-
PDF
- Split View
-
Views
-
Cite
Cite
René M’Pembele, Sebastian Roth, Freya Jenkins, Vincent Hettlich, Anthony Nucaro, Alexandra Stroda, Theresa Tenge, Amin Polzin, Bedri Ramadani, Giovanna Lurati Buse, Hug Aubin, Artur Lichtenberg, Ragnar Huhn, Udo Boeken, Association between early postoperative hypoalbuminaemia and outcome after orthotopic heart transplantation, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 38, Issue 1, January 2024, ivae012, https://doi.org/10.1093/icvts/ivae012
Close - Share Icon Share
Abstract
In patients undergoing heart transplantation (HTX), preoperative liver impairment and consecutive hypoalbuminaemia are associated with increased mortality. The role of early postoperative hypoalbuminaemia after HTX is unclear. This study investigated the association between early postoperative hypoalbuminaemia and 1-year mortality as well as ‘days alive and out of hospital’ (DAOH) after HTX.
This retrospective cohort study included patients who underwent HTX at the University Hospital Duesseldorf, Germany, between 2010 and 2022. The main exposure was serum albumin concentration at intensive care unit (ICU) arrival. The primary endpoints were mortality and DAOH within 1 year after surgery. Receiver operating characteristic (ROC) curve analysis was performed and logistic and quantile regression models with adjustment for 13 a priori defined clinical risk factors were conducted.
Out of 241 patients screened, 229 were included in the analysis (mean age 55 ± 11 years, 73% male). ROC analysis showed moderate discrimination for 1-year mortality by postoperative serum albumin after HTX [AUC = 0.74; 95% confidence interval (CI): 0.66–0.83]. The cutoff for serum albumin at ICU arrival was 3.0 g/dl. According to multivariate logistic and quantile regression, there were independent associations between hypoalbuminaemia and mortality/DAOH [odds ratio of 4.76 (95% CI: 1.94–11.67) and regression coefficient of −46.97 (95% CI: −83.81 to −10.13)].
Postoperative hypoalbuminaemia <3.0 g/dl is associated with 1-year mortality and reduced DAOH after HTX and therefore might be used for early postoperative risk re-assessment in clinical practice.
INTRODUCTION
Postoperative hypoalbuminaemia is frequent after cardiac surgery with the use of cardiopulmonary bypass (CPB) and can result from intraoperative blood loss, dilution, increased inflammatory response or postoperative capillary leak amongst other reasons [1]. Recent studies showed an association of postoperative hypoalbuminaemia with poor short- and long-term survival in patients undergoing cardiac surgery with and without CPB [2]. In patients undergoing heart transplantation (HTX), preoperative risk assessment is crucial to identify patients with poor prognosis. In this context, preoperative liver impairment was identified as a factor which is associated with increased mortality after HTX. Therefore, biomarkers of liver impairment are included in risk prediction tools like the Index for Mortality Prediction After Cardiac Transplantation (IMPACT) score [3, 4]. Recently, the use of the model for end-stage liver disease (MELD) score was proposed for preoperative risk assessment in heart transplant patients [5]. Preoperative serum albumin is another biomarker that is decreased when liver function is impaired. Previous research showed that low preoperative albumin levels as the sole marker or when added to the MELD score were a strong predictor of poor outcome in HTX and heart failure patients [6–9]. However, the role of early postoperative hypoalbuminaemia in risk re-assessment and prognosis after HTX is unclear. Therefore, the aim of this study was to investigate the association between early postoperative hypoalbuminaemia and 1-year mortality as well as ‘days alive and out of hospital’ (DAOH) as a patient-centred outcome after HTX. Furthermore, the additional predictive value of postoperative hypoalbuminaemia for 1-year mortality was assessed as compared to the postoperative MELD score, as a measure for early postoperative liver dysfunction, and the preoperative IMPACT score.
MATERIALS AND METHODS
Ethics statement
This analysis was conducted as a retrospective single-centre cohort study. Approval was obtained from the institutional review board of the University of Duesseldorf (reference number: 4567). The extracted data for this analysis were available in the local prospective HTX database. All patients included in this database had given written informed consent to be enrolled. Reporting of the results follows STROBE guidelines [10].
Patient population and inclusion criteria
Consecutive adult patients (≥18 years) undergoing HTX at a tertiary care University Hospital in Duesseldorf, Germany, from September 2010 to April 2022 with completed 1-year follow-up were screened for inclusion. Patients with missing data regarding the primary endpoints or postoperative serum albumin measurements were excluded from the analysis.
Perioperative fluid and transfusion management
Perioperative fluid and transfusion management of HTX patients is highly standardized in our institution and guided by continuous haemodynamic monitoring. Intraoperative blood management during and post CPB targets haemoglobin levels around 10 g/dl. Serum albumin levels of 2.0 g/dl or below are used as a threshold for albumin substitution. Albumin substitution is considered at serum albumin levels below 2.5 g/dl if the patient shows signs of peripheral oedema.
Serum albumin measurements
Main exposure was the first postoperative serum albumin concentration measured in g/dl within the first 12 h at ICU. Albumin values were determined by the local central laboratory.
IMPACT score calculation
The risk index for IMPACT was calculated for each patient as described previously [3, 4, 11]. It assigns varying points for the variables age, serum bilirubin, creatinine clearance, dialysis, sex, heart failure aetiology, preoperative infection, race, circulatory support and type of ventricular assist device and was calculated for a previously published analysis [11]. Data were received by the prospective local database that stored manually extracted information from electronic clinical charts by trained personnel.
MELD score calculation
The MELD score was calculated for each patient as described previously using the following formula: 10 × [0.957 × Ln(Creatinine) + 0.378 × Ln(total bilirubin) + 1.12 × Ln(international normalized ratio) + 0.643] [12]. For calculation, immediate postoperative values of these biomarkers were used.
Outcomes
The primary endpoint was all-cause mortality during the first year after surgery. The secondary endpoint was the number of DAOH within the first year after HTX. DAOH were calculated by subtraction of all days spent in the hospital from 365 days. In case of death, the days the patient did not survive were added to the time spent in the hospital which was then subtracted from 365 days [13, 14]. DAOH is a more patient-centred outcome as it includes mortality, length of hospital stay and hospital readmissions and is known for its correlation with measures of quality of life [11].
Statistical analysis
Statistical software used for the present analysis were IBM SPSS software version 25.0 (Armonk, NY, USA), GraphPad Prism version 8.02 (La Jolla, CA, USA) and MedCalc Statistical Software version 20.114 (MedCalc Software Ltd, Ostend, Belgium). Descriptive statistics are presented as number (n) with corresponding percentages (%) in brackets for categorical variables and as mean ± standard deviation (SD) for continuous variables. Fisher’s exact test or unpaired t-tests were used to compare continuous or dichotomous variables between groups. To evaluate the prognostic value of postoperative albumin receiver operating characteristic curve (ROC) analysis was conducted (dependent variable: 1-year mortality). The Youden index was used to determine a cutoff value for albumin level. Kaplan–Meier curves were conducted for survival analysis depending on albumin cutoff value. Univariate logistic regression was conducted for postoperative albumin level and 1-year mortality. In a multivariate logistic regression model, odds ratios (ORs) were adjusted for clinical risk factors from baseline characteristics that might be associated with 1-year mortality. To evaluate if postoperative albumin could improve risk stratification of mortality prediction models, the net reclassification improvement (NRI) and the net absolute reclassification improvement (NARI) of two mortality prediction models adding postoperative albumin level to either MELD score or IMPACT score were calculated as previously performed [11]. Discrimination of these models with and without the addition of albumin was assessed (ROC-AUC) and compared using the Delong test. DAOH of patients with albumin values higher and lower than Youden index derived cutoff were compared in univariate analysis using Mann–Whitney U test. In a multivariate quantile regression model of the lowest DAOH centile, association of continuous postoperative albumin values with DAOH was adjusted for clinical risk factors from baseline characteristics. For all statistical tests, a P-value of <0.05 was considered significant. Similar methods were used in previous publications [11].
RESULTS
Study cohort and characteristics
A total of 241 patients underwent HTX from 2010 to 2022 at the University Hospital of Duesseldorf and completed 1-year follow-up. Based on the inclusion and exclusion criteria 12 patients had to be excluded and 229 patients were included in the analysis. Mean age of the study group was 55 ± 11 years. Overall 1-year mortality was 17.4% (40 patients) and 30-day mortality was 7.8% (18 patients). Median DAOH were 299 (230–322) at 1 year after HTX. Detailed patient characteristics are presented in Table 1 (Supplementary Table S1, Figure S1).
| . | Albumin ≥3 g/dl (N = 173) . | Albumin <3 g/dl (N = 56) . | P-value . |
|---|---|---|---|
| Preoperative recipient characteristics | |||
| Male sex | 129 (74.6) | 38 (67.9) | 0.387 |
| Age (years) | 55.1 ± 10.8 | 55.3 ± 11 | 0.904 |
| BMI (kg/m2) | 25.8 ± 4.5 | 25.4 ± 4.7 | 0.614 |
| Smoker | 45 (26.2) | 12 (21.4) | 0.594 |
| Diabetes | 32 (18.7) | 18 (32.1) | 0.042 |
| Arterial hypertension | 98 (57) | 32 (57.1) | >0.999 |
| Pulmonary hypertension | 14 (8.1) | 7 (12.5) | 0.424 |
| Prior cardiothoracic surgery | 101 (58.4) | 44 (78.6) | 0.007 |
| LVAD | 78 (45.1) | 37 (66.1) | 0.009 |
| ICM | 73 (42.4) | 25 (44.6) | 0.877 |
| DCM | 81 (47.1) | 28 (50) | 0.759 |
| ARVC | 7 (4.1) | 1 (1.8) | 0.683 |
| RCM | 0 (0) | 1 (1.8) | 0.246 |
| HCM | 3 (1.7) | 1 (1.8) | >0.999 |
| Myocarditis | 2 (1.2) | 0 (0) | >0.999 |
| Preoperative dialysis | 10 (5.8) | 2 (3.7) | 0.736 |
| IMPACT score | 8.1 ± 4.7 | 9.4 ± 4.1 | 0.096 |
| Albumin (g/dl) | 3.9 ± 0.7 | 3.7 ± 0.7 | 0.185 |
| MELD score | 14.3 ± 7.6 | 13.7 ± 6.5 | 0.652 |
| Donor characteristics | |||
| Male sex | 98 (56.6) | 28 (50) | 0.441 |
| Age (years) | 43.1 ± 12.2 | 44.3 ± 12.4 | 0.526 |
| BMI (kg/m²) | 26.1 ± 4.9 | 26.0 ± 3.7 | 0.945 |
| Cardiopulmonary resuscitation | 53 (30.6) | 15 (26.8) | 0.618 |
| Intraoperative characteristics (min) | |||
| Duration of surgery | 401 ± 95 | 490 ± 148 | <0.001 |
| Duration of CPB | 240 ± 57 | 294 ± 94 | <0.001 |
| Total ischaemia time | 212 ± 48 | 220 ± 51 | 0.330 |
| PRBC (l) | 2.9 ± 2.4 | 4.9 ± 3.2 | <0.001 |
| Platelets (l) | 1.0 ± 0.8 | 1.5 ± 1.1 | 0.001 |
| FFP (l) | 1.5 ± 1.6 | 1.9 ± 2.3 | 0.218 |
| Postoperative laboratory values | |||
| Creatinine (mg/dl) | 1.5 ± 0.8 | 1.3 ± 0.5 | 0.230 |
| Bilirubin (mg/dl) | 2.6 ± 1.9 | 2.5 ± 1.3 | 0.576 |
| INR | 1.2 ± 1.9 | 1.3 ± 2.2 | 0.280 |
| Albumin (g/dl) | 3.5 ± 0.4 | 2.4 ± 0.4 | <0.001 |
| Postoperative characteristics | |||
| ECMO | 33 (19.2) | 28 (50) | <0.001 |
| Renal replacement therapy | 90 (57.7) | 35 (62.5) | 0.635 |
| MELD score | 14.6 ± 5.3 | 14.3 ± 5.1 | 0.711 |
| Albumin substitution within first 24 h | 47 (28.7) | 29 (54.7) | 0.001 |
| Days in ICU | 24 ± 25 | 25 ± 26 | 0.677 |
| Duration of mechanical ventilation (hours) | 110 ± 167 | 223 ± 216 | 0.001 |
| 30-day mortality | 6 (3.5) | 12 (21.4) | <0.001 |
| 1-year mortality | 18 (10.4) | 22 (39.3) | <0.001 |
| . | Albumin ≥3 g/dl (N = 173) . | Albumin <3 g/dl (N = 56) . | P-value . |
|---|---|---|---|
| Preoperative recipient characteristics | |||
| Male sex | 129 (74.6) | 38 (67.9) | 0.387 |
| Age (years) | 55.1 ± 10.8 | 55.3 ± 11 | 0.904 |
| BMI (kg/m2) | 25.8 ± 4.5 | 25.4 ± 4.7 | 0.614 |
| Smoker | 45 (26.2) | 12 (21.4) | 0.594 |
| Diabetes | 32 (18.7) | 18 (32.1) | 0.042 |
| Arterial hypertension | 98 (57) | 32 (57.1) | >0.999 |
| Pulmonary hypertension | 14 (8.1) | 7 (12.5) | 0.424 |
| Prior cardiothoracic surgery | 101 (58.4) | 44 (78.6) | 0.007 |
| LVAD | 78 (45.1) | 37 (66.1) | 0.009 |
| ICM | 73 (42.4) | 25 (44.6) | 0.877 |
| DCM | 81 (47.1) | 28 (50) | 0.759 |
| ARVC | 7 (4.1) | 1 (1.8) | 0.683 |
| RCM | 0 (0) | 1 (1.8) | 0.246 |
| HCM | 3 (1.7) | 1 (1.8) | >0.999 |
| Myocarditis | 2 (1.2) | 0 (0) | >0.999 |
| Preoperative dialysis | 10 (5.8) | 2 (3.7) | 0.736 |
| IMPACT score | 8.1 ± 4.7 | 9.4 ± 4.1 | 0.096 |
| Albumin (g/dl) | 3.9 ± 0.7 | 3.7 ± 0.7 | 0.185 |
| MELD score | 14.3 ± 7.6 | 13.7 ± 6.5 | 0.652 |
| Donor characteristics | |||
| Male sex | 98 (56.6) | 28 (50) | 0.441 |
| Age (years) | 43.1 ± 12.2 | 44.3 ± 12.4 | 0.526 |
| BMI (kg/m²) | 26.1 ± 4.9 | 26.0 ± 3.7 | 0.945 |
| Cardiopulmonary resuscitation | 53 (30.6) | 15 (26.8) | 0.618 |
| Intraoperative characteristics (min) | |||
| Duration of surgery | 401 ± 95 | 490 ± 148 | <0.001 |
| Duration of CPB | 240 ± 57 | 294 ± 94 | <0.001 |
| Total ischaemia time | 212 ± 48 | 220 ± 51 | 0.330 |
| PRBC (l) | 2.9 ± 2.4 | 4.9 ± 3.2 | <0.001 |
| Platelets (l) | 1.0 ± 0.8 | 1.5 ± 1.1 | 0.001 |
| FFP (l) | 1.5 ± 1.6 | 1.9 ± 2.3 | 0.218 |
| Postoperative laboratory values | |||
| Creatinine (mg/dl) | 1.5 ± 0.8 | 1.3 ± 0.5 | 0.230 |
| Bilirubin (mg/dl) | 2.6 ± 1.9 | 2.5 ± 1.3 | 0.576 |
| INR | 1.2 ± 1.9 | 1.3 ± 2.2 | 0.280 |
| Albumin (g/dl) | 3.5 ± 0.4 | 2.4 ± 0.4 | <0.001 |
| Postoperative characteristics | |||
| ECMO | 33 (19.2) | 28 (50) | <0.001 |
| Renal replacement therapy | 90 (57.7) | 35 (62.5) | 0.635 |
| MELD score | 14.6 ± 5.3 | 14.3 ± 5.1 | 0.711 |
| Albumin substitution within first 24 h | 47 (28.7) | 29 (54.7) | 0.001 |
| Days in ICU | 24 ± 25 | 25 ± 26 | 0.677 |
| Duration of mechanical ventilation (hours) | 110 ± 167 | 223 ± 216 | 0.001 |
| 30-day mortality | 6 (3.5) | 12 (21.4) | <0.001 |
| 1-year mortality | 18 (10.4) | 22 (39.3) | <0.001 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BMI, body mass index; CPB, cardiopulmonary bypass; DCM, dilative cardiomyopathy; ECMO; extracorporeal membrane oxygenation; FFP, fresh frozen plasma; HCM, hypertrophic cardiomyopathy; ICM, ischaemic cardiomyopathy; IMPACT, risk index for mortality prediction after cardiac transplantation; ICU, intensive care unit; INR, international normalized ratio; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RCM, restrictive cardiomyopathy [11].
Significant results are marked in bold.
| . | Albumin ≥3 g/dl (N = 173) . | Albumin <3 g/dl (N = 56) . | P-value . |
|---|---|---|---|
| Preoperative recipient characteristics | |||
| Male sex | 129 (74.6) | 38 (67.9) | 0.387 |
| Age (years) | 55.1 ± 10.8 | 55.3 ± 11 | 0.904 |
| BMI (kg/m2) | 25.8 ± 4.5 | 25.4 ± 4.7 | 0.614 |
| Smoker | 45 (26.2) | 12 (21.4) | 0.594 |
| Diabetes | 32 (18.7) | 18 (32.1) | 0.042 |
| Arterial hypertension | 98 (57) | 32 (57.1) | >0.999 |
| Pulmonary hypertension | 14 (8.1) | 7 (12.5) | 0.424 |
| Prior cardiothoracic surgery | 101 (58.4) | 44 (78.6) | 0.007 |
| LVAD | 78 (45.1) | 37 (66.1) | 0.009 |
| ICM | 73 (42.4) | 25 (44.6) | 0.877 |
| DCM | 81 (47.1) | 28 (50) | 0.759 |
| ARVC | 7 (4.1) | 1 (1.8) | 0.683 |
| RCM | 0 (0) | 1 (1.8) | 0.246 |
| HCM | 3 (1.7) | 1 (1.8) | >0.999 |
| Myocarditis | 2 (1.2) | 0 (0) | >0.999 |
| Preoperative dialysis | 10 (5.8) | 2 (3.7) | 0.736 |
| IMPACT score | 8.1 ± 4.7 | 9.4 ± 4.1 | 0.096 |
| Albumin (g/dl) | 3.9 ± 0.7 | 3.7 ± 0.7 | 0.185 |
| MELD score | 14.3 ± 7.6 | 13.7 ± 6.5 | 0.652 |
| Donor characteristics | |||
| Male sex | 98 (56.6) | 28 (50) | 0.441 |
| Age (years) | 43.1 ± 12.2 | 44.3 ± 12.4 | 0.526 |
| BMI (kg/m²) | 26.1 ± 4.9 | 26.0 ± 3.7 | 0.945 |
| Cardiopulmonary resuscitation | 53 (30.6) | 15 (26.8) | 0.618 |
| Intraoperative characteristics (min) | |||
| Duration of surgery | 401 ± 95 | 490 ± 148 | <0.001 |
| Duration of CPB | 240 ± 57 | 294 ± 94 | <0.001 |
| Total ischaemia time | 212 ± 48 | 220 ± 51 | 0.330 |
| PRBC (l) | 2.9 ± 2.4 | 4.9 ± 3.2 | <0.001 |
| Platelets (l) | 1.0 ± 0.8 | 1.5 ± 1.1 | 0.001 |
| FFP (l) | 1.5 ± 1.6 | 1.9 ± 2.3 | 0.218 |
| Postoperative laboratory values | |||
| Creatinine (mg/dl) | 1.5 ± 0.8 | 1.3 ± 0.5 | 0.230 |
| Bilirubin (mg/dl) | 2.6 ± 1.9 | 2.5 ± 1.3 | 0.576 |
| INR | 1.2 ± 1.9 | 1.3 ± 2.2 | 0.280 |
| Albumin (g/dl) | 3.5 ± 0.4 | 2.4 ± 0.4 | <0.001 |
| Postoperative characteristics | |||
| ECMO | 33 (19.2) | 28 (50) | <0.001 |
| Renal replacement therapy | 90 (57.7) | 35 (62.5) | 0.635 |
| MELD score | 14.6 ± 5.3 | 14.3 ± 5.1 | 0.711 |
| Albumin substitution within first 24 h | 47 (28.7) | 29 (54.7) | 0.001 |
| Days in ICU | 24 ± 25 | 25 ± 26 | 0.677 |
| Duration of mechanical ventilation (hours) | 110 ± 167 | 223 ± 216 | 0.001 |
| 30-day mortality | 6 (3.5) | 12 (21.4) | <0.001 |
| 1-year mortality | 18 (10.4) | 22 (39.3) | <0.001 |
| . | Albumin ≥3 g/dl (N = 173) . | Albumin <3 g/dl (N = 56) . | P-value . |
|---|---|---|---|
| Preoperative recipient characteristics | |||
| Male sex | 129 (74.6) | 38 (67.9) | 0.387 |
| Age (years) | 55.1 ± 10.8 | 55.3 ± 11 | 0.904 |
| BMI (kg/m2) | 25.8 ± 4.5 | 25.4 ± 4.7 | 0.614 |
| Smoker | 45 (26.2) | 12 (21.4) | 0.594 |
| Diabetes | 32 (18.7) | 18 (32.1) | 0.042 |
| Arterial hypertension | 98 (57) | 32 (57.1) | >0.999 |
| Pulmonary hypertension | 14 (8.1) | 7 (12.5) | 0.424 |
| Prior cardiothoracic surgery | 101 (58.4) | 44 (78.6) | 0.007 |
| LVAD | 78 (45.1) | 37 (66.1) | 0.009 |
| ICM | 73 (42.4) | 25 (44.6) | 0.877 |
| DCM | 81 (47.1) | 28 (50) | 0.759 |
| ARVC | 7 (4.1) | 1 (1.8) | 0.683 |
| RCM | 0 (0) | 1 (1.8) | 0.246 |
| HCM | 3 (1.7) | 1 (1.8) | >0.999 |
| Myocarditis | 2 (1.2) | 0 (0) | >0.999 |
| Preoperative dialysis | 10 (5.8) | 2 (3.7) | 0.736 |
| IMPACT score | 8.1 ± 4.7 | 9.4 ± 4.1 | 0.096 |
| Albumin (g/dl) | 3.9 ± 0.7 | 3.7 ± 0.7 | 0.185 |
| MELD score | 14.3 ± 7.6 | 13.7 ± 6.5 | 0.652 |
| Donor characteristics | |||
| Male sex | 98 (56.6) | 28 (50) | 0.441 |
| Age (years) | 43.1 ± 12.2 | 44.3 ± 12.4 | 0.526 |
| BMI (kg/m²) | 26.1 ± 4.9 | 26.0 ± 3.7 | 0.945 |
| Cardiopulmonary resuscitation | 53 (30.6) | 15 (26.8) | 0.618 |
| Intraoperative characteristics (min) | |||
| Duration of surgery | 401 ± 95 | 490 ± 148 | <0.001 |
| Duration of CPB | 240 ± 57 | 294 ± 94 | <0.001 |
| Total ischaemia time | 212 ± 48 | 220 ± 51 | 0.330 |
| PRBC (l) | 2.9 ± 2.4 | 4.9 ± 3.2 | <0.001 |
| Platelets (l) | 1.0 ± 0.8 | 1.5 ± 1.1 | 0.001 |
| FFP (l) | 1.5 ± 1.6 | 1.9 ± 2.3 | 0.218 |
| Postoperative laboratory values | |||
| Creatinine (mg/dl) | 1.5 ± 0.8 | 1.3 ± 0.5 | 0.230 |
| Bilirubin (mg/dl) | 2.6 ± 1.9 | 2.5 ± 1.3 | 0.576 |
| INR | 1.2 ± 1.9 | 1.3 ± 2.2 | 0.280 |
| Albumin (g/dl) | 3.5 ± 0.4 | 2.4 ± 0.4 | <0.001 |
| Postoperative characteristics | |||
| ECMO | 33 (19.2) | 28 (50) | <0.001 |
| Renal replacement therapy | 90 (57.7) | 35 (62.5) | 0.635 |
| MELD score | 14.6 ± 5.3 | 14.3 ± 5.1 | 0.711 |
| Albumin substitution within first 24 h | 47 (28.7) | 29 (54.7) | 0.001 |
| Days in ICU | 24 ± 25 | 25 ± 26 | 0.677 |
| Duration of mechanical ventilation (hours) | 110 ± 167 | 223 ± 216 | 0.001 |
| 30-day mortality | 6 (3.5) | 12 (21.4) | <0.001 |
| 1-year mortality | 18 (10.4) | 22 (39.3) | <0.001 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BMI, body mass index; CPB, cardiopulmonary bypass; DCM, dilative cardiomyopathy; ECMO; extracorporeal membrane oxygenation; FFP, fresh frozen plasma; HCM, hypertrophic cardiomyopathy; ICM, ischaemic cardiomyopathy; IMPACT, risk index for mortality prediction after cardiac transplantation; ICU, intensive care unit; INR, international normalized ratio; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RCM, restrictive cardiomyopathy [11].
Significant results are marked in bold.
Pre- and postoperative serum albumin levels in survivors and non-survivors
There was no significant difference in preoperative serum albumin levels between survivors and non-survivors (survivors: 3.9 ± 0.7 g/dl vs non-survivors: 3.7 ± 0.7 g/dl, P = 0.084). Postoperative serum albumin levels were significantly lower in patients who died in the first year after HTX (survivors: 3.3 ± 0.6 g/dl vs non-survivors: 2.8 ± 0.6 g/dl, P < 0.001) (Fig. 1).
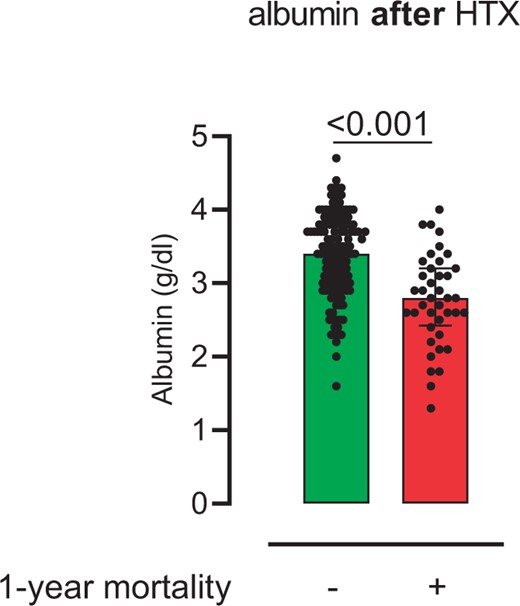
Postoperative serum albumin levels. The figure shows postoperative serum albumin levels after heart transplantation in survivors compared with non-survivors 1-year follow-up (survivors: 3.3 ± 0.6 g/dl vs non-survivors: 2.8 ± 0.6 g/dl; P < 0.001)
Discrimination and association of postoperative albumin and 1-year mortality
In ROC analysis, postoperative albumin levels showed a significant discrimination for 1-year mortality with an area under the curve (AUC) of 0.75 and 95% CI of 0.66–0.83. Youden index determined a cutoff of 2.95 g/dl for postoperative albumin levels. In a univariate logistic regression model, there was a significant association between postoperative serum albumin values and 1-year mortality (OR: 4.54, 95% CI: 2.34–8.78, P≤0.001). In Kaplan–Meier analysis, patients with postoperative albumin levels below cutoff showed lower survival rates as compared to controls (hazard ratio 7.95, 95% CI: 3.7–17.1, P < 0.001). Of note, preoperative hypoalbuminaemia occurred in 19 patients (8%) and was not associated with the incidence of postoperative hypoalbuminaemia. After adjustment for 13 covariables in multivariate logistic regression analysis, only postoperative albumin and donor age remained independently associated with 1-year mortality (postoperative albumin—OR: 4.76, 95% CI: 1.94–11.67, P = 0.001 and donor age—OR: 1.11, 95% CI: 1.05–1.17, P≤0.001] (Figs 2 and 3, Table 2).
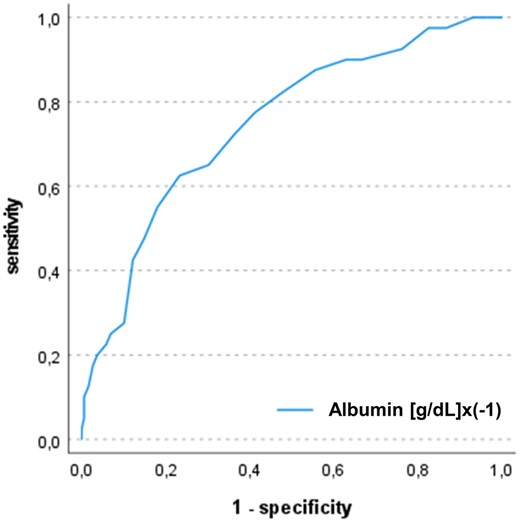
Receiver operating characteristic curves of postoperative albumin levels and 1-year mortality. The figure shows the ROC curves for association of postoperative albumin levels with 1-year mortality after heart transplantation. The AUC was 0.75 (95% CI: 0.66–0.83)
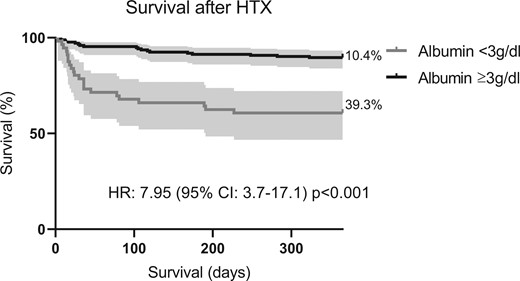
Postoperative survival of patients based on albumin levels. The figure shows the Kaplan–Meier curves of patients after HTX classified by postoperative albumin levels above and below cutoff of 3 g/dl. Survival in patients with postoperative albumin levels >3 g/dl was higher as compared to controls (HR 7.95, 95% CI: 3.7–17.1; P<0.001)
Multivariate binary logistic regression for the association of postoperative albumin and 1-year mortality
| Parameter . | Adjusted odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Albumin (g/dl) × (−1) | 4.76 | 1.94–11.67 | 0.001 |
| IMPACT | 1.04 | 0.91–1.19 | 0.549 |
| Postoperative MELD | 1.05 | 0.94–1.17 | 0.424 |
| Recipient diabetes | 1.09 | 0.31–3.78 | 0.892 |
| Prior LVAD | 0.86 | 0.20–3.77 | 0.838 |
| Prior cardiothoracic surgery | 1.50 | 0.31–7.33 | 0.618 |
| Donor age (years) | 1.11 | 1.05–1.17 | <0.001 |
| Duration of surgery (min) | 0.99 | 0.98–0.99 | 0.015 |
| Duration of CPB (min) | 1.01 | 0.99–1.02 | 0.221 |
| mechanical ventilation (h) | 1.00 | 1.00–1.01 | 0.073 |
| Postoperative RRT | 1.78 | 0.47–6.68 | 0.396 |
| Postoperative ECMO | 1.96 | 0.54–7.07 | 0.305 |
| PRBC transfusion (ml) | 1.00 | 1.00–1.00 | 0.774 |
| Platelet transfusion (ml) | 1.00 | 1.00–1.00 | 0.154 |
| Parameter . | Adjusted odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Albumin (g/dl) × (−1) | 4.76 | 1.94–11.67 | 0.001 |
| IMPACT | 1.04 | 0.91–1.19 | 0.549 |
| Postoperative MELD | 1.05 | 0.94–1.17 | 0.424 |
| Recipient diabetes | 1.09 | 0.31–3.78 | 0.892 |
| Prior LVAD | 0.86 | 0.20–3.77 | 0.838 |
| Prior cardiothoracic surgery | 1.50 | 0.31–7.33 | 0.618 |
| Donor age (years) | 1.11 | 1.05–1.17 | <0.001 |
| Duration of surgery (min) | 0.99 | 0.98–0.99 | 0.015 |
| Duration of CPB (min) | 1.01 | 0.99–1.02 | 0.221 |
| mechanical ventilation (h) | 1.00 | 1.00–1.01 | 0.073 |
| Postoperative RRT | 1.78 | 0.47–6.68 | 0.396 |
| Postoperative ECMO | 1.96 | 0.54–7.07 | 0.305 |
| PRBC transfusion (ml) | 1.00 | 1.00–1.00 | 0.774 |
| Platelet transfusion (ml) | 1.00 | 1.00–1.00 | 0.154 |
CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; IMPACT, risk index for mortality prediction after cardiac transplantation; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RRT, renal replacement therapy [11].
Multivariate binary logistic regression for the association of postoperative albumin and 1-year mortality
| Parameter . | Adjusted odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Albumin (g/dl) × (−1) | 4.76 | 1.94–11.67 | 0.001 |
| IMPACT | 1.04 | 0.91–1.19 | 0.549 |
| Postoperative MELD | 1.05 | 0.94–1.17 | 0.424 |
| Recipient diabetes | 1.09 | 0.31–3.78 | 0.892 |
| Prior LVAD | 0.86 | 0.20–3.77 | 0.838 |
| Prior cardiothoracic surgery | 1.50 | 0.31–7.33 | 0.618 |
| Donor age (years) | 1.11 | 1.05–1.17 | <0.001 |
| Duration of surgery (min) | 0.99 | 0.98–0.99 | 0.015 |
| Duration of CPB (min) | 1.01 | 0.99–1.02 | 0.221 |
| mechanical ventilation (h) | 1.00 | 1.00–1.01 | 0.073 |
| Postoperative RRT | 1.78 | 0.47–6.68 | 0.396 |
| Postoperative ECMO | 1.96 | 0.54–7.07 | 0.305 |
| PRBC transfusion (ml) | 1.00 | 1.00–1.00 | 0.774 |
| Platelet transfusion (ml) | 1.00 | 1.00–1.00 | 0.154 |
| Parameter . | Adjusted odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Albumin (g/dl) × (−1) | 4.76 | 1.94–11.67 | 0.001 |
| IMPACT | 1.04 | 0.91–1.19 | 0.549 |
| Postoperative MELD | 1.05 | 0.94–1.17 | 0.424 |
| Recipient diabetes | 1.09 | 0.31–3.78 | 0.892 |
| Prior LVAD | 0.86 | 0.20–3.77 | 0.838 |
| Prior cardiothoracic surgery | 1.50 | 0.31–7.33 | 0.618 |
| Donor age (years) | 1.11 | 1.05–1.17 | <0.001 |
| Duration of surgery (min) | 0.99 | 0.98–0.99 | 0.015 |
| Duration of CPB (min) | 1.01 | 0.99–1.02 | 0.221 |
| mechanical ventilation (h) | 1.00 | 1.00–1.01 | 0.073 |
| Postoperative RRT | 1.78 | 0.47–6.68 | 0.396 |
| Postoperative ECMO | 1.96 | 0.54–7.07 | 0.305 |
| PRBC transfusion (ml) | 1.00 | 1.00–1.00 | 0.774 |
| Platelet transfusion (ml) | 1.00 | 1.00–1.00 | 0.154 |
CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; IMPACT, risk index for mortality prediction after cardiac transplantation; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RRT, renal replacement therapy [11].
Improvement of risk prediction models by postoperative albumin
We analysed if risk prediction of either IMPACT or MELD score for 1-year mortality could be improved by the addition of postoperative albumin levels to the prognostic models (based on logistic regression). The NRI for the model including IMPACT and albumin was 15.87% (95% CI: 9.43–23.53) for non-events and 5% (95% CI: 1.64–11.28) for events. The NRI for the model including MELD and albumin was 4.23% (95% CI: 1.64–9.93) for non-events and 25% (95% CI: 16.88–34.66) for events. The assessment of NARI showed that both models including postoperative albumin were able to detect 139/1000 and 78/1000 patients more at risk for 1-year mortality, respectively. ROC analysis showed that the models including postoperative albumin levels had significantly higher AUC as compared to the two baseline models only including IMPACT score (IMPACT score—AUC = 0.65, 95% CI: 0.57–0.74; IMPACT score with albumin—AUC = 0.77, 95% CI: 0.70–0.84; difference between areas: 0.12, 95% CI: 0.02–0.21, P = 0.016) or MELD score (MELD score—AUC = 0.60, 95% CI: 0.50–0.70; MELD score with albumin—AUC = 0.78, 95% CI: 0.70–0.85; difference between areas: 0.17, 95% CI: 0.06–0.29, P = 0.002) (Fig. 4, Supplementary Tables S2 and S3).
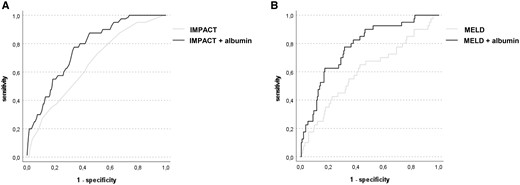
Receiver operating characteristic curves of two different prediction models for 1-year mortality including postoperative albumin. ROC curves using the IMPACT score (A) or the MELD score (B) alone compared to models which added postoperative albumin levels (dependent variable: 1-year mortality). Models including postoperative albumin showed better discrimination for 1-year mortality (IMPACT score—AUC = 0.65, 95% CI: 0.57–0.74 vs IMPACT score with albumin—AUC = 0.77, 95% CI: 0.70–0.84; difference between areas: 0.12, 95% CI: 0.02–0.21, P = 0.016; MELD score—AUC = 0.60, 95% CI: 0.50–0.70 vs. MELD score with albumin—AUC = 0.78, 95% CI: 0.70–0.85; difference between areas: 0.17, 95% CI: 0.06–0.29, P = 0.002)
Association of postoperative albumin with DAOH
In univariate analysis, DAOH of patients with postoperative albumin levels ≥3 g/dl were significantly higher than DAOH of patients with albumin levels <3 g/dl [albumin—above cut-off 308 (271–325) days vs below cutoff 253 (0–305) days, P ≤ 0.001]. Of note, after the exclusion of patients who died during 1-year follow-up, univariate findings for DAOH remained significant in a sensitivity analysis (Supplementary Figure S2). A multivariate quantile regression model was performed in which postoperative albumin levels were independently associated with poor DAOH after adjustment for 13 covariables (postoperative albumin—coefficient: −46.97, 95% CI: −83.81 to −10.13, P = 0.013) (Table 3, Supplementary Figure S3).
Multivariate quantile regression model for the association of postoperative albumin with Days alive and out of hospital
| Parameter . | Coefficient . | Standard error . | 95% CI . | P-value . |
|---|---|---|---|---|
| Constant | 163.39 | 95.20 | −24.49 to 351.27 | 0.088 |
| Albumin (g/dl) × (−1) | −46.97 | 18.67 | −83.81 to −10.13 | 0.013 |
| MELD | −0.09 | 0.39 | −0.86–0.68 | 0.821 |
| IMPACT | −3.42 | 2.67 | −8.70 to 1.85 | 0.202 |
| Recipient diabetes | −73.40 | 25.26 | −123.24 to −23.56 | 0.004 |
| Prior LVAD | 13.75 | 30.82 | −47.07 to 74.58 | 0.656 |
| Prior cardiothoracic surgery | 18.73 | 31.40 | −43.25 to 80.70 | 0.552 |
| Donor age (years) | −2.80 | 0.81 | −4.40 to −1.20 | <0.001 |
| Duration of surgery (min) | 0.08 | 0.15 | −0.21 to 0.37 | 0.607 |
| Duration of CPB (min) | 0.21 | 0.23 | −0.24 to 0.67 | 0.355 |
| mechanical ventilation (h) | −0.14 | 0.07 | −0.28 to 0.01 | 0.056 |
| Postoperative RRT | −120.91 | 22.59 | −165.49 to −76.34 | <0.001 |
| Postoperative ECMO | −3.60 | 29.22 | −61.26 to 54.05 | 0.902 |
| PRBC transfusion (ml) | −0.016 | 0.01 | −0.03 to −0.01 | 0.006 |
| Platelet transfusion (ml) | 0.01 | 0.02 | −0.02 to 0.05 | 0.405 |
| Parameter . | Coefficient . | Standard error . | 95% CI . | P-value . |
|---|---|---|---|---|
| Constant | 163.39 | 95.20 | −24.49 to 351.27 | 0.088 |
| Albumin (g/dl) × (−1) | −46.97 | 18.67 | −83.81 to −10.13 | 0.013 |
| MELD | −0.09 | 0.39 | −0.86–0.68 | 0.821 |
| IMPACT | −3.42 | 2.67 | −8.70 to 1.85 | 0.202 |
| Recipient diabetes | −73.40 | 25.26 | −123.24 to −23.56 | 0.004 |
| Prior LVAD | 13.75 | 30.82 | −47.07 to 74.58 | 0.656 |
| Prior cardiothoracic surgery | 18.73 | 31.40 | −43.25 to 80.70 | 0.552 |
| Donor age (years) | −2.80 | 0.81 | −4.40 to −1.20 | <0.001 |
| Duration of surgery (min) | 0.08 | 0.15 | −0.21 to 0.37 | 0.607 |
| Duration of CPB (min) | 0.21 | 0.23 | −0.24 to 0.67 | 0.355 |
| mechanical ventilation (h) | −0.14 | 0.07 | −0.28 to 0.01 | 0.056 |
| Postoperative RRT | −120.91 | 22.59 | −165.49 to −76.34 | <0.001 |
| Postoperative ECMO | −3.60 | 29.22 | −61.26 to 54.05 | 0.902 |
| PRBC transfusion (ml) | −0.016 | 0.01 | −0.03 to −0.01 | 0.006 |
| Platelet transfusion (ml) | 0.01 | 0.02 | −0.02 to 0.05 | 0.405 |
CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; IMPACT, risk index for mortality prediction after cardiac transplantation; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RRT, renal replacement therapy [11].
Multivariate quantile regression model for the association of postoperative albumin with Days alive and out of hospital
| Parameter . | Coefficient . | Standard error . | 95% CI . | P-value . |
|---|---|---|---|---|
| Constant | 163.39 | 95.20 | −24.49 to 351.27 | 0.088 |
| Albumin (g/dl) × (−1) | −46.97 | 18.67 | −83.81 to −10.13 | 0.013 |
| MELD | −0.09 | 0.39 | −0.86–0.68 | 0.821 |
| IMPACT | −3.42 | 2.67 | −8.70 to 1.85 | 0.202 |
| Recipient diabetes | −73.40 | 25.26 | −123.24 to −23.56 | 0.004 |
| Prior LVAD | 13.75 | 30.82 | −47.07 to 74.58 | 0.656 |
| Prior cardiothoracic surgery | 18.73 | 31.40 | −43.25 to 80.70 | 0.552 |
| Donor age (years) | −2.80 | 0.81 | −4.40 to −1.20 | <0.001 |
| Duration of surgery (min) | 0.08 | 0.15 | −0.21 to 0.37 | 0.607 |
| Duration of CPB (min) | 0.21 | 0.23 | −0.24 to 0.67 | 0.355 |
| mechanical ventilation (h) | −0.14 | 0.07 | −0.28 to 0.01 | 0.056 |
| Postoperative RRT | −120.91 | 22.59 | −165.49 to −76.34 | <0.001 |
| Postoperative ECMO | −3.60 | 29.22 | −61.26 to 54.05 | 0.902 |
| PRBC transfusion (ml) | −0.016 | 0.01 | −0.03 to −0.01 | 0.006 |
| Platelet transfusion (ml) | 0.01 | 0.02 | −0.02 to 0.05 | 0.405 |
| Parameter . | Coefficient . | Standard error . | 95% CI . | P-value . |
|---|---|---|---|---|
| Constant | 163.39 | 95.20 | −24.49 to 351.27 | 0.088 |
| Albumin (g/dl) × (−1) | −46.97 | 18.67 | −83.81 to −10.13 | 0.013 |
| MELD | −0.09 | 0.39 | −0.86–0.68 | 0.821 |
| IMPACT | −3.42 | 2.67 | −8.70 to 1.85 | 0.202 |
| Recipient diabetes | −73.40 | 25.26 | −123.24 to −23.56 | 0.004 |
| Prior LVAD | 13.75 | 30.82 | −47.07 to 74.58 | 0.656 |
| Prior cardiothoracic surgery | 18.73 | 31.40 | −43.25 to 80.70 | 0.552 |
| Donor age (years) | −2.80 | 0.81 | −4.40 to −1.20 | <0.001 |
| Duration of surgery (min) | 0.08 | 0.15 | −0.21 to 0.37 | 0.607 |
| Duration of CPB (min) | 0.21 | 0.23 | −0.24 to 0.67 | 0.355 |
| mechanical ventilation (h) | −0.14 | 0.07 | −0.28 to 0.01 | 0.056 |
| Postoperative RRT | −120.91 | 22.59 | −165.49 to −76.34 | <0.001 |
| Postoperative ECMO | −3.60 | 29.22 | −61.26 to 54.05 | 0.902 |
| PRBC transfusion (ml) | −0.016 | 0.01 | −0.03 to −0.01 | 0.006 |
| Platelet transfusion (ml) | 0.01 | 0.02 | −0.02 to 0.05 | 0.405 |
CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; IMPACT, risk index for mortality prediction after cardiac transplantation; LVAD, left ventricular assist device; MELD, model for end-stage liver disease; PRBC, packed red blood cells; RRT, renal replacement therapy [11].
DISCUSSION
This study revealed an independent association between low postoperative serum albumin levels and increased 1-year mortality as well as poor DAOH after HTX. Additionally, risk prediction for mortality by IMPACT score or MELD score was significantly improved when postoperative albumin was added to the models.
The role of preoperative serum albumin in patients undergoing HTX
Previous investigations described that preoperative serum albumin levels were associated with unfavourable outcome after HTX. Kato et al. [8] showed in a retrospective analysis of 822 HTX patients that preoperative serum albumin levels <3.5 g/dl were associated with increased 1-year mortality. Additionally, the authors used the same cutoff for analysis of data of 13 671 HTX patients from the united network of organ sharing database. Again preoperative serum albumin levels below cutoff were associated with poor 1-year survival in a parametric survival model [15]. Other studies showed similar associations between preoperative albumin levels and 1-year mortality after HTX [6]. Previously preoperative albumin levels were also included in risk scores to assess postoperative survival after HTX. In this context, Schulze et al. [16] proposed the CARRS score including prior stroke, albumin, retransplantation, glomerular filtration rate and prior thoracic surgeries. This score showed a good discriminative ability with an ROC-AUC of 0.77. Another study by Chokshi et al. [7] investigated the role of liver dysfunction on outcome after HTX. Therefore, preoperative serum albumin values were added to the MELD score. Higher modified MELD score and lower albumin values were associated with poor survival.
The role of postoperative serum albumin after HTX
The role of postoperative serum albumin levels after HTX has not been investigated until now. Our current data suggest that low postoperative serum albumin values are strongly associated with poor outcome regarding 1-year survival and DAOH after HTX. This is in line with the findings of previous studies for cardiac and non-cardiac surgery [2, 17]. Berbel-Franco et al. [1] showed a strong association of low postoperative serum albumin levels (within the first 24 h at ICU) with in-hospital and long-term mortality in 2818 cardiac surgery patients. Interestingly risk for mortality was not linear but showed a progressively steeper increase when serum albumin levels were below 3.0 g/dl which goes in line with the cutoff from our ROC analysis [1]. However, heart transplant patients were not included in the above-mentioned study. It is well known that postoperative serum albumin mimics intra- and postoperative course as it is influenced by numerous factors like increased inflammatory response resulting from surgical trauma, long CPB times or ischaemia reperfusion injury and postoperative liver dysfunction, making it a suitable biomarker for postoperative prognosis [1, 2, 18–20]. Within our patient cohort, we could show that patients with serum albumin levels below the cutoff of 3 g/dl had significantly longer durations of surgery and CPB, as well as significantly higher transfusion requirements, supporting this relationship. This might be the reason why postoperative albumin showed a strong independent association with mortality and DAOH in our cohort. Additionally, we adjusted our findings for postoperative MELD score, which shows that low albumin values are not solely influenced by postoperative liver dysfunction and impaired synthesis after HTX. Although, preoperative factors are more useful for risk prediction than postoperative variables, postoperative albumin can be used for early risk re-assessment after HTX, as a widely available biomarker in clinical practice. If low serum albumin levels themselves have negative effects on patient’s prognosis after HTX is unclear, but could be an interesting therapeutic approach for upcoming investigations. Targeting postoperative serum albumin levels >3.0 g/dl after HTX by substitution of human albumin might be a feasible approach and should be investigated in prospective trials, as data concerning this topic are lacking. However, as in our cohort patients with low albumin levels had higher rates of albumin substitution but also higher mortality, benefit of this therapeutic approach is questionable and cannot be drawn from our data, as negative effects should also be considered.
Strengths and limitations
This work has several limitations that we are aware of. First, this was a retrospective single-centre study with a limited number of patients and events. However, data were obtained from the local HTX database and we had a full dataset regarding the endpoints and covariates in 95% of all patients. As our patients are closely connected to our centre we could provide not only data on mortality but also DAOH which represents a more patient-centred outcome. Nevertheless, we cannot be sure if patients were hospitalized externally during 1-year follow-up, although the risk is very limited. Finally, we had to assess the postoperative albumin values during the first 12 h after arrival ICU, as albumin was not measured systematically at arrival in ICU in all patients. Regarding our statistical approach, all analyses were exploratory in nature and 95% CIs were not adjusted for multiple comparisons, hence inferences drawn from them may not be reproducible.
CONCLUSIONS
Early postoperative hypoalbuminaemia <3.0 g/dl is associated with 1-year mortality and poor DAOH after HTX. This makes postoperative albumin a suitable marker for early risk re-assessment after HTX.
Abstracts including data from the same data set have been submitted to the annual meeting 2023 of the European Association for Cardio-Thoracic Surgery (EACTS; no presentation) and to the annual meeting 2023 of the European Society of Anaesthesiology and Intensive Care (ESAIC; oral presentation).
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
FUNDING
This research received no external funding.
Conflict of interest: None declared.
DATA AVAILABILITY
All relevant data are included in the present manuscript or in the supplements. Raw data are available upon reasonable request by the first author R.M.
Author contributions
René M’Pembele: Conceptualization; Formal analysis; Investigation; Writing—original draft. Sebastian Roth: Conceptualization; Investigation; Writing—original draft; Writing—review & editing. Freya Jenkins: Investigation; Writing—review & editing. Vincent Hettlich: Conceptualization; Investigation; Writing—review & editing. Anthony Nucaro: Data curation; Investigation; Writing—review & editing. Alexandra Stroda: Investigation; Writing—review & editing. Theresa Tenge: Investigation; Writing—review & editing. Amin Polzin: Investigation; Writing—review & editing. Bedri Ramadani: Investigation; Writing—review & editing. Giovanna Lurati Buse: Investigation; Methodology; Supervision; Writing—review & editing. Hug Aubin: Investigation; Writing—review & editing. Artur Lichtenberg: Investigation; Supervision; Writing—review & editing. Ragnar Huhn: Investigation; Supervision; Writing—review & editing. Udo Boeken: Conceptualization; Investigation; Supervision; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Thomas Martens and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- heart transplantation
- cardiac surgery procedures
- albumins
- biological markers
- germany
- hypoalbuminemia
- hospitals, university
- intensive care unit
- preoperative care
- roc curve
- surgical procedures, operative
- liver
- mortality
- serum albumin
- surgery specialty
- liver function
- stratification
- surrogate endpoints
- orthotopic allotransplant of heart





