-
PDF
- Split View
-
Views
-
Cite
Cite
Shigemitsu Iwai, Koji Miwa, Toshiaki Nagashima, Norwood procedure for hypoplastic left heart syndrome associated with valvular pulmonary stenosis, Interactive CardioVascular and Thoracic Surgery, Volume 34, Issue 5, May 2022, Pages 930–932, https://doi.org/10.1093/icvts/ivac022
Close - Share Icon Share
Abstract
Association between hypoplastic left heart syndrome and valvular pulmonary stenosis is very rare. Severity of valvular pulmonary stenosis in this setting limits management options. Consequently, patients with this condition are considered poor candidates for Norwood stage one reconstruction. Herein, we describe a newborn with hypoplastic left heart syndrome and significantly dysplastic pulmonary valve who successfully underwent the Norwood procedure with neoaortic valve reconstruction. Therefore, the Norwood procedure with neoaortic valve reconstruction might be an option for this difficult condition.
INTRODUCTION
Association between hypoplastic left heart syndrome (HLHS) and valvular pulmonary stenosis is rare and has not been well described in the literature. Due to outflow obstruction, neonates with HLHS and significant pulmonary stenosis are considered poor candidates for Norwood stage one reconstruction. Herein, we report a newborn with HLHS and significantly dysplastic pulmonary valve who underwent the Norwood procedure with neoaortic valve reconstruction wherein survival was achieved until infancy.
CASE REPORT
A male patient was diagnosed with HLHS on prenatal echocardiography. Foetal cardiography showed features of HLHS with aortic valve atresia and hypoplasia of the mitral valve and left ventricle. The pulmonary valve was dysplastic, and flow velocity through the pulmonary valve was heightened. He was born at 39 weeks of gestation, with a birthweight of 2780 g. Transthoracic echocardiography confirmed HLHS diagnosis (aortic valve atresia/mitral valve stenosis); moderate regurgitation; and stenotic pulmonary valve with 3 dysplastic and thickened leaflets. Peak pressure gradient across the pulmonary valve was 86 mmHg (Fig. 1A).
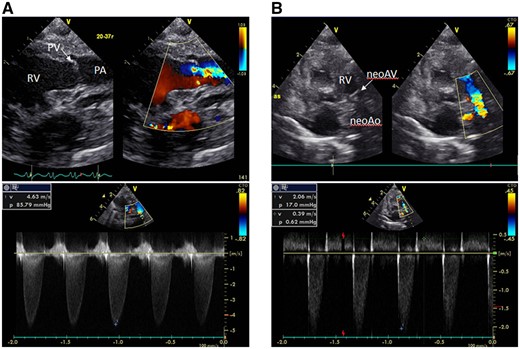
(A) A transthoracic echocardiogram after birth reveals a severely stenotic pulmonary valve with 3 dysplastic and thickened leaflets with a peak pressure gradient of 86 mmHg across the pulmonary valve. (B) Postoperative echocardiography shows a pressure gradient of 17 mmHg across the neoaortic valve, with an improved neoaortic valve regurgitation. neoAo: neoaorta; neoAV: neoaortic valve; PA: pulmonary artery; PV: pulmonary valve ; RV: right ventricle.
Norwood stage one operation was performed the following day. Cardiopulmonary bypass was established with the brachiocephalic artery, descending aorta and bicaval cannulation. The main pulmonary artery was transected, and the stump was connected to a 5 mm-ringed Gore-Tex graft (W.L. Gore & Assoc; Flagstaff, AZ, USA). The pulmonary valve was tricuspid with thickened whole leaflets, and the opening was extremely restricted without commissural fusion (Fig. 2A). Therefore neoaortic valve leaflet replacement using glutaraldehyde-treated autologous pericardium (0.6%, 5-min treatment) was performed. After cardiac arrest was induced by antegrade cold blood cardioplegia infusion, 3 thickened leaflets were meticulously excised. Width and height of the 3 new pericardial leaflets were equal to the annulus diameter (7 mm), and the cusp free edge was trimmed to a length of 1.4× the annulus diameter. The new leaflets were sutured with a 7–0 monofilament to each annulus (Fig. 2B). Each commissure was suspended with an additional 7–0 monofilament suture. Another glutaraldehyde-treated autologous pericardial patch was used to connect the neoaorta to the descending aorta. After atrial septectomy, the 5-mm Gore-Tex graft was connected to the right ventricle using the dunk technique. Two days after extracorporeal membrane oxygenation support, the patient was weaned off mechanical circulatory support smoothly. Delayed sternal closure was performed on postoperative Day 7. Postoperative echocardiography showed a pressure gradient across the neoaortic valve (17 mmHg). Moreover, the neoaortic valve regurgitation was trivial to mild (Fig. 1B). However, at 5 months, recurrence of neoaortic stenosis and insufficiency at a peak velocity of 3.8 m/s were observed with moderate regurgitation. Re-neoaortic valve reconstruction (bicuspidization using bovine pericardium) was performed. Afterwards, his general condition worsened due to bloodstream infection and heart failure. He did not respond to anti-heart failure therapy and died at 9 months.
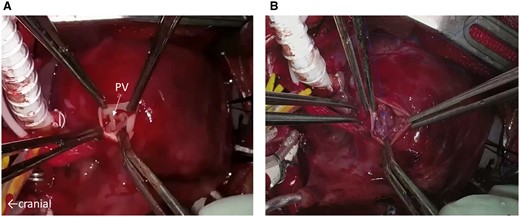
(A) The pulmonary valve as shown during the Norwood procedure. Three thickened and dysplastic leaflets make the orifice opening limited. (B) Neoaortic valve leaflet replacement using glutaraldehyde-treated autologous pericardium. The new 3 pericardial leaflets are sutured to each annulus. PV: pulmonary valve.
DISCUSSION
This report presents the first reported case of survival in a neonate who underwent the Norwood procedure concomitant with neoaortic valve reconstruction for HLHS with valvular pulmonary stenosis and insufficiency. Association between HLHS and valvular pulmonary stenosis is extremely rare, with a reported incidence of 0.4% in aortic tract complex hypoplasia [1]. Most of the reported cases were fatal, and there have been only a few reports of survival.
Farra and Kort [2] described successful palliation using transcatheter pulmonary valvotomy followed by heart transplantation a few days after birth. In the presence of moderate pulmonary insufficiency, a transcatheter pulmonary valvotomy was contraindicated due to possible risks of exacerbating pulmonary valve insufficiency and right ventricular dysfunction. Since cardiac transplant is less available for infants in our country, we had to perform the Norwood procedure as single-ventricle palliation in the patient.
If valvular pulmonary stenosis is less than moderate and right ventricular function is maintained, the bilateral pulmonary artery banding procedure could have been a management option to stabilize the patient at the relatively early onset of pulmonary overcirculation. Nakamura et al. [3] reported a newborn with borderline hypoplastic left heart complex and valvular pulmonary stenosis who underwent a successful Norwood procedure after bilateral pulmonary artery banding. However, the persistent labile haemodynamic state observed in the patient after this, characterized by low diastolic arterial pressure, also suggested a need to eliminate the patent ductus arteriosus and repair the aortic arch. In our case, this strategy would have left the right ventricle pressurized due to valvular pulmonary stenosis and led to early deterioration in right ventricular function.
Huang et al. [4] reported a successful Norwood stage one reconstruction without valvuloplasty of the pulmonary valve. In their case, only mild insufficiency and pressure gradient of 36 mmHg across the pulmonary valve were noted after birth. They described that ‘thinning’ of the 3 thickened leaflets in the neonatal stage was difficult and might have resulted in severe regurgitation, which would be more difficult to tolerate than valve stenosis. We initially planned to perform simple slicing of thickened valve. However, preoperative echocardiography showed moderate regurgitation, and the intraoperative findings showed poor leaflet coaptation. Therefore, we decided that neoaortic valve leaflet reconstruction was necessary for survival during the perioperative period. This method has achieved good outcomes in adult patients using the Ozaki procedure [5] and has recently been adopted in paediatric patients. Although its durability remained questionable, this aortic valvuloplasty might have been possible even in neonatal cases. Reportedly, regurgitation could be suppressed to some extent, and timing of reoperation or valve replacement could be delayed.
In our case, although diameter of the pulmonary artery annulus was 7 mm, which was remarkably narrow, tricuspid reconstruction was performed in the first operation. Consequently, neoaortic stenosis and insufficiency recurred 4 months after the operation due to degeneration of the reconstructed leaflet. In this case, reoperation for degenerative neoaortic valve was delayed due to temporary deterioration in his general condition associated with infection. In addition, in cases of remarkably narrow annulus, to delay reoperation for progression of neoaortic valve stenosis, bicuspid reconstruction or annulus enlargement might be necessary.
CONCLUSIONS
The Norwood procedure concomitant with neoaortic valve reconstruction could be a treatment option even in neonates with HLHS and valvular pulmonary stenosis.
Conflict of interest: none declared.
Data availability statement
The data underlying this article are available in the article.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Tim Attmann and the other anonymous reviewers for their contribution to the peer review process of this article.
ACKNOWLEDGEMENTS
We thank Editage (www.editage.jp) for English language editing.




