-
PDF
- Split View
-
Views
-
Cite
Cite
Jinmiao Chen, Minzhi Lv, Jiahui Fu, Chen He, Yingqiang Guo, Liang Tao, Xinmin Zhou, Tianxiang Gu, Krzysztof Bartus, Lai Wei, Tao Hong, Chunsheng Wang, Five-year outcomes of surgical aortic valve replacement with a novel bovine pericardial bioprosthesis, Interdisciplinary CardioVascular and Thoracic Surgery, Volume 38, Issue 1, January 2024, ivad209, https://doi.org/10.1093/icvts/ivad209
Close - Share Icon Share
Abstract
The short-term performance of the Cingular bovine pericardial aortic valve was proven. This study evaluated its 5-year safety and haemodynamic outcomes.
It enrolled 148 patients who underwent surgical aortic valve replacement with the Cingular bovine pericardial aortic valve between March 2016 and October 2017 in 5 clinical centres in China. Safety and haemodynamic outcomes were followed up to 5 years. The incidence of all-cause mortality, structural valve deterioration and reintervention was estimated by Kaplan–Meier analysis.
The mean age of patients was 67.7 [standard deviation (SD) 5.1] years, and 36.5% of patients were female. The mean follow-up was 5.3 (SD 1.2) years. Five-year freedom from all-cause mortality, structural valve deterioration and all-cause reintervention were 91.2%, 100% and 99.3%, respectively. At 5 years, the mean gradient and effective orifice area of all sizes combined were 14.0 (SD 5.5) mmHg and 1.9 (SD 0.3) cm2, respectively. For 19- and 21-mm sizes of aortic prostheses, the mean gradients and effective orifice area at 5 years were 17.5 (SD 7.0) mmHg and 1.6 (SD 0.2) cm2 and 13.7 (SD 6.7) mmHg and 1.8 (SD 0.3) cm2, respectively. The incidence of moderate or severe patient–prosthesis mismatch was 4.1% and 0.0% patients at 5 years, respectively.
The 5-year safety and haemodynamic outcomes of Cingular bovine pericardial aortic valve are encouraging. Longer-term follow-up is warranted to assess its true durability.
INTRODUCTION
Despite advances in transcatheter aortic valve replacement, surgical aortic valve replacement (SAVR) is still the standard of care for patients with aortic valve disease. With the development of transcatheter valve-in-valve technique, tissue valves are becoming more and more an attractive clinical option in relatively young patients [1]. The selection of surgical or transcatheter valves depends on safety and haemodynamic performance. Thus, it is critical to have the data on the contemporary surgical bioprosthetic valve as the benchmark for the transcatheter valve.
The Cingular bovine pericardial valve (Cingular Biotech, Shanghai, China) whose design is based on the Carpentier-Edwards Perimount valve (Edwards Lifesciences, Irvine, CA, USA) incorporates certain optimizations and innovations [2]. The performance of this study valve was evaluated in juvenile sheep [3]. The 1- and 2-year follow-up outcomes of this bioprosthesis were reported to be safe and associated with good haemodynamic performance [4, 5]. We herein reported the 5-year safety and haemodynamic outcomes of the Cingular bovine pericardial aortic valve in 148 patients who underwent SAVR.
METHODS AND MATERIALS
Ethics statement
The study protocol was reviewed and approved by Zhongshan Hospital, Fudan University (number 2015-69R, date of approval 3 December 2015), West China Hospital of Sichuan University (number 2016–003, date of approval 28 January 2016), Wuhan Asia Heart Hospital (number 2016-YXGCP-001, date of approval 19 January 2016), The Second Xiangya Hospital of Central South University (number 2016-006, date of approval 13 May 2016) and The First Hospital of China Medical University (number 2016QL004, date of approval 26 August 2016). The patients provided their written informed consent to participate in this study.
Study design
A prospective, multicentre, single-arm clinical trial was conducted to assess the safety and effectiveness of the Cingular bovine pericardial valve in both aortic and mitral positions (Clinical Trial Number: NCT02755220). Details regarding patient inclusion and exclusion criteria have been reported previously [4]. In order to compare with other contemporary aortic bioprostheses [6–9], we herein reported the 5-year outcomes of 148 patients who underwent SAVR.
Study device and surgical aortic valve replacement
Based on the original design of the Carpentier-Edwards Perimount valve, the Cingular bovine pericardial valve incorporates new optimizations and innovations being a three-layer stent structure, improved leaflet matching and redesign of the sewing ring. The details of optimizations and innovations were described previously [2]. These modifications were likely to improve the stability and durability of valve structure, reduce the possibility and the extent of triangular leaflet opening and increase the effective orifice area (EOA) [2].
SAVR was performed using either median sternotomy or upper hemisternotomy at the discretion of the surgeons. Details of the used surgical technique were previously reported [4, 5]. Postoperative anticoagulation therapy with warfarin was adopted to achieve an International Normalized Ratio target (range 2.0–3.0) for 3–6 months.
Study end points
Patients were clinically assessed at 1 month, 6 months and annually post-implant. Safety end points included all-cause mortality, structural valve deterioration (SVD) and reintervention [10, 11]. Based on patient-level source documents acquired from each centre, an independent Clinical Events Committee assessed all safety end-point events. According to the guideline, the definition of SVD was dysfunction or deterioration of the operated valve (exclusive of infection or thrombosis), as determined by reoperation, autopsy, or clinical investigation [11]. The SVD refers to changes intrinsic to the valve, such as wear, fracture, poppet escape, calcification, leaflet tear, stent creep and suture line disruption of components of a prosthetic valve.
Transthoracic echocardiography was performed at 1 month, 1 year, 2 years and 5 years after implant. Haemodynamic end points included mean transvalvular pressure gradient and EOA. An independent echocardiographic core laboratory (Department of Echocardiography, Zhongshan Hospital, Fudan University) assessed the haemodynamic performance data. The mean gradient across the bioprosthetic valve was calculated using the modified Bernoulli equation. In order to determine whether patient–prosthesis mismatch (PPM) existed, the EOA was calculated using the continuity equation and indexed to body surface area. PPM was deemed not clinically significant if the indexed EOA was larger than 0.85 cm2/m2; moderate if it was between 0.65 and 0.85 cm2/m2; and severe if it was lower than 0.65 cm2/m2 [12].
Data management and statistics
The trial was overseen by an independent Contract Research Organization. The statistical analysis and data management were under the supervision of the National Center for Cardiovascular Diseases in Beijing, China. Continuous variables were summarized as mean and standard deviation (SD) or median and interquartile range (IQR) [13]. Categorical variables were presented as the number and percentage of subjects in each category. Early safety events were those that occurred within a period of 30 days after the implant and were reported as the number of events divided by the number of total implanted patients. The Kaplan–Meier method was used to estimate the rate and 95% confidence interval (CI) of freedom from death, SVD and reintervention at 5 years after valve implantation [14]. The competing risk analyses were also performed on SVD and reintervention accounting for the competing risk of death, respectively. The Mann–Kendall trend test was used to test the increasing or decreasing trend of the haemodynamic performance after valve implantation. Complete case analysis was performed to address missing data for echocardiographic outcomes [15]. Two-sided P-value 0.05 was considered statistically significant. Tests were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Baseline characteristics
This report included a total of 148 patients who underwent SAVR with the Cingular bovine pericardial aortic valve at 5 clinical centres in China. Table 1 summarizes the patient baseline characteristics. Overall, the mean age was 67.7 (SD 5.1) years with 36.5% female patients. At baseline, patients were in New York Heart Association functional class II, III and IV at 24.3%, 75.0% and 0.7%, respectively. Among comorbidities, 41.9% of patients had systemic hypertension, 17.6% had chronic obstructive pulmonary disease and 4.1% had diabetes mellitus. The mean logistic European System for Cardiac Operative Risk Evaluation II and Society of Thoracic Surgeons Predicted Risk of Mortality score were 3.0 (SD 2.5)% and 1.6 (SD 1.1)%, respectively.
| Variables . | Implanted (N = 148) . |
|---|---|
| Baseline characteristics | |
| Age (years) | 67.7 (SD 5.1) |
| 60–69 | 97(65.5%) |
| 70–79 | 48(32.4%) |
| ≥80 | 3(2.0%) |
| Female | 54 (36.5%) |
| BMI (kg/m2) | 23.2 (SD 3.7) |
| NYHA functional class | |
| II | 36 (24.3%) |
| III | 111 (75.0%) |
| IV | 1 (0.7%) |
| Systemic hypertension | 62 (41.9%) |
| Coronary artery disease | 7 (4.7%) |
| COPD | 26 (17.6%) |
| Diabetes mellitus | 6 (4.1%) |
| STS predicted risk of mortality (%) | 1.6 (SD 1.1) |
| STS predicted risk of morbidity or mortality (%) | 12.5 (SD 5.4) |
| Logistic EuroSCORE II (%) | 3.0 (SD 2.5) |
| Variables . | Implanted (N = 148) . |
|---|---|
| Baseline characteristics | |
| Age (years) | 67.7 (SD 5.1) |
| 60–69 | 97(65.5%) |
| 70–79 | 48(32.4%) |
| ≥80 | 3(2.0%) |
| Female | 54 (36.5%) |
| BMI (kg/m2) | 23.2 (SD 3.7) |
| NYHA functional class | |
| II | 36 (24.3%) |
| III | 111 (75.0%) |
| IV | 1 (0.7%) |
| Systemic hypertension | 62 (41.9%) |
| Coronary artery disease | 7 (4.7%) |
| COPD | 26 (17.6%) |
| Diabetes mellitus | 6 (4.1%) |
| STS predicted risk of mortality (%) | 1.6 (SD 1.1) |
| STS predicted risk of morbidity or mortality (%) | 12.5 (SD 5.4) |
| Logistic EuroSCORE II (%) | 3.0 (SD 2.5) |
Data were presented as N (%) or mean (SD).
BMI: body mass index; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; SD: standard deviation; STS: Society of Thoracic Surgeons.
| Variables . | Implanted (N = 148) . |
|---|---|
| Baseline characteristics | |
| Age (years) | 67.7 (SD 5.1) |
| 60–69 | 97(65.5%) |
| 70–79 | 48(32.4%) |
| ≥80 | 3(2.0%) |
| Female | 54 (36.5%) |
| BMI (kg/m2) | 23.2 (SD 3.7) |
| NYHA functional class | |
| II | 36 (24.3%) |
| III | 111 (75.0%) |
| IV | 1 (0.7%) |
| Systemic hypertension | 62 (41.9%) |
| Coronary artery disease | 7 (4.7%) |
| COPD | 26 (17.6%) |
| Diabetes mellitus | 6 (4.1%) |
| STS predicted risk of mortality (%) | 1.6 (SD 1.1) |
| STS predicted risk of morbidity or mortality (%) | 12.5 (SD 5.4) |
| Logistic EuroSCORE II (%) | 3.0 (SD 2.5) |
| Variables . | Implanted (N = 148) . |
|---|---|
| Baseline characteristics | |
| Age (years) | 67.7 (SD 5.1) |
| 60–69 | 97(65.5%) |
| 70–79 | 48(32.4%) |
| ≥80 | 3(2.0%) |
| Female | 54 (36.5%) |
| BMI (kg/m2) | 23.2 (SD 3.7) |
| NYHA functional class | |
| II | 36 (24.3%) |
| III | 111 (75.0%) |
| IV | 1 (0.7%) |
| Systemic hypertension | 62 (41.9%) |
| Coronary artery disease | 7 (4.7%) |
| COPD | 26 (17.6%) |
| Diabetes mellitus | 6 (4.1%) |
| STS predicted risk of mortality (%) | 1.6 (SD 1.1) |
| STS predicted risk of morbidity or mortality (%) | 12.5 (SD 5.4) |
| Logistic EuroSCORE II (%) | 3.0 (SD 2.5) |
Data were presented as N (%) or mean (SD).
BMI: body mass index; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; SD: standard deviation; STS: Society of Thoracic Surgeons.
Procedural outcomes
All 148 patients were successfully implanted with the Cingular bovine pericardial aortic valve at the first attempt (100% technical success). In 98.0% of cases, a full sternotomy was performed, and in 2.0% of cases, an upper hemisternotomy was done. Among them, 18 patients (12.2%) were implanted with a valve of 19 mm, 36 patients (24.3%) with a 21-mm valve, 61 patients (41.2%) with a 23-mm valve and 33 patients (22.3%) with a 25-mm valve. For 148 patients, 38.5% of patients had concomitant procedure and the proportion of each concomitant procedure is reported in Table 2. The mean time of aortic cross-clamp and cardiopulmonary bypass times during surgery were 67.9 (SD 26.0) min and 99.0 (SD 31.4) min, respectively.
| Variables . | Implanted (N = 148) . |
|---|---|
| CPB time (min) | 99.0 (SD 31.4) |
| Cross-clamp time (min) | 67.9 (SD 26.0) |
| Surgical approach | |
| Median sternotomy | 145 (98.0%) |
| Minimal sternotomy | 3 (2.0%) |
| Implanted valve sizes | 22.5 (SD 1.9) |
| 19 mm | 18 (12.2%) |
| 21 mm | 36 (24.3%) |
| 23 mm | 61 (41.2%) |
| 25 mm | 33 (22.3%) |
| Concomitant procedures | |
| Tricuspid valve repair | 13 (8.8%) |
| Atrial fibrillation ablation | 9 (6.1%) |
| Mitral valve repair | 22 (14.9%) |
| Ascending aortoplasty | 14 (9.5%) |
| Bentall procedure | 15 (10.1%) |
| LVOT myectomy | 1 (0.7%) |
| ASD repair | 1 (0.7%) |
| VSD repair | 1 (0.7%) |
| Variables . | Implanted (N = 148) . |
|---|---|
| CPB time (min) | 99.0 (SD 31.4) |
| Cross-clamp time (min) | 67.9 (SD 26.0) |
| Surgical approach | |
| Median sternotomy | 145 (98.0%) |
| Minimal sternotomy | 3 (2.0%) |
| Implanted valve sizes | 22.5 (SD 1.9) |
| 19 mm | 18 (12.2%) |
| 21 mm | 36 (24.3%) |
| 23 mm | 61 (41.2%) |
| 25 mm | 33 (22.3%) |
| Concomitant procedures | |
| Tricuspid valve repair | 13 (8.8%) |
| Atrial fibrillation ablation | 9 (6.1%) |
| Mitral valve repair | 22 (14.9%) |
| Ascending aortoplasty | 14 (9.5%) |
| Bentall procedure | 15 (10.1%) |
| LVOT myectomy | 1 (0.7%) |
| ASD repair | 1 (0.7%) |
| VSD repair | 1 (0.7%) |
Data were presented as N (%) or mean (SD).
ASD: atrial septal defect; CPB: cardiopulmonary bypass; LVOT: Left ventricular outflow trace; SD: standard deviation; VSD: Ventricular septal defect.
| Variables . | Implanted (N = 148) . |
|---|---|
| CPB time (min) | 99.0 (SD 31.4) |
| Cross-clamp time (min) | 67.9 (SD 26.0) |
| Surgical approach | |
| Median sternotomy | 145 (98.0%) |
| Minimal sternotomy | 3 (2.0%) |
| Implanted valve sizes | 22.5 (SD 1.9) |
| 19 mm | 18 (12.2%) |
| 21 mm | 36 (24.3%) |
| 23 mm | 61 (41.2%) |
| 25 mm | 33 (22.3%) |
| Concomitant procedures | |
| Tricuspid valve repair | 13 (8.8%) |
| Atrial fibrillation ablation | 9 (6.1%) |
| Mitral valve repair | 22 (14.9%) |
| Ascending aortoplasty | 14 (9.5%) |
| Bentall procedure | 15 (10.1%) |
| LVOT myectomy | 1 (0.7%) |
| ASD repair | 1 (0.7%) |
| VSD repair | 1 (0.7%) |
| Variables . | Implanted (N = 148) . |
|---|---|
| CPB time (min) | 99.0 (SD 31.4) |
| Cross-clamp time (min) | 67.9 (SD 26.0) |
| Surgical approach | |
| Median sternotomy | 145 (98.0%) |
| Minimal sternotomy | 3 (2.0%) |
| Implanted valve sizes | 22.5 (SD 1.9) |
| 19 mm | 18 (12.2%) |
| 21 mm | 36 (24.3%) |
| 23 mm | 61 (41.2%) |
| 25 mm | 33 (22.3%) |
| Concomitant procedures | |
| Tricuspid valve repair | 13 (8.8%) |
| Atrial fibrillation ablation | 9 (6.1%) |
| Mitral valve repair | 22 (14.9%) |
| Ascending aortoplasty | 14 (9.5%) |
| Bentall procedure | 15 (10.1%) |
| LVOT myectomy | 1 (0.7%) |
| ASD repair | 1 (0.7%) |
| VSD repair | 1 (0.7%) |
Data were presented as N (%) or mean (SD).
ASD: atrial septal defect; CPB: cardiopulmonary bypass; LVOT: Left ventricular outflow trace; SD: standard deviation; VSD: Ventricular septal defect.
Safety outcomes
The proportion of these 148 patients whose follow-up through 5 years was complete (i.e. death, reintervention or complete 5-year clinical follow-up) was 100%. The mean follow-up time was 5.3 (SD 1.2) years, with an aggregate follow-up of 783.0 patient-years. Early all-cause mortality (<30 days) was 2.0% (3/148). At late follow-up, 16 all-cause deaths were reported, of which 3 death cases occurred beyond 5 years. Five-year freedom from all-cause mortality was 91.2% (95% CI 86.8–95.9%) (Fig. 1). There were no events of SVD in either the early or late period (Fig. 1). One reintervention occurred in a 72-year-old man implanted with a 21-mm study valve, concomitant mitral valve repair and ascending aortoplasty. On postoperative day 1687, the patient complained of shortness of breath. Transthoracic echocardiogram revealed valve thrombosis and a mean gradient of 41 mmHg and moderate regurgitation of the study valve. On postoperative day 1794, a transcatheter aortic valve-in-valve replacement was successfully performed. Five-year freedom from all-cause reintervention was 99.3% (95% CI 95.3–99.9%). Considering death as a competing risk event, the 5-year cumulative incidence of reintervention was 0.7% (95% CI 0.1–4.9%) (Supplementary Material, Fig. S1).
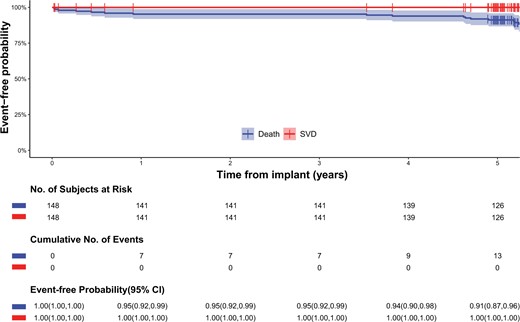
Kaplan–Meier curves for all-cause mortality and structural valve deterioration. CI: confidence interval.
| Time point . | Parameters . | All sizes . | 19 mm . | 21 mm . | 23 mm . | 25 mm . |
|---|---|---|---|---|---|---|
| 1 month | N | 145 | 17 | 36 | 60 | 32 |
| MG (mmHg) | 11.6 (SD 3.6) | 15.9 (SD 3.4) | 12.4 (SD 3.7) | 11.1 (SD 2.8) | 9.4 (SD 2.8) | |
| 11.0 (IQR 9.0–14.0) | 16.0 (IQR 14.0–18.0) | 11.9 (IQR 9.8–15.0) | 11.0 (IQR 9.3–13.1) | 9.3 (IQR 7.4–10.4) | ||
| EOA (cm2) | 2.0 (SD 0.3) | 1.6 (SD 0.3) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.8–2.2) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 2.1 (IQR 1.9–2.1) | 2.3 (IQR 2.2–2.4) | ||
| 1 year | N | 140 | 16 | 35 | 58 | 31 |
| MG (mmHg) | 12.7 (SD 4.3) | 17.1 (SD 3.6) | 13.5 (SD 4.1) | 12.5 (SD 4.3) | 10.0 (SD 3.0) | |
| 12.0 (IQR 9.9–15.0) | 17.0 (IQR 14.7–18.5) | 13.8 (IQR 11.0–16.1) | 11.6 (IQR 10.0–14.0) | 9.9 (IQR 7.8–11.4) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.1) | 1.9 (SD 0.2) | 2.2 (SD 0.2) | |
| 1.9 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 1.9 (IQR 1.8–2.0) | 2.2 (IQR 2.1–2.4) | ||
| 2 years | N | 135 | 15 | 35 | 55 | 30 |
| MG (mmHg) | 12.5 (SD 4.1) | 15.9 (SD 4.3) | 13.3 (SD 4.1) | 12.5 (SD 3.5) | 9.9 (SD 3.3) | |
| 12.0 (IQR 9.3–15.0) | 15.4 (IQR 13.0–19.5) | 13.0 (IQR 10.7–15.6) | 12.0 (IQR 10.5–14.0) | 8.5 (IQR 7.7–11.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.7 (SD 0.2) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.7 (IQR 1.6–1.9) | 2.0 (IQR 1.8–2.1) | 2.3 (IQR 2.1–2.4) | ||
| 5 years | N | 127 | 16 | 28 | 52 | 31 |
| MG (mmHg) | 14.0 (SD 5.5) | 17.5 (SD 7.0) | 13.7 (SD 6.7) | 13.6 (SD 4.7) | 13.0 (SD 4.5) | |
| 13.0 (IQR 10.0–16.0) | 15.4 (IQR 12.0–23.5) | 12.0 (IQR 10.0–15.0) | 13.0 (IQR 10.2–16.0) | 12.0 (IQR 10.0–14.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.3) | 1.9 (SD 0.3) | 2.0 (SD 0.3) | |
| 1.8 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.8 (IQR 1.7–2.0) | 1.9 (IQR 1.7–2.1) | 2.1 (IQR 1.9–2.2) | ||
| P for trenda | MG (mmHg) | 0.308 | 0.308 | 0.308 | 0.149 | 0.308 |
| EOA (cm2) | 0.308 | 1.000 | 1.000 | 0.308 | 0.308 |
| Time point . | Parameters . | All sizes . | 19 mm . | 21 mm . | 23 mm . | 25 mm . |
|---|---|---|---|---|---|---|
| 1 month | N | 145 | 17 | 36 | 60 | 32 |
| MG (mmHg) | 11.6 (SD 3.6) | 15.9 (SD 3.4) | 12.4 (SD 3.7) | 11.1 (SD 2.8) | 9.4 (SD 2.8) | |
| 11.0 (IQR 9.0–14.0) | 16.0 (IQR 14.0–18.0) | 11.9 (IQR 9.8–15.0) | 11.0 (IQR 9.3–13.1) | 9.3 (IQR 7.4–10.4) | ||
| EOA (cm2) | 2.0 (SD 0.3) | 1.6 (SD 0.3) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.8–2.2) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 2.1 (IQR 1.9–2.1) | 2.3 (IQR 2.2–2.4) | ||
| 1 year | N | 140 | 16 | 35 | 58 | 31 |
| MG (mmHg) | 12.7 (SD 4.3) | 17.1 (SD 3.6) | 13.5 (SD 4.1) | 12.5 (SD 4.3) | 10.0 (SD 3.0) | |
| 12.0 (IQR 9.9–15.0) | 17.0 (IQR 14.7–18.5) | 13.8 (IQR 11.0–16.1) | 11.6 (IQR 10.0–14.0) | 9.9 (IQR 7.8–11.4) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.1) | 1.9 (SD 0.2) | 2.2 (SD 0.2) | |
| 1.9 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 1.9 (IQR 1.8–2.0) | 2.2 (IQR 2.1–2.4) | ||
| 2 years | N | 135 | 15 | 35 | 55 | 30 |
| MG (mmHg) | 12.5 (SD 4.1) | 15.9 (SD 4.3) | 13.3 (SD 4.1) | 12.5 (SD 3.5) | 9.9 (SD 3.3) | |
| 12.0 (IQR 9.3–15.0) | 15.4 (IQR 13.0–19.5) | 13.0 (IQR 10.7–15.6) | 12.0 (IQR 10.5–14.0) | 8.5 (IQR 7.7–11.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.7 (SD 0.2) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.7 (IQR 1.6–1.9) | 2.0 (IQR 1.8–2.1) | 2.3 (IQR 2.1–2.4) | ||
| 5 years | N | 127 | 16 | 28 | 52 | 31 |
| MG (mmHg) | 14.0 (SD 5.5) | 17.5 (SD 7.0) | 13.7 (SD 6.7) | 13.6 (SD 4.7) | 13.0 (SD 4.5) | |
| 13.0 (IQR 10.0–16.0) | 15.4 (IQR 12.0–23.5) | 12.0 (IQR 10.0–15.0) | 13.0 (IQR 10.2–16.0) | 12.0 (IQR 10.0–14.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.3) | 1.9 (SD 0.3) | 2.0 (SD 0.3) | |
| 1.8 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.8 (IQR 1.7–2.0) | 1.9 (IQR 1.7–2.1) | 2.1 (IQR 1.9–2.2) | ||
| P for trenda | MG (mmHg) | 0.308 | 0.308 | 0.308 | 0.149 | 0.308 |
| EOA (cm2) | 0.308 | 1.000 | 1.000 | 0.308 | 0.308 |
Data were presented as N (%), mean (SD) or median (IQR).
Mann–Kendall test.
EOA: effective orifice area; IQR: interquartile range; MG: mean gradient; SD: standard deviation.
| Time point . | Parameters . | All sizes . | 19 mm . | 21 mm . | 23 mm . | 25 mm . |
|---|---|---|---|---|---|---|
| 1 month | N | 145 | 17 | 36 | 60 | 32 |
| MG (mmHg) | 11.6 (SD 3.6) | 15.9 (SD 3.4) | 12.4 (SD 3.7) | 11.1 (SD 2.8) | 9.4 (SD 2.8) | |
| 11.0 (IQR 9.0–14.0) | 16.0 (IQR 14.0–18.0) | 11.9 (IQR 9.8–15.0) | 11.0 (IQR 9.3–13.1) | 9.3 (IQR 7.4–10.4) | ||
| EOA (cm2) | 2.0 (SD 0.3) | 1.6 (SD 0.3) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.8–2.2) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 2.1 (IQR 1.9–2.1) | 2.3 (IQR 2.2–2.4) | ||
| 1 year | N | 140 | 16 | 35 | 58 | 31 |
| MG (mmHg) | 12.7 (SD 4.3) | 17.1 (SD 3.6) | 13.5 (SD 4.1) | 12.5 (SD 4.3) | 10.0 (SD 3.0) | |
| 12.0 (IQR 9.9–15.0) | 17.0 (IQR 14.7–18.5) | 13.8 (IQR 11.0–16.1) | 11.6 (IQR 10.0–14.0) | 9.9 (IQR 7.8–11.4) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.1) | 1.9 (SD 0.2) | 2.2 (SD 0.2) | |
| 1.9 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 1.9 (IQR 1.8–2.0) | 2.2 (IQR 2.1–2.4) | ||
| 2 years | N | 135 | 15 | 35 | 55 | 30 |
| MG (mmHg) | 12.5 (SD 4.1) | 15.9 (SD 4.3) | 13.3 (SD 4.1) | 12.5 (SD 3.5) | 9.9 (SD 3.3) | |
| 12.0 (IQR 9.3–15.0) | 15.4 (IQR 13.0–19.5) | 13.0 (IQR 10.7–15.6) | 12.0 (IQR 10.5–14.0) | 8.5 (IQR 7.7–11.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.7 (SD 0.2) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.7 (IQR 1.6–1.9) | 2.0 (IQR 1.8–2.1) | 2.3 (IQR 2.1–2.4) | ||
| 5 years | N | 127 | 16 | 28 | 52 | 31 |
| MG (mmHg) | 14.0 (SD 5.5) | 17.5 (SD 7.0) | 13.7 (SD 6.7) | 13.6 (SD 4.7) | 13.0 (SD 4.5) | |
| 13.0 (IQR 10.0–16.0) | 15.4 (IQR 12.0–23.5) | 12.0 (IQR 10.0–15.0) | 13.0 (IQR 10.2–16.0) | 12.0 (IQR 10.0–14.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.3) | 1.9 (SD 0.3) | 2.0 (SD 0.3) | |
| 1.8 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.8 (IQR 1.7–2.0) | 1.9 (IQR 1.7–2.1) | 2.1 (IQR 1.9–2.2) | ||
| P for trenda | MG (mmHg) | 0.308 | 0.308 | 0.308 | 0.149 | 0.308 |
| EOA (cm2) | 0.308 | 1.000 | 1.000 | 0.308 | 0.308 |
| Time point . | Parameters . | All sizes . | 19 mm . | 21 mm . | 23 mm . | 25 mm . |
|---|---|---|---|---|---|---|
| 1 month | N | 145 | 17 | 36 | 60 | 32 |
| MG (mmHg) | 11.6 (SD 3.6) | 15.9 (SD 3.4) | 12.4 (SD 3.7) | 11.1 (SD 2.8) | 9.4 (SD 2.8) | |
| 11.0 (IQR 9.0–14.0) | 16.0 (IQR 14.0–18.0) | 11.9 (IQR 9.8–15.0) | 11.0 (IQR 9.3–13.1) | 9.3 (IQR 7.4–10.4) | ||
| EOA (cm2) | 2.0 (SD 0.3) | 1.6 (SD 0.3) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.8–2.2) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 2.1 (IQR 1.9–2.1) | 2.3 (IQR 2.2–2.4) | ||
| 1 year | N | 140 | 16 | 35 | 58 | 31 |
| MG (mmHg) | 12.7 (SD 4.3) | 17.1 (SD 3.6) | 13.5 (SD 4.1) | 12.5 (SD 4.3) | 10.0 (SD 3.0) | |
| 12.0 (IQR 9.9–15.0) | 17.0 (IQR 14.7–18.5) | 13.8 (IQR 11.0–16.1) | 11.6 (IQR 10.0–14.0) | 9.9 (IQR 7.8–11.4) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.1) | 1.9 (SD 0.2) | 2.2 (SD 0.2) | |
| 1.9 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.7) | 1.8 (IQR 1.7–1.9) | 1.9 (IQR 1.8–2.0) | 2.2 (IQR 2.1–2.4) | ||
| 2 years | N | 135 | 15 | 35 | 55 | 30 |
| MG (mmHg) | 12.5 (SD 4.1) | 15.9 (SD 4.3) | 13.3 (SD 4.1) | 12.5 (SD 3.5) | 9.9 (SD 3.3) | |
| 12.0 (IQR 9.3–15.0) | 15.4 (IQR 13.0–19.5) | 13.0 (IQR 10.7–15.6) | 12.0 (IQR 10.5–14.0) | 8.5 (IQR 7.7–11.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.7 (SD 0.2) | 1.8 (SD 0.2) | 2.0 (SD 0.2) | 2.3 (SD 0.2) | |
| 2.0 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.7 (IQR 1.6–1.9) | 2.0 (IQR 1.8–2.1) | 2.3 (IQR 2.1–2.4) | ||
| 5 years | N | 127 | 16 | 28 | 52 | 31 |
| MG (mmHg) | 14.0 (SD 5.5) | 17.5 (SD 7.0) | 13.7 (SD 6.7) | 13.6 (SD 4.7) | 13.0 (SD 4.5) | |
| 13.0 (IQR 10.0–16.0) | 15.4 (IQR 12.0–23.5) | 12.0 (IQR 10.0–15.0) | 13.0 (IQR 10.2–16.0) | 12.0 (IQR 10.0–14.0) | ||
| EOA (cm2) | 1.9 (SD 0.3) | 1.6 (SD 0.2) | 1.8 (SD 0.3) | 1.9 (SD 0.3) | 2.0 (SD 0.3) | |
| 1.8 (IQR 1.7–2.1) | 1.6 (IQR 1.5–1.8) | 1.8 (IQR 1.7–2.0) | 1.9 (IQR 1.7–2.1) | 2.1 (IQR 1.9–2.2) | ||
| P for trenda | MG (mmHg) | 0.308 | 0.308 | 0.308 | 0.149 | 0.308 |
| EOA (cm2) | 0.308 | 1.000 | 1.000 | 0.308 | 0.308 |
Data were presented as N (%), mean (SD) or median (IQR).
Mann–Kendall test.
EOA: effective orifice area; IQR: interquartile range; MG: mean gradient; SD: standard deviation.
Haemodynamic outcomes
Among 148 patients, 127 patients (85.8%) completed the 5-year echocardiographic follow-up. The 21 patients missed 5-year echocardiography due to unwillingness to receive echocardiography (n = 5) and death (n = 16). The mean echocardiographic follow-up was 67.4 (SD 6.1) months. Haemodynamic performances with mean gradient and EOA over 5 years by valve sizes were detailed in Table 3. The mean gradient and EOA for all implanted aortic valves at 1 month were 11.6 (SD 3.6) mmHg, and 2.0 (SD 0.3) cm2, respectively. The haemodynamic performance of these valves remained stable throughout the 5-year follow-up period with a mean gradient and EOA of 14.0 (SD 5.5) mmHg and 1.9 (SD 0.3) cm2 at 5 years, respectively. In particular, for the 19- and 21-mm size bioprostheses, the mean gradients and EOA at 5 years were 17.5 (SD 7.0) mmHg and 1.6 (SD 0.2) cm2 and 13.7 (SD 6.7) mmHg and 1.8 (SD 0.3) cm2, respectively (Fig. 2). At 5 years, only 4.1% and 0.0% of patients had moderate or severe PPM. The percentage of patients with New York Heart Association functional classes III and IV decreased from 75.7% before surgery to 0.8% at 5 years after surgery (Fig. 3).
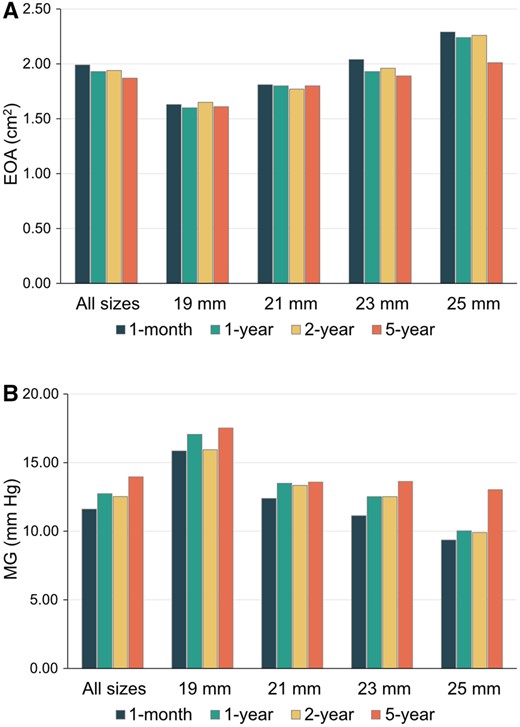
(A) Mean gradients by valve size over 5 years. (B) Mean effective orifice areas by valve size over 5 years.
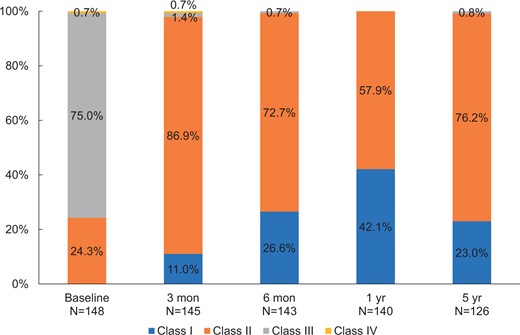
Improvement of NYHA heart functional class in patients receiving Cingular bovine pericardial valve implantations during 5-year follow-up compared with preoperative status. NYHA: New York Heart Association.
DISCUSSION
This study evaluated the safety outcome and haemodynamic performance of the Cingular bovine pericardial aortic valve in 148 patients who underwent SAVR over 5-year follow-up. Valve haemodynamics and safety outcomes were very encouraging. In addition, no events of SVD during the 5-year follow-up period were seen. Longer-term follow-up is ongoing and the results are to be reported in a few years.
In so-called border-line patients aged 50–69 years, it is still uncertain whether a mechanical or biological aortic valve prosthesis should be utilized [16–19]. However, as transcatheter technologies develop, the application of tissue valves is progressive in younger patients [20]. Thus, lifetime management of aortic valve disease is critically important for low-risk and young patients [21, 22]. The durability and haemodynamic performance are 2 key considerations during decision-making. The contemporary surgical tissue valves have undergone modifications and optimizations in valve stent and leaflet tissue processing over the years and exhibit good 5-year outcomes with very low rates of SVD [6–8].
The Cingular bovine pericardial aortic valve also benefits from the technological progress and knowledge that can significantly improve its durability. Based on the Carpentier-Edwards Perimount valve, it features certain innovations and optimizations. The novel three-layer stent can remain its structure annular both in stationary and stressed states [2]. In addition, the alloy wire can be fixed in the groove created by the three-layer stent, thereby avoiding the malposition between the stent and the alloy wire [2]. The malposition would decrease stability of the entire bioprosthesis and cause uneven stress on the tissue leaflets, resulting in wrinkles and expediting wear of the tissue leaflets. Thus, these optimizations are expected to increase bioprosthetic stability and decrease triangular leaflet opening. Based on the 5-year results reported in this study, its performance is comparable to other valves available [6, 7, 23, 24]. The genuine long-term durability will be validated by considerably longer-term follow-up.
Haemodynamic performance is another key feature for a tissue valve. Based on the findings of this trial, the haemodynamics of the study valve are very encouraging. Mean gradients were between 10 and 15 mmHg for all valve sizes except 19 mm, which were also below 20 mmHg. These were consistent with recent reports of 5-year outcomes of the Perimount Magna Ease valve with Resilia leaflets (Edwards Lifesciences, Irvine, CA, USA) and the Avalus aortic bioprosthesis (Medtronic, Minneapolis, MN, USA) [6–8]. PPM is associated with short-term and long-term mortality [25, 26]. Although aortic annular or root enlargement is useful for SAVR patients with small annuli [27, 28], it is still technically challenging in elderly patients. As shown in our data, 19- and 21-mm size valves were very common in our study. Particularly, for 19- and 21-mm sizes of the Cingular bovine pericardial aortic valve, the EOAs at 5 years were 1.6 (SD 0.2) and 1.8 (SD 0.3) cm2, respectively. Overall, the incidence of PPM was very low for the study valve in comparison to data published despite the high percentage of small-size aortic valves in our study [29, 30], which may be partially due to lower average body surface area in Chinese patients [31].
Limitations
This study had some limitations. First, this trial was a nonrandomized, single-arm study without a concurrent control group, making comparisons with other valves challenging. Thus, it was susceptible to selection bias. Second, although the mid-term results were promising, SVD is actually infrequent in the first few years. Thus, the longer-term follow-up is ongoing to assess the long-term durability of this valve, especially for the small prosthesis. Third, although our definition of SVD is consistent with other SAVR studies, it is different from that of the Valve Academic Research Consortium-3 which includes the detailed criteria for 3 stages of SVD based on haemodynamic changes and is used in many modern transcatheter aortic valve replacement studies [32].
CONCLUSIONS
The Cingular bovine pericardial aortic valve exhibited good safety and haemodynamic outcomes over a 5-year follow-up period. Good haemodynamic performance was sustained for the small bioprostheses. The longer-term follow-up is warranted to assess its durability.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 82200526), the Shanghai Rising-Star Program (No. 23QB1400900), the Shanghai ‘Rising Stars of Medical Talents’ Youth Development Program (No. SHWRS2023-62), the Shanghai Sailing Program (No. 20YF1405400), the Clinical Research Fund of Shanghai Municipal Health Commission (No. 20224Y0286) and Shanghai Cingular Biotech Corporation.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Jinmiao Chen: Conceptualization; Formal analysis; Writing—original draft; Writing—review & editing. Minzhi Lv: Data curation; Formal analysis; Methodology; Writing—original draft. Jiahui Fu: Data curation; Formal analysis. Chen He: Data curation; Formal analysis. Yingqiang Guo: Conceptualization; Resources; Supervision. Liang Tao: Conceptualization; Investigation; Supervision. Xinmin Zhou: Conceptualization; Investigation; Resources. Tianxiang Gu: Conceptualization; Investigation; Supervision. Krzysztof Bartus: Conceptualization; Investigation; Methodology; Writing—review & editing. Lai Wei: Conceptualization; Methodology; Resources; Supervision. Tao Hong: Formal analysis; Methodology; Resources; Supervision; Writing—review & editing. Chunsheng Wang: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Yutaka Okita, Anders Jeppsson, Tom C. Nguyen and the other, anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- CI
Confidence interval
- EOA
Effective orifice area
- PPM
Patient–prosthesis mismatch
- SAVR
Surgical aortic valve replacement
- SD
Standard deviation
- SVD
Structural valve deterioration
Author notes
Jinmiao Chen and Minzhi Lv authors contributed equally to this work.





