV dysfunction (n = 19) . | P-value . |
|---|---|---|---|
| Sex (female) | 14 (24) | 5 (26) | 1 |
| Age at angiography (months) | 3.2 (2.6–4.0) | 2.9 (2.4–3.7) | 0.44 |
| Weight (kg) | 5.1 (4.8–5.6) | 4.6 (4.5–5.1) | 0.017 |
| Mitral atresia | 31 (53) | 6 (32) | 0.18 |
| Norwood procedure | |||
| RV-PA conduit | 42 (71) | 17 (89) | 0.19 |
| Systemic-to-pulmonary shunt | 17 (29) | 2 (11) | 0.19 |
| CPB time (min) | 131 (105–160) | 146 (134–141) | 0.17 |
| Coronary fistulas | 10 (17) | 3 (16) | 1 |
| Re-coarctation | 10 (17) | 1 (5) | 0.37 |
| Atrioventricular valve regurgitation ≥ moderate | 15 (25) | 4 (21) | 0.93 |
| RV dysfunction ≥ moderate prior to stage II | 2 (3) | 7 (37) | <0.001 |
| Morphology of the nAAo | |||
| Diameter (mm) | 3.2 (2.6–3.7) | 3.1 (2.5–4.0) | 0.91 |
| Length (mm) | 15.7 (13.7–18.1) | 15.1 (12.7–17.7) | 0.50 |
| nAAo–PPA anastomosis angle | 42° (34°–50 °) | 49° (44°–52°) | 0.04 |
| Stage III (total cavopulmonary anastomosis) | 44 (75) | 11 (58) | 0.27 |
| Age at stage III (years) | 1.8 (1.6–2.1) | 1.8 (1.5–2.1) | 0.37 |
| Death | 3 (5) | 5 (26) | 0.03 |
CPB: cardiopulmonary bypass; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV: right ventricle; RV-PA: right ventricle-to-pulmonary artery.
Bold values indicate statistical significance.
Figure 3 illustrates the distribution of nAAo–PPA anastomosis angles in patients with and without RV dysfunction.
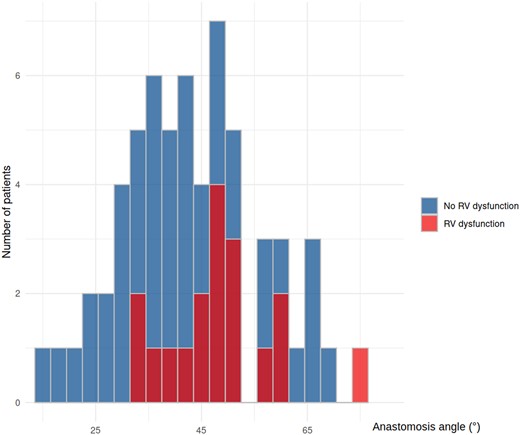
Distribution of native ascending aorta–proximal pulmonary artery anastomosis angles in patients with and without right ventricular dysfunction after stage II palliation.
Risk factor analysis: right ventricular dysfunction
The risk factor analysis is detailed in Table 3. The nAAo-PPA anastomosis angle was a significant risk factor for RV dysfunction (odds ratio 1.07, 95% confidence interval 1.01–1.14, P = 0.021) after stage II. Parameters such as AV valve regurgitation, coronary fistulas and intervention for re-coarctation were not significantly associated.
Risk-factor multivariable analysis for right ventricular dysfunction after stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| RV-PA conduit | 6.04 | 1.16–55.41 | 0.06 |
| Coronary fistulas | 1.15 | 0.21–5.4 | 0.86 |
| CPB time (min) | 1.01 | 0.99–1.02 | 0.26 |
| Re-coarctation | 0.16 | 0.01–1.23 | 0.13 |
| Atrioventricular valve regurgitation ≥ moderate | 0.61 | 0.11–2.59 | 0.52 |
| nAAo diameter (mm) | 1.37 | 0.7–2.72 | 0.36 |
| nAAo length (mm) | 0.88 | 0.73–1.03 | 0.12 |
| nAAo–PPA anastomosis angle (°) | 1.07 | 1.01–1.14 | 0.02 |
| Variable . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| RV-PA conduit | 6.04 | 1.16–55.41 | 0.06 |
| Coronary fistulas | 1.15 | 0.21–5.4 | 0.86 |
| CPB time (min) | 1.01 | 0.99–1.02 | 0.26 |
| Re-coarctation | 0.16 | 0.01–1.23 | 0.13 |
| Atrioventricular valve regurgitation ≥ moderate | 0.61 | 0.11–2.59 | 0.52 |
| nAAo diameter (mm) | 1.37 | 0.7–2.72 | 0.36 |
| nAAo length (mm) | 0.88 | 0.73–1.03 | 0.12 |
| nAAo–PPA anastomosis angle (°) | 1.07 | 1.01–1.14 | 0.02 |
CI: confidence interval; CPB: cardiopulmonary bypass; nAAo: native ascending aorta; OR: odds ratio; PPA: proximal pulmonary artery; RV-PA: right ventricle-to-pulmonary artery.
Bold values indicate statistical significance.
Risk-factor multivariable analysis for right ventricular dysfunction after stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| RV-PA conduit | 6.04 | 1.16–55.41 | 0.06 |
| Coronary fistulas | 1.15 | 0.21–5.4 | 0.86 |
| CPB time (min) | 1.01 | 0.99–1.02 | 0.26 |
| Re-coarctation | 0.16 | 0.01–1.23 | 0.13 |
| Atrioventricular valve regurgitation ≥ moderate | 0.61 | 0.11–2.59 | 0.52 |
| nAAo diameter (mm) | 1.37 | 0.7–2.72 | 0.36 |
| nAAo length (mm) | 0.88 | 0.73–1.03 | 0.12 |
| nAAo–PPA anastomosis angle (°) | 1.07 | 1.01–1.14 | 0.02 |
| Variable . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| RV-PA conduit | 6.04 | 1.16–55.41 | 0.06 |
| Coronary fistulas | 1.15 | 0.21–5.4 | 0.86 |
| CPB time (min) | 1.01 | 0.99–1.02 | 0.26 |
| Re-coarctation | 0.16 | 0.01–1.23 | 0.13 |
| Atrioventricular valve regurgitation ≥ moderate | 0.61 | 0.11–2.59 | 0.52 |
| nAAo diameter (mm) | 1.37 | 0.7–2.72 | 0.36 |
| nAAo length (mm) | 0.88 | 0.73–1.03 | 0.12 |
| nAAo–PPA anastomosis angle (°) | 1.07 | 1.01–1.14 | 0.02 |
CI: confidence interval; CPB: cardiopulmonary bypass; nAAo: native ascending aorta; OR: odds ratio; PPA: proximal pulmonary artery; RV-PA: right ventricle-to-pulmonary artery.
Bold values indicate statistical significance.
Risk factor analysis: mortality
The risk factor analysis is detailed in Table 4. The multivariable analysis could not detect any significant association between the selected parameters and mortality.
Risk-factor multivariable analysis for death after stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Sex | 0.36 | 0.04–3.45 | 0.379 |
| Mitral atresia | 0.97 | 0.15–6.33 | 0.973 |
| RV-PA conduit | 2.81 | 0.31–25.54 | 0.36 |
| Coronary fistulas | 1.84 | 0.27–12.39 | 0.531 |
| CPB time (min) | 1 | 0.99–1.02 | 0.622 |
| Re-coarctation | 2.35 | 0.35–15.87 | 0.38 |
| Atrioventricular valve regurgitation ≥ moderate | 2.6 | 0.44–15.45 | 0.293 |
| nAAo diameter (mm) | 1.6 | 0.65–3.92 | 0.302 |
| nAAo length (mm) | 0.9 | 0.72–1.13 | 0.366 |
| nAAo–PPA anastomosis angle (°) | 1.04 | 0.96–1.12 | 0.34 |
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Sex | 0.36 | 0.04–3.45 | 0.379 |
| Mitral atresia | 0.97 | 0.15–6.33 | 0.973 |
| RV-PA conduit | 2.81 | 0.31–25.54 | 0.36 |
| Coronary fistulas | 1.84 | 0.27–12.39 | 0.531 |
| CPB time (min) | 1 | 0.99–1.02 | 0.622 |
| Re-coarctation | 2.35 | 0.35–15.87 | 0.38 |
| Atrioventricular valve regurgitation ≥ moderate | 2.6 | 0.44–15.45 | 0.293 |
| nAAo diameter (mm) | 1.6 | 0.65–3.92 | 0.302 |
| nAAo length (mm) | 0.9 | 0.72–1.13 | 0.366 |
| nAAo–PPA anastomosis angle (°) | 1.04 | 0.96–1.12 | 0.34 |
CI: confidence interval; CPB: cardiopulmonary bypass; HR: hazard ratio; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV-PA: right ventricle-to-pulmonary artery.
Risk-factor multivariable analysis for death after stage II palliation (bidirectional cavopulmonary shunt).
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Sex | 0.36 | 0.04–3.45 | 0.379 |
| Mitral atresia | 0.97 | 0.15–6.33 | 0.973 |
| RV-PA conduit | 2.81 | 0.31–25.54 | 0.36 |
| Coronary fistulas | 1.84 | 0.27–12.39 | 0.531 |
| CPB time (min) | 1 | 0.99–1.02 | 0.622 |
| Re-coarctation | 2.35 | 0.35–15.87 | 0.38 |
| Atrioventricular valve regurgitation ≥ moderate | 2.6 | 0.44–15.45 | 0.293 |
| nAAo diameter (mm) | 1.6 | 0.65–3.92 | 0.302 |
| nAAo length (mm) | 0.9 | 0.72–1.13 | 0.366 |
| nAAo–PPA anastomosis angle (°) | 1.04 | 0.96–1.12 | 0.34 |
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Sex | 0.36 | 0.04–3.45 | 0.379 |
| Mitral atresia | 0.97 | 0.15–6.33 | 0.973 |
| RV-PA conduit | 2.81 | 0.31–25.54 | 0.36 |
| Coronary fistulas | 1.84 | 0.27–12.39 | 0.531 |
| CPB time (min) | 1 | 0.99–1.02 | 0.622 |
| Re-coarctation | 2.35 | 0.35–15.87 | 0.38 |
| Atrioventricular valve regurgitation ≥ moderate | 2.6 | 0.44–15.45 | 0.293 |
| nAAo diameter (mm) | 1.6 | 0.65–3.92 | 0.302 |
| nAAo length (mm) | 0.9 | 0.72–1.13 | 0.366 |
| nAAo–PPA anastomosis angle (°) | 1.04 | 0.96–1.12 | 0.34 |
CI: confidence interval; CPB: cardiopulmonary bypass; HR: hazard ratio; nAAo: native ascending aorta; PPA: proximal pulmonary artery; RV-PA: right ventricle-to-pulmonary artery.
DISCUSSION
In this retrospective study of patients with HLHS/AA after the Norwood procedure, we could not detect any significant association between the native aortic morphology and mortality after stage II palliation. However, considering the low rate of events (10%) and the small number of patients, our study was not adequately powered to detect any significance with the statistical effects observed. Therefore, these results should be interpreted with caution.
Although we observed no association between nAAo length and nAAo diameter, the larger nAAo–PPA anastomosis angle was a risk factor for RV dysfunction. Interestingly, the presence of a systemic-to-pulmonary shunt at stage I was a significant protective factor for RV dysfunction after stage II, which is consistent with our previous report on our extended (n = 146) HLHS/AA cohort and with several other studies [15–17]. However, recent data from a study on HLHS teenagers (n = 168) revealed no association between the initial shunt type at the Norwood procedure and RV function at the age of 10 years (10.4–11.5) [18]. Of note, this study did not focus on HLHS/AA types. More data are still sought to state the long-term superiority of 1 shunt type over the other concerning the RV function in these patients.
Impact of native ascending aorta diameter
Aortic diameter has been extensively studied as a prognostic factor for patients with HLHS. In line with our findings, several retrospective cohort studies—including 1 from our centre—reported no significant interaction between nAAo diameter and short-term survival after the Norwood procedure [7, 9, 19–21]. Other groups have observed an increase in short-term mortality in patients with a smaller diameter of nAAo after the Norwood procedure, albeit with variable cut-offs of aortic diameter [6, 22, 23]. Of note, no subgroup analysis for patients with HLHS/AA was included in these studies. Whether the anatomy of the aortic valve has affected the prognosis more negatively than the aortic diameter itself, in these heterogeneous populations of both HLHS/AA and HLHS/aortic stenosis subtypes, remains hypothetical. In 2020, Carvajal et al. reported an excessive number of 30-day deaths after the Norwood procedure in patients who were exclusively HLHS/AA with an aortic diameter smaller than or equal to 1.5 mm [8]. Although this diameter is found in a minor proportion of patients with HLHS, the hypothesis of impaired coronary perfusion due to a restrictive aortic diameter has led some groups to undertake a prophylactic augmentation of the nAAo during the Norwood operation in selected patients. Hoganson et al. recently demonstrated a reduction in a composite end point of transplant-free survival, extracorporeal membrane oxygenator or more than moderate right ventricular dysfunction at the time of stage II palliation in 11 patients with HLHS with patch augmentation of the nAAo during the Norwood procedure compared to 115 patients without patch augmentation (100% vs 61.8%, P = 0.008) [24].Consistent with Carvajal et al., the authors also observed an increase in 30-day deaths in patients with an nAAo diameter ≤ 1.5 mm.
Our findings regarding RV dysfunction were in line with those of Carvajal et al., who observed no impact of nAAo diameter on RV function 14 months after the Norwood procedure in patients with HLHS/AA [8].
Impact of native ascending aorta length and native ascending aorta–proximal pulmonary artery anastomosis angle
Morphologic features of the nAAo in patients with HLHS/AA after the Norwood that are distinct from its diameter have been rarely, if ever, studied. Interestingly, whereas nAAo length had no detectable impact on either survival or RV dysfunction within the limited scope of this study, a larger nAAo–PPA anastomosis angle was associated with RV dysfunction. The statistical effect of this angle on the outcome was modest. Nonetheless, a strict interpretation of the statistical model would mean that, with a 10° increase in the nAAo–PPA anastomosis angle, the odds of RV dysfunction would be almost 2 times (1.0710 = 1.97) higher, which may be considered clinically significant. We interpreted this finding as being the result of an increased resistance to the diastolic back-flow in the nAAo with a larger inclination angle to the neo-aorta. By reducing the flow into the aortic root, this increased resistance may affect myocardial perfusion negatively and ultimately cause RV dysfunction.
Vulnerability of the coronary circulation and clinical implications of the present study
The vulnerability of the coronary circulation in patients with HLHS after the Norwood procedure is well documented. Using positron-emission tomography, Donelly et al. observed an inferior resting coronary flow as well as limited coronary flow reserve in patients with HLHS after Norwood palliation compared with patients after repair of biventricular congenital heart disease [25]. Of note, this study focused on patients with a systemic-to-pulmonary shunt, whereas our study was mainly composed of patients with an RV-PA shunt. Using invasive measurements, Saiki et al. demonstrated a reduced myocardial oxygen supply–demand balance in patients with HLHS after Norwood, compared with control patients as well as patients with shunt-dependent pulmonary atresia, reflecting an insufficient coronary flow intrinsic to the Norwood circulation [10]. The authors identified aortic stiffness (i.e. ratio of pulse pressure to stroke volume) and increased neo-aortic size discrepancy (i.e. a smaller neo-aorta relative to the native descending aorta) as risk factors for this myocardial oxygen supply–demand imbalance. Our observations suggest that a larger angle between the nAAo and the PA/neo-aorta may be another risk factor for impaired coronary perfusion, as reflected by the higher occurrence of RV dysfunction in our cohort.
Though the position of the great vessels on hypoplastic left hearts is variable and the spatial relationship between nAAo and PA is fixed on a given heart, these observations could improve the clinical outcomes of patients with HLHS by refining the surgical design of the neo-aorta during the Norwood procedure. Indeed, our data suggest that coronary perfusion after the Norwood procedure is affected by the angle formed between the nAAo and the PA and not by the residual length of the nAAo itself. To which extent the residual segment of the native aorta should be lengthened in favour of a narrower nAAo–PPA anastomosis angle for an optimal effective coronary perfusion, remains to be defined.
Limitations
This study was limited by the small number of patients and its retrospective design. Although the median 20-year follow-up was satisfactory, a minority of patients were lost to follow-up within 5 years of stage II, which may be an insufficient observation period in which to capture the outcomes of interest of this study.
The estimation of RV dysfunction is also problematic for several reasons. First, RV function varies over time and can be affected by transitory factors such as hypervolaemia or dysrhythmia and can improve with appropriate therapy. Secondly, semi-quantitative assessment of RV function by echocardiography, even with a single experienced cardiologist, is affected by intra-observer variability and by the technical limitations intrinsic to echocardiography. Cardiac magnetic resonance imaging would be the examination of choice for a more reliable assessment, but this examination is not routinely performed in our patients. Finally, in the presence of significant AV regurgitation, the RV function is necessarily overestimated.
We did not perform either invasive measurements or flow imaging to corroborate our hypothesis of impaired coronary perfusion. This statement was extrapolated from the RV dysfunction, for which we found no other causal factor, to the extent of the data available for this cohort. Further studies with 4-dimensional imaging of the ascending aortic flow in patients with HLHS after the Norwood procedure could shed some light on this point. We are planning a study based on this technology.
Technically, the measurement of the nAAo–PPA anastomosis angle was restricted to a single plane and may have been underestimated, depending on the position of the large vessels specific to each patient. However, given the low variability in the spatial relationship of the great vessels in patients with HLHS, predominantly antero-posterior, this ‘parallax’ effect was considered negligible.
Also, because we did not include patients who died prior to stage II palliation, critical cases of coronary ischaemia leading to interstage death were absent from the study collective, so no conclusions can be drawn concerning the role of their aortic morphology. These missing data, together with the missing aortographies at the preoperative catheter, are responsible for the lack of statistical power of our study with regard to the analysis of mortality risk factors.
Finally, this study focused on patients with HLHS/AA, and its findings may not be generalizable to patients with HLHS/aortic stenosis. Due to its exclusively retrograde flow and usually smaller diameter, we considered the nAAo in HLHS/AA as the Achilles’ heel of coronary perfusion and chose to focus primarily on the aortic morphology in this subgroup of patients.
CONCLUSIONS
In survivors of the Norwood procedure for HLHS/AA, no significant association between the native aortic morphology and mortality could be demonstrated after stage II palliation, within the scope of this limited study. A larger anastomosis angle between the nAAo and the proximal pulmonary artery emerged as a risk factor for RV dysfunction. Further data and functional imaging of the proximal aortic flow are warranted to corroborate this potentially important surgical issue.
Presented at the 37th annual meeting of the European Association for Cardiothoracic Surgery, Vienna, Austria, 4–7 October 2023.
FUNDING
This study was not supported by any grants.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Thibault Schaeffer: Data curation; Formal analysis; Methodology; Writing—original draft. Paul Philipp Heinisch: Data curation; Methodology; Writing—review and editing. Helena Staehler: Data curation. Stanimir Georgiev: Supervision; Validation; Writing—review and editing. Christoph Röhlig: Data curation; Validation. Alfred Hager: Methodology; Validation; Writing—review and editing. Peter Ewert: Supervision. Jürgen Hörer: Conceptualization; Methodology; Supervision; Writing—review and editing. Masamichi Ono: Methodology; Supervision; Validation; Writing—review and editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Hannah Rosemary Bellsham-Revell, Arun Gopalakrishnan and Christian Pizarro for their contributions to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- AA
Aortic atresia
- AV
Atrioventricular
- EF
Ejection fraction
- HLHS
Hypoplastic left heart syndrome
- nAAo
Native ascending aorta
- PPA
Proximal pulmonary artery
- RV
Right ventricle
- angiogram
- aorta
- hypoplastic left heart syndrome
- pulmonary artery
- ventricular dysfunction, right
- ascending aorta
- repair of single ventricle with aortic outflow obstruction and aortic arch hypoplasia (hypoplastic left heart syndrome) (eg, norwood procedure)
- anastomosis, surgical
- ventricular function, right
- mortality
- palliative care
- congenital atresia of aorta





