-
PDF
- Split View
-
Views
-
Cite
Cite
Alan G Dawson, Cathy J Richards, Leonidas Hadjinikolaou, Apostolos Nakas, Metastatic renal cell carcinoma extending to the left atrium through the inferior pulmonary vein, Interactive CardioVascular and Thoracic Surgery, Volume 32, Issue 6, June 2021, Pages 991–992, https://doi.org/10.1093/icvts/ivab018
Close - Share Icon Share
Abstract
Metastatic renal cell carcinoma with involvement through the pulmonary veins to the left atrium is very rare. We report the case of a 70-year-old male with metastatic renal cell carcinoma to the right lower lobe of the lung abutting the inferior pulmonary vein with extension to the left atrium without pre-operative evidence. Surgical resection was achieved through a posterolateral thoracotomy. Lung masses that abut the pulmonary veins should prompt further investigation with a pre-operative transoesophageal echocardiogram to minimize unexpected intraoperative findings.
INTRODUCTION
Renal cancer accounts for 4% of new cancer cases in the UK, of which renal cell carcinoma (RCC) is the most common [1]. Cardiac metastases from RCC are infrequent and commonly manifest with extension to the right atrium via the renal vein and inferior vena cava [2]. Involvement of the pulmonary veins is exceedingly rare [3]. We report a case of metastatic RCC to the right lower lobe of the lung with extension to the left atrium (LA) through the right inferior pulmonary vein (IPV) despite Sunitinib therapy.
CASE REPORT
A 70-year-old male ex-smoker was referred to the Thoracic Surgery Department for the management of right middle and lower lobe lung masses in 2017. His past medical history included: right nephrectomy secondary to complications from pyelonephritis, secondary hypertension and hyperlipidaemia. In 2003, he underwent a left partial nephrectomy for RCC. Five years later, a right middle lobe lung metastasis was identified and he was commenced on Sunitinib 25 mg once daily. In 2016, he developed end-stage renal failure requiring haemodialysis.
Computerized tomographic (CT) scan of the chest demonstrated a stable 1.6-cm right middle lobe nodule and a new 3.9-cm right lower lobe mass abutting the IPV (Fig. 1A). Positron emission tomography/CT with 2-deoxy-2-[fluorine-18]fluoro-d-glucose measured the standardized uptake value of 1.5 in the middle lobe nodule and 4.4 in the lower lobe mass (Fig. 1B). A pre-operative transthoracic echocardiogram did not show any abnormality. He was reviewed and provided informed consent for a right middle lobe wedge resection and lower lobectomy via thoracotomy.
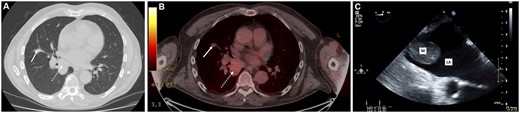
Non-contrast computerized tomographic (A) and positron emission tomographic (B) scans demonstrating the middle lobe nodule (solid arrow) and lower lobe mass (broken arrow). Two-dimensional transoesophageal echocardiogram (C) demonstrating the tumour mass in the left atrium.
A right posterolateral thoracotomy was performed and the middle lobe nodule was excised by wedge resection. During dissection, a firm mass was palpated within the IPV lumen. An intraoperative transoesophageal echocardiogram revealed a 2.2 cm × 2.8 cm pedunculated LA mass with no atrial wall attachments or obstruction to the mitral valve orifice (Fig. 1C). A left atriotomy was performed using cardiopulmonary bypass (CPB) with aortic and bicaval cannulation through the posterolateral thoracotomy and the mass removed. The right lower lobectomy and systematic lymph node dissection was completed. He was transferred to the intensive care unit.
Histopathological analysis confirmed metastatic RCC without lymph node involvement. Immunohistochemistry showed positivity for RCC, CD10 and Epithelial Membrane Antigen (EMA) with focal staining for PAX-8 (Fig. 2).
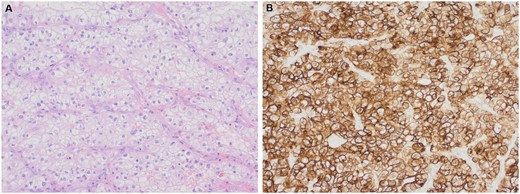
Histological examination of the tumour by haematoxylin and eosin stain (A) and renal cell carcinoma immunohistochemistry showing strong positive staining of the tumour cells (B). Magnification ×200.
The patient was extubated on postoperative day 1. On the following day, he developed abdominal pain and an abdominal and pelvic CT scan confirmed ischaemic bowel. It was felt that a laparotomy would carry a significant mortality risk and he died on postoperative day 8.
DISCUSSION
Left atrial metastases from RCC are exceedingly rare and this is the 11th such case in the literature and the first reported patient in the UK. This case is unique in that the patient presented 13 years following nephrectomy and that it invaded the IPV with LA extension without pre-operative diagnosis. Furthermore, the lower lobe metastasis developed despite treatment with Sunitinib.
During pre-operative work-up, no investigation demonstrated IPV or LA involvement; therefore, surgical approach was by the way of posterolateral thoracotomy. Owing to renal failure, the CT scan was performed without iodinated contrast and, although the positron emission tomography scan showed tracer uptake around the LA, this was thought to represent physiological blood pool activity and not raised as a concern. Despite LA masses resulting in systemic embolization, syncope, arrhythmia or congestive heart failure, there is no accepted management for cardiac metastases from RCC [4]. As the LA mass was pedunculated, CPB was employed to allow the isolation of the LA ensuring a complete oncological resection, to minimize the risk of tumour embolization mitigating the aforementioned complications, and for the provision of safety in the event of severe bleeding.
In conclusion, this case illustrates that pulmonary metastases from RCC can involve the LA via the pulmonary veins. If pre-operative investigations suggest a close relationship of the metastatic deposit to a pulmonary vein, a high index of suspicion of LA involvement must be considered and assessed using transoesophageal echocardiogram.
Conflict of interest: none declared.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Lucio Cagini, Nuria M Novoa and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES




