-
PDF
- Split View
-
Views
-
Cite
Cite
Chun-Yang Huang, Po-Lin Chen, Hsin-Ying Lu, Hung-Lung Hsu, Tzu-Ting Kuo, I.-Ming Chen, Chiao-Po Hsu, Chun-Che Shih, Midterm result of custom-made iliac branch device for common iliac aneurysm with and without abdominal aortic aneurysm, Interactive CardioVascular and Thoracic Surgery, Volume 32, Issue 1, January 2021, Pages 97–105, https://doi.org/10.1093/icvts/ivaa229
Close - Share Icon Share
Abstract
Although commercial iliac branch devices offer a new and valid endovascular approach to treating iliac aneurysm and effectively preserve antegrade flow of the internal iliac artery, their use may not be suited for all types of challenging anatomy, especially isolated common iliac artery aneurysm. Our custom-made iliac bifurcation device has a unique design and excludes both combined and isolated iliac branch aneurysm. This study validated the efficacy and safety of the custom device by comparing clinical outcomes between groups receiving commercial and custom devices.
Data of consecutive patients receiving iliac bifurcation device implantation for iliac aneurysm with or without concomitant endovascular repair for abdominal aortic aneurysm from January 2010 to May 2019 were reviewed.
Iliac bifurcation device implantation with or without concomitant abdominal aortic aneurysm stent grafting was completed in 46 patients (commercial, n = 35; custom, n = 11). No significant differences were observed regarding postoperative complications, occlusion or endoleak. Comparisons of primary (80.8% vs 85.7%, P = 0.88) and secondary (86.5% vs 85.7%, P = 0.85) patency and freedom from reintervention (88.2% vs 100%, P = 0.33), all-cause mortality (78.6% vs 100%, P = 0.25) and aneurysm-related mortality (100% vs 100%, P = 1.00) also indicated no differences at a 5-year surveillance point. Furthermore, the iliac aneurysms of the groups displayed similar shrinkage 1 year after procedures.
For iliac aneurysm, the novel custom-made iliac bifurcation device is an adaptable design not inferior to commercial devices with regard to postoperative complications, bridge occlusion, endoleak and short-term aneurysm remodelling. It provides an alternative for treatment, particularly when certain anatomic challenges are present.
2018-07-050BC, 2017-01-023ACF.
INTRODUCTION
Common iliac artery aneurysms (CIAAs) tend to develop in patients with an abdominal aortic aneurysm (AAA), whereas isolated iliac artery aneurysms (IAAs) are rare, constituting ~2% of all abdominal aneurysms [1]. Individuals with an isolated CIAA are usually asymptomatic, and diagnosis is incidental during imaging studies. Similar to AAAs, IAAs are prone to rupture when they reach a critical size and cause high mortality (50–100%) [2]. McCready et al. [3] recommended repair when an aneurysm reaches 3 cm in size because of a high growth rate of 2.9–4 mm/years.
An IAA can be treated using either a surgical or endovascular approach. The main goal of treatment is to exclude the aneurysm sac and maintain internal iliac artery perfusion. However, surgical treatment is associated with high mortality and morbidity (10%) and may cause injury to adjacent organs during exposure of the vessels [4]. A commercial iliac branch device (cIBD) has been introduced as a new endovascular approach for treating IAAs concomitant with AAA; such devices effectively preserve antegrade flow in the internal iliac artery [5]. However, challenging iliac anatomy, such as a short CIAA neck or small external iliac artery (EIA) or internal iliac artery (IIA) diameter, restricts cIBD application. Hence, chimney technique are recommended for patients who are not suitable candidates for treatment with the cIBD. However, the alternative is sometimes inapplicable in practice and may not provide favourable outcomes [6]. The fenestrated/branched endograft method has been used in aortic arch and thoraco-abdominal aorta repair [7]. It is associated with lower mortality risk than open or other hybrid surgery. Our custom-made iliac bifurcation device (cmIBD) enables modified compliant application of an iliac stent graft. In the present study, the in situ fenestration device was inserted through an in vitro technique and was designed and adjusted according to preoperative computed tomography (CT).
We compared cIBD and cmIBD by evaluating postoperative complications and midterm follow-up outcomes.
MATERIALS AND METHODS
Patient selection
This study is a retrospective single-centre analysis. From January 2010 to May 2019, the data of consecutive patients with a CIAA who received IBD treatment with or without concomitant AAA stent grafting were reviewed. Indications for the treatment included the presence of a unilateral or bilateral CIA ≧35 mm in diameter or the occurrence of a CIA ≧25 mm in diameter with an AAA of ≧50 mm in diameter. Patient characteristics as well as preoperative, intraoperative and follow-up clinical and CT imaging data were obtained from a Taipei Veterans General Hospital database. Preoperative comorbidities and aneurysm anatomy were graded according to comorbidity and anatomic factor severity scoring proposed by the Vascular Surgery/American Association for Vascular Surgery in 2002 [8]. Informed consent was obtained from patients, and the study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Severity scoring systems
Comorbidity
Vascular Surgery/American Association for Vascular Surgery scoring of comorbidity includes major morbidity and mortality associated with endovascular or surgical treatment of aortic aneurysm. A 0–3 scale for grading severity (0 = absent, 1 = mild, 2 = moderate and 3 = severe) ensures simplicity and uniformity of scoring [8]. Patient characteristics considered were the following risk factors: age, hypertension and cardiac, pulmonary and renal diseases. Cardiac (weighted × 4), pulmonary (weighted × 2) and renal (weighted × 2) risk factors are regarded as major components of the score, whereas hypertension (weighted × 1) and age (weighted × 1) are minor components. Because the 5 risk factors are weighted, the maximum total score is 30. This score is divided by 10 to yield a comorbidity severity score on the 3-point comorbidity severity scale.
Aneurysm anatomy
The evaluation of vascular morphology was based primarily on data derived from CT imaging. The aortic neck is measured from the lowest renal artery to the aneurysm. Length, diameter, angle and calcification are evaluated using the same 3-point scale. Components of the evaluation of an aortic aneurysm are angulation measurement, a tortuosity index (TI), assessment of intramural thrombus status and measurement of branch vessels. Pelvic perfusion is evaluated to investigate whether the bilateral or single internal iliac artery is patent. The calcification, diameter, angulation, TI, length and diameter in the sealing zone of the iliac artery are evaluated. In all, 15 factors are summarized, with a maximum score of 45. This score is divided by 15 to yield an anatomic severity score on the 3-point scale. Surgery difficulty was therefore described by a scale corresponding to no, mild, moderate and severe.
Surgical procedure
Commercial iliac bifurcation device
A Cook iliac bifurcation device (IBD) (Cook, Bloomington, IN, USA) was used for a CIA length of >40 mm, an iliac bifurcation diameter >16 mm, an EIA diameter >8 mm and an IIA diameter >10 mm. If the EIA diameter was 6.5–8 mm and the IIA diameter was 6.5–10 mm, a Gore IBE (W. L. Gore and Associates, Flagstaff, AZ, USA) was considered. The surgical procedure was performed under general anaesthesia. Each procedure began with the administration of 3000 U of unfractionated heparin for an activated clotting time of >200 s. The bilateral inguinal areas were incised, and the superficial femoral artery was exposed. The left brachial artery or subclavian artery was exposed if the patient had undergone prior abdominal aortic stent grafting. The femoral artery of the iliac aneurysm side was punctured, and the sheath was inserted. Aortography was performed, and the location of the aneurysm was identified. The main body of the IBD was delivered and partially deployed. Another guidewire was inserted from the contralateral femoral artery and advanced to the IBD using a 12-Fr long sheath and the crossover method. For patients with previous abdominal aortic stent grafting, the guidewire and long sheath were delivered from the left axillary or brachial artery. The branch of the IBD was cannulated, and a bridge stent graft (VIABHAN, W. L. Gore and Associates; AdvantaV12, Atrium Medical, Hudson, NH, USA; LifeStream, C.R. Bard Peripheral Vascular Inc., Murray Hill, NJ, USA; Fluency, C.R. Bard Peripheral Vascular Inc., Murray Hill, NJ, USA) was deployed. Regarding the selection of the bridge stent graft, a self-expandable stent was used if the iliac TI was >1.25, and otherwise, a balloon-expandable stent was deployed in the lower index iliac artery. If the iliac aneurysm was concomitant with an AAA, an abdominal aortic stent graft was implanted as in our previous study [9]. Poststenting balloons were moulded for the stent junction and overlapping portion. Angiography was used to ascertain stent graft patency and any endoleak. The bilateral superficial or axillary artery was repaired, and the wound was closed.
Custom-made iliac bifurcation device
For patients with indications of difficult anatomy, such as a CIA length ˂40 mm, iliac bifurcation diameter under 16 mm, an EIA or IIA landing diameter smaller than 6.5 mm, or an isolated iliac aneurysm with a proper proximal landing zone, or with previous abdominal EVAR stenting, a cmIBD was used. The off-label device application was approved by IRB. Anaesthesia and exposure for the cmIBDs were identical to those for the cIBD procedure. An iliac stent graft (ETLW, Endurant, Medtronic Inc., Minneapolis, MN, USA) was used because of its easy reloading and polyester material with low porosity. The stent graft was partially deployed at the back table, and a 5–6-mm fenestrated hole was created on the stent graft through electrocauterization according to preoperative CT calculation (Fig. 1A). The fenestrated hole was reinforced with radiopaque tip wire cut from the 0.014″ guidewire by a 7-0 Prolene (Ethicon, Somerville, NJ, USA) running suture fixation (Fig. 1B). This modified fenestrated iliac stent graft was reloaded into the delivery sheath (Fig. 1C–E). The sheath was inserted from the superficial femoral artery on the lesion side and was advanced to a suitable position. The iliac stent graft was partially deployed until the radiopaque wire marker of the fenestrated hole was visible and fully open under fluorescence. This fenestrated hole was cannulated using the contralateral femoral guidewire by the crossover method or guidewire from axillary artery access. After passing the fenestrated hole, the guidewire was advanced into the orifice of the internal iliac artery. Bridging stent grafts were sequentially delivered and deployed (Fig. 2A and B). The balloon-expandable stent was preferred because it can be deployed with greater precision. These bridging stents were secured in place by allowing a significant portion of the stent, typically between 3 and 5 mm, to protrude into the main iliac stent graft. The protruding section of the stent was then dilated using a balloon catheter to form a funnel-shaped conduit for blood flow into the internal iliac artery and to ensure intimate contact between the main body and bridging stent grafts. The abdominal aortic stent graft was used in concomitant AAAs (Video 1). The final steps were the same as those used for cIBDs. Technical success was defined as the uneventful implantation of a stent graft with accompanying branch patency and without any endoleak by the end of the intervention period.
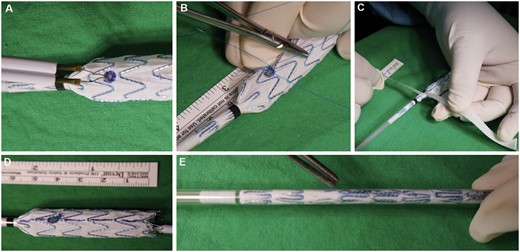
Custom-made iliac bifurcation device table procedure (A–E). (A) Fenestration hole creation. (B) 014″ wire reinforcement. (C) Tape-assisted reloading. (D) Alignment mark for reloading. (E) Complete reloading.
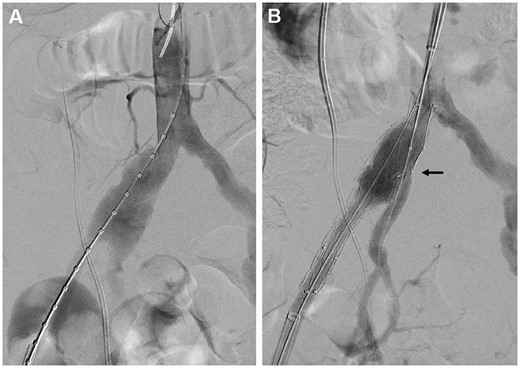
Custom-made iliac bifurcation device deployment under fluorescence (A, B). (A) Aneurysm length measurement. (B) Custom-made iliac bifurcation device deployment and internal iliac artery stent grafting by axillary cannulation.
A 71-year-old male with right common iliac aneurysm and mild abdominal aorta aneurysm was treated by custom-made iliac branch device. It was created on the back table, including electrocauterizing fenestration, 0.014″ wire reinforcement and reloading. The device was inserted and deployed by endovascular skill as usual. No significant complication was recorded postoperatively.
Surveillance protocol
All patients underwent CT (GE Healthcare, Waukesha, WI, USA) surveillance and received an initial scan within 1 month of the procedure, followed by a repeat scan after 6 months and annual scans thereafter [10]. All scan images were saved for further evaluation. Iliac aneurysm remodelling and imaging data were transferred to OsiriX MD (OsiriX Version1.1). Aneurysm diameters and volumes were automatically calculated using region of interest computed volumes. Preoperative and postoperative 1-year results were compared within and between the groups; postoperative complications, endoleak, stent graft occlusion and reinterventions were identified and recorded.
Statistical analysis
All variables presented in the table are expressed as median (Q1–Q3). The normality of variable distribution was tested using the Kolmogorov–Smirnov method. The Mann–Whitney U-test was used to compare continuous variables between groups, and Fisher’s exact test was used to compare preoperative and postoperative nominal variables. Analysis of covariance was used to compare continuous variables between the groups at preoperation and 1-year follow-up. Kaplan–Meier analysis was used to calculate rates for primary and secondary patency as well as freedom from reintervention, all-cause mortality and aneurysm-related mortality. A P-value <0.05 was considered statistically significant. Data were analysed using SPSS statistical software (Version 22; IBM Corp., Armonk, NY, USA).
RESULTS
IBD treatment with or without concomitant abdominal aortic stent grafting was completed in 46 patients. The patients were assigned to 2 groups: cIBD and cmIBD. The baseline demographics and comorbidities of both groups are presented in Table 1. The median age of the patients was 73 years, and 46 of 47 (97.9%) patients were male. Except for the prevalence of diabetes, no significant differences between the groups’ characteristics were identified. Comorbidity severity scores did not differ significantly after further weighting calculation. In addition, most patients had concomitant AAA (100% in the cIBD group and 82% in the cmIBD group) and displayed similar iliac aneurysm anatomic allocation (Table 1). In preoperative assessments of aneurysm characteristics, the cIBD group had a higher severe bilateral iliac TI mean score than the cmIBD group (1 vs 0.5, P=0.001; Table 2). Anatomic severity scores did not indicate significant differences between the groups. In IBD side anatomic evaluation, CIA diameter also did not display a significant difference between the groups. However, the cIBD group had longer common iliac artery length than the cmIBD group (59.4 vs 41.4 mm, P = 0.003). Moreover, the cIBD group had larger iliac bifurcation, EIA and IIA diameter and longer EIA and IIA sealing length than those of the cmIBD group (20.3 vs 16.3 mm, P = 0.005 for bifurcation; 10.3 vs 7.0 mm, P < 0.001 for EIA diameter; 132.1 vs 101.6 mm, P = 0.001 for EIA length; 9.7 vs 8.3 mm, P = 0.043 for IIA diameter; 47.8 vs 33.8 mm, P = 0.013 for IIA length; Table 2).
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Age (years), median (range) | 73 (66–81) | 72 (64–79) | 0.75 |
| Gender (M/F), n (%) | 34/1 (97/3) | 11/0 (100/0) | 1.00 |
| Smoking, n (%) | 16 (46) | 5 (46) | 0.99 |
| Hypertension, n (%) | 29 (83) | 10 (91) | 1.00 |
| Diabetes, n (%) | 4 (11) | 5 (46) | 0.025 |
| Hyperlipidaemia, n (%) | 6 (17) | 4 (36) | 0.22 |
| Coronary artery disease, n (%) | 10 (29) | 1 (9) | 0.25 |
| Stroke, n (%) | 4 (11) | 0 | 0.56 |
| Haemodialysis, n (%) | 1 (3) | 0 | 1.00 |
| Comorbidity severity score, median (range) | |||
| Heart | 0 (0–1) | 0 (0) | 0.32 |
| Pulmonary | 0 (0) | 0 (0) | 0.76 |
| Renal | 0 (0) | 0 (0) | 0.78 |
| Hypertension | 1 (1–1) | 1 (1–1) | 0.54 |
| Age | 2 (1–3) | 2 (1–2) | 0.69 |
| Average score, median (range) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.32 |
| Aneurysm distribution, n (%) | |||
| Abdominal aortic aneurysm | 35 (100) | 9 (82) | 0.053 |
| CIA aneurysm (R/l/Bil) | 11/6/17 (31/17/49) | 3/2/6 (27/18/55) | 0.88 |
| EIA aneurysm (R/l/Bil) | 1/1/0 (3/3/0) | 0/0/0 (0/0/0) | 0.57 |
| IIA aneurysm (R/l/Bil) | 3/5/3 (9/14/9) | 0/1/0 (0/9/0) | 0.26 |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Age (years), median (range) | 73 (66–81) | 72 (64–79) | 0.75 |
| Gender (M/F), n (%) | 34/1 (97/3) | 11/0 (100/0) | 1.00 |
| Smoking, n (%) | 16 (46) | 5 (46) | 0.99 |
| Hypertension, n (%) | 29 (83) | 10 (91) | 1.00 |
| Diabetes, n (%) | 4 (11) | 5 (46) | 0.025 |
| Hyperlipidaemia, n (%) | 6 (17) | 4 (36) | 0.22 |
| Coronary artery disease, n (%) | 10 (29) | 1 (9) | 0.25 |
| Stroke, n (%) | 4 (11) | 0 | 0.56 |
| Haemodialysis, n (%) | 1 (3) | 0 | 1.00 |
| Comorbidity severity score, median (range) | |||
| Heart | 0 (0–1) | 0 (0) | 0.32 |
| Pulmonary | 0 (0) | 0 (0) | 0.76 |
| Renal | 0 (0) | 0 (0) | 0.78 |
| Hypertension | 1 (1–1) | 1 (1–1) | 0.54 |
| Age | 2 (1–3) | 2 (1–2) | 0.69 |
| Average score, median (range) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.32 |
| Aneurysm distribution, n (%) | |||
| Abdominal aortic aneurysm | 35 (100) | 9 (82) | 0.053 |
| CIA aneurysm (R/l/Bil) | 11/6/17 (31/17/49) | 3/2/6 (27/18/55) | 0.88 |
| EIA aneurysm (R/l/Bil) | 1/1/0 (3/3/0) | 0/0/0 (0/0/0) | 0.57 |
| IIA aneurysm (R/l/Bil) | 3/5/3 (9/14/9) | 0/1/0 (0/9/0) | 0.26 |
Medical severity score = (heart × 4 + pulmonary × 2 + renal × 2 + hypertension × 1 + age × 1)/10.
cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Age (years), median (range) | 73 (66–81) | 72 (64–79) | 0.75 |
| Gender (M/F), n (%) | 34/1 (97/3) | 11/0 (100/0) | 1.00 |
| Smoking, n (%) | 16 (46) | 5 (46) | 0.99 |
| Hypertension, n (%) | 29 (83) | 10 (91) | 1.00 |
| Diabetes, n (%) | 4 (11) | 5 (46) | 0.025 |
| Hyperlipidaemia, n (%) | 6 (17) | 4 (36) | 0.22 |
| Coronary artery disease, n (%) | 10 (29) | 1 (9) | 0.25 |
| Stroke, n (%) | 4 (11) | 0 | 0.56 |
| Haemodialysis, n (%) | 1 (3) | 0 | 1.00 |
| Comorbidity severity score, median (range) | |||
| Heart | 0 (0–1) | 0 (0) | 0.32 |
| Pulmonary | 0 (0) | 0 (0) | 0.76 |
| Renal | 0 (0) | 0 (0) | 0.78 |
| Hypertension | 1 (1–1) | 1 (1–1) | 0.54 |
| Age | 2 (1–3) | 2 (1–2) | 0.69 |
| Average score, median (range) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.32 |
| Aneurysm distribution, n (%) | |||
| Abdominal aortic aneurysm | 35 (100) | 9 (82) | 0.053 |
| CIA aneurysm (R/l/Bil) | 11/6/17 (31/17/49) | 3/2/6 (27/18/55) | 0.88 |
| EIA aneurysm (R/l/Bil) | 1/1/0 (3/3/0) | 0/0/0 (0/0/0) | 0.57 |
| IIA aneurysm (R/l/Bil) | 3/5/3 (9/14/9) | 0/1/0 (0/9/0) | 0.26 |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Age (years), median (range) | 73 (66–81) | 72 (64–79) | 0.75 |
| Gender (M/F), n (%) | 34/1 (97/3) | 11/0 (100/0) | 1.00 |
| Smoking, n (%) | 16 (46) | 5 (46) | 0.99 |
| Hypertension, n (%) | 29 (83) | 10 (91) | 1.00 |
| Diabetes, n (%) | 4 (11) | 5 (46) | 0.025 |
| Hyperlipidaemia, n (%) | 6 (17) | 4 (36) | 0.22 |
| Coronary artery disease, n (%) | 10 (29) | 1 (9) | 0.25 |
| Stroke, n (%) | 4 (11) | 0 | 0.56 |
| Haemodialysis, n (%) | 1 (3) | 0 | 1.00 |
| Comorbidity severity score, median (range) | |||
| Heart | 0 (0–1) | 0 (0) | 0.32 |
| Pulmonary | 0 (0) | 0 (0) | 0.76 |
| Renal | 0 (0) | 0 (0) | 0.78 |
| Hypertension | 1 (1–1) | 1 (1–1) | 0.54 |
| Age | 2 (1–3) | 2 (1–2) | 0.69 |
| Average score, median (range) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.32 |
| Aneurysm distribution, n (%) | |||
| Abdominal aortic aneurysm | 35 (100) | 9 (82) | 0.053 |
| CIA aneurysm (R/l/Bil) | 11/6/17 (31/17/49) | 3/2/6 (27/18/55) | 0.88 |
| EIA aneurysm (R/l/Bil) | 1/1/0 (3/3/0) | 0/0/0 (0/0/0) | 0.57 |
| IIA aneurysm (R/l/Bil) | 3/5/3 (9/14/9) | 0/1/0 (0/9/0) | 0.26 |
Medical severity score = (heart × 4 + pulmonary × 2 + renal × 2 + hypertension × 1 + age × 1)/10.
cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Anatomic severity score | |||
| Neck, median (range) | |||
| Diameter | 0 (0–1) | 0 (0–3) | 0.50 |
| Length | 0 (0) | 0 (0) | 0.81 |
| Angle | 0 (0–1) | 0 (0) | 0.35 |
| Calcification/thrombus | 0 (0) | 0 (0) | 0.60 |
| AAA, median (range) | |||
| TI | 1 (0–2) | 1 (1–1) | 0.51 |
| Angle | 1 (0–2) | 1 (0–2) | 0.82 |
| Thrombus | 1 (0–1) | 0 (0–1) | 0.21 |
| Branch vessels | 2 (2–3) | 2 (0–3) | 0.36 |
| Iliac artery (bilateral mean score), median (range) | |||
| Internal iliac artery | 0 (0–0) | 0 (0–0) | 0.890 |
| Calcification | 1 (1–2) | 1 (0–1) | 0.20 |
| Diameter/occlusive disease | 0.5 (0–1) | 1 (0–1) | 0.68 |
| TI | 1 (1–2) | 0.5 (0–1) | 0.001 |
| Angle | 1.5 (1–2) | 1 (0.5–1.5) | 0.31 |
| Seal length | 1 (0.5–1.5) | 0.5 (0–1.5) | 0.26 |
| Seal diameter | 2.5 (2–3) | 1.5 (1–2) | 0.022 |
| Average score, median (range) | 1.3 (1.1–1.5) | 1.1 (0.6–1.3) | 0.078 |
| Iliac artery parameter (IBD side) | n = 37 | n = 11 | |
| CIA, median (range) | |||
| Diameter (mm) | 36.7 (30.6–40.7) | 39.8 (23.3–45.1) | 0.97 |
| Length (mm) | 59.4 (48.4–69.2) | 41.4 (37.4–54.1) | 0.003 |
| Bifurcation diameter (mm), median (range) | 20.3 (17.2–24.1) | 16.3 (14.6–17.6) | 0.005 |
| EIA, median (range) | |||
| Diameter (mm) | 10.3 (9.3–11.9) | 7.0 (6.6–7.9) | <0.001 |
| Sealing length (mm) | 132.1 (123.6–142.1) | 101.6 (87.7–126.5) | 0.001 |
| IIA, median (range) | |||
| Diameter (mm) | 9.7 (8.1–11.1) | 8.3 (6.6–10.0) | 0.043 |
| Sealing length (mm) | 47.8 (33.9–57.0) | 33.8 (27.2–35.6) | 0.013 |
| IBD side IIA (patent/stenosis/occlusion), n (%) | 17/20/0 (46/54/0) | 3/8/0 (27/73/0) | 0.52 |
| Contralateral IIA (patent/stenosis/occlusion) n (%) | 16/19/2 (43/51/6) | 2/7/2 (18/64/18) | 0.25 |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Anatomic severity score | |||
| Neck, median (range) | |||
| Diameter | 0 (0–1) | 0 (0–3) | 0.50 |
| Length | 0 (0) | 0 (0) | 0.81 |
| Angle | 0 (0–1) | 0 (0) | 0.35 |
| Calcification/thrombus | 0 (0) | 0 (0) | 0.60 |
| AAA, median (range) | |||
| TI | 1 (0–2) | 1 (1–1) | 0.51 |
| Angle | 1 (0–2) | 1 (0–2) | 0.82 |
| Thrombus | 1 (0–1) | 0 (0–1) | 0.21 |
| Branch vessels | 2 (2–3) | 2 (0–3) | 0.36 |
| Iliac artery (bilateral mean score), median (range) | |||
| Internal iliac artery | 0 (0–0) | 0 (0–0) | 0.890 |
| Calcification | 1 (1–2) | 1 (0–1) | 0.20 |
| Diameter/occlusive disease | 0.5 (0–1) | 1 (0–1) | 0.68 |
| TI | 1 (1–2) | 0.5 (0–1) | 0.001 |
| Angle | 1.5 (1–2) | 1 (0.5–1.5) | 0.31 |
| Seal length | 1 (0.5–1.5) | 0.5 (0–1.5) | 0.26 |
| Seal diameter | 2.5 (2–3) | 1.5 (1–2) | 0.022 |
| Average score, median (range) | 1.3 (1.1–1.5) | 1.1 (0.6–1.3) | 0.078 |
| Iliac artery parameter (IBD side) | n = 37 | n = 11 | |
| CIA, median (range) | |||
| Diameter (mm) | 36.7 (30.6–40.7) | 39.8 (23.3–45.1) | 0.97 |
| Length (mm) | 59.4 (48.4–69.2) | 41.4 (37.4–54.1) | 0.003 |
| Bifurcation diameter (mm), median (range) | 20.3 (17.2–24.1) | 16.3 (14.6–17.6) | 0.005 |
| EIA, median (range) | |||
| Diameter (mm) | 10.3 (9.3–11.9) | 7.0 (6.6–7.9) | <0.001 |
| Sealing length (mm) | 132.1 (123.6–142.1) | 101.6 (87.7–126.5) | 0.001 |
| IIA, median (range) | |||
| Diameter (mm) | 9.7 (8.1–11.1) | 8.3 (6.6–10.0) | 0.043 |
| Sealing length (mm) | 47.8 (33.9–57.0) | 33.8 (27.2–35.6) | 0.013 |
| IBD side IIA (patent/stenosis/occlusion), n (%) | 17/20/0 (46/54/0) | 3/8/0 (27/73/0) | 0.52 |
| Contralateral IIA (patent/stenosis/occlusion) n (%) | 16/19/2 (43/51/6) | 2/7/2 (18/64/18) | 0.25 |
Anatomic severity score= (neck item + AAA item + iliac item)/15.
AAA: abdominal aortic aneurysm; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device; TI: tortuosity index.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Anatomic severity score | |||
| Neck, median (range) | |||
| Diameter | 0 (0–1) | 0 (0–3) | 0.50 |
| Length | 0 (0) | 0 (0) | 0.81 |
| Angle | 0 (0–1) | 0 (0) | 0.35 |
| Calcification/thrombus | 0 (0) | 0 (0) | 0.60 |
| AAA, median (range) | |||
| TI | 1 (0–2) | 1 (1–1) | 0.51 |
| Angle | 1 (0–2) | 1 (0–2) | 0.82 |
| Thrombus | 1 (0–1) | 0 (0–1) | 0.21 |
| Branch vessels | 2 (2–3) | 2 (0–3) | 0.36 |
| Iliac artery (bilateral mean score), median (range) | |||
| Internal iliac artery | 0 (0–0) | 0 (0–0) | 0.890 |
| Calcification | 1 (1–2) | 1 (0–1) | 0.20 |
| Diameter/occlusive disease | 0.5 (0–1) | 1 (0–1) | 0.68 |
| TI | 1 (1–2) | 0.5 (0–1) | 0.001 |
| Angle | 1.5 (1–2) | 1 (0.5–1.5) | 0.31 |
| Seal length | 1 (0.5–1.5) | 0.5 (0–1.5) | 0.26 |
| Seal diameter | 2.5 (2–3) | 1.5 (1–2) | 0.022 |
| Average score, median (range) | 1.3 (1.1–1.5) | 1.1 (0.6–1.3) | 0.078 |
| Iliac artery parameter (IBD side) | n = 37 | n = 11 | |
| CIA, median (range) | |||
| Diameter (mm) | 36.7 (30.6–40.7) | 39.8 (23.3–45.1) | 0.97 |
| Length (mm) | 59.4 (48.4–69.2) | 41.4 (37.4–54.1) | 0.003 |
| Bifurcation diameter (mm), median (range) | 20.3 (17.2–24.1) | 16.3 (14.6–17.6) | 0.005 |
| EIA, median (range) | |||
| Diameter (mm) | 10.3 (9.3–11.9) | 7.0 (6.6–7.9) | <0.001 |
| Sealing length (mm) | 132.1 (123.6–142.1) | 101.6 (87.7–126.5) | 0.001 |
| IIA, median (range) | |||
| Diameter (mm) | 9.7 (8.1–11.1) | 8.3 (6.6–10.0) | 0.043 |
| Sealing length (mm) | 47.8 (33.9–57.0) | 33.8 (27.2–35.6) | 0.013 |
| IBD side IIA (patent/stenosis/occlusion), n (%) | 17/20/0 (46/54/0) | 3/8/0 (27/73/0) | 0.52 |
| Contralateral IIA (patent/stenosis/occlusion) n (%) | 16/19/2 (43/51/6) | 2/7/2 (18/64/18) | 0.25 |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Anatomic severity score | |||
| Neck, median (range) | |||
| Diameter | 0 (0–1) | 0 (0–3) | 0.50 |
| Length | 0 (0) | 0 (0) | 0.81 |
| Angle | 0 (0–1) | 0 (0) | 0.35 |
| Calcification/thrombus | 0 (0) | 0 (0) | 0.60 |
| AAA, median (range) | |||
| TI | 1 (0–2) | 1 (1–1) | 0.51 |
| Angle | 1 (0–2) | 1 (0–2) | 0.82 |
| Thrombus | 1 (0–1) | 0 (0–1) | 0.21 |
| Branch vessels | 2 (2–3) | 2 (0–3) | 0.36 |
| Iliac artery (bilateral mean score), median (range) | |||
| Internal iliac artery | 0 (0–0) | 0 (0–0) | 0.890 |
| Calcification | 1 (1–2) | 1 (0–1) | 0.20 |
| Diameter/occlusive disease | 0.5 (0–1) | 1 (0–1) | 0.68 |
| TI | 1 (1–2) | 0.5 (0–1) | 0.001 |
| Angle | 1.5 (1–2) | 1 (0.5–1.5) | 0.31 |
| Seal length | 1 (0.5–1.5) | 0.5 (0–1.5) | 0.26 |
| Seal diameter | 2.5 (2–3) | 1.5 (1–2) | 0.022 |
| Average score, median (range) | 1.3 (1.1–1.5) | 1.1 (0.6–1.3) | 0.078 |
| Iliac artery parameter (IBD side) | n = 37 | n = 11 | |
| CIA, median (range) | |||
| Diameter (mm) | 36.7 (30.6–40.7) | 39.8 (23.3–45.1) | 0.97 |
| Length (mm) | 59.4 (48.4–69.2) | 41.4 (37.4–54.1) | 0.003 |
| Bifurcation diameter (mm), median (range) | 20.3 (17.2–24.1) | 16.3 (14.6–17.6) | 0.005 |
| EIA, median (range) | |||
| Diameter (mm) | 10.3 (9.3–11.9) | 7.0 (6.6–7.9) | <0.001 |
| Sealing length (mm) | 132.1 (123.6–142.1) | 101.6 (87.7–126.5) | 0.001 |
| IIA, median (range) | |||
| Diameter (mm) | 9.7 (8.1–11.1) | 8.3 (6.6–10.0) | 0.043 |
| Sealing length (mm) | 47.8 (33.9–57.0) | 33.8 (27.2–35.6) | 0.013 |
| IBD side IIA (patent/stenosis/occlusion), n (%) | 17/20/0 (46/54/0) | 3/8/0 (27/73/0) | 0.52 |
| Contralateral IIA (patent/stenosis/occlusion) n (%) | 16/19/2 (43/51/6) | 2/7/2 (18/64/18) | 0.25 |
Anatomic severity score= (neck item + AAA item + iliac item)/15.
AAA: abdominal aortic aneurysm; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device; TI: tortuosity index.
All elective surgeries were included in both groups. No significant difference was observed in perioperative characteristics, including IBD stent side, between the groups. The cIBD group had fewer previous abdominal aortic endografts (11% vs 46%, P = 0.025), more self-expandable (60% vs 9%, P = 0.003) and larger (10.0 vs 8.0 mm, P = 0.004) bridge stents, less axillary access cannulation of the bridge stent (14% vs 73%, P = 0.001), higher contralateral IIA embolization (34% vs 0%, P = 0.009) and higher concomitant AAA stent grafting (89% vs 36%, P = 0.001) than the cmIBD group had (Table 3). Both groups had a technical success rate higher than 90% (94% in the cIBD group and 100% in the cmIBD group; Table 3). The 2 technical failures in the cIBD group were an IIA bridge stent graft occlusion and a type 3 endoleak. During the postoperative surveillance period, no mortality or major complication occurred in either group (Table 3).
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Perioperative characteristics, n (%) | |||
| Previous EVAR stenting | 4 (11) | 5 (46) | 0.025 |
| IMA (patent/stenosis/occlusion) | 28/3/4 (80/9/11) | 4/2/5 (36/18/46) | 0.011 |
| IBD manufacture, Cook/Gore/Medtronic | 33/2/0 (94/6/0) | 0/0/11 (0/0/100) | <0.001 |
| IBD stent side (R/l/Bil) | 18/15/2 (51/43/6) | 8/3/0 (75/25/0) | 0.32 |
| Bridge stent (SE/BE) | 21/14 (60/40) | 1/10 (9/91) | 0.003 |
| Bridge stent diameter (mm) | 10.0 (10.0–11.0) | 8.0 (7.0–10.0) | 0.004 |
| Bridge stent cannulation access (axilla/femoral) | 5/30 (14/86) | 8/3 (73/27) | 0.001 |
| Contralateral IIA embolization (no/yes/prior) | 22/12/1 (63/34/3) | 9/0/2 (82/0/18) | 0.009 |
| Concomitant AAA stenting | 31 (89) | 4 (36) | 0.001 |
| Technical successa | 33 (94) | 11 (100) | 1.00 |
| Surgical complication, n | |||
| Retroperitoneal haematoma | 0 | 0 | |
| Stroke | 0 | 0 | |
| Buttock claudication | 0 | 0 | |
| Sexual claudication | 0 | 0 | |
| 30-Day mortality | 0 | 0 | |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Perioperative characteristics, n (%) | |||
| Previous EVAR stenting | 4 (11) | 5 (46) | 0.025 |
| IMA (patent/stenosis/occlusion) | 28/3/4 (80/9/11) | 4/2/5 (36/18/46) | 0.011 |
| IBD manufacture, Cook/Gore/Medtronic | 33/2/0 (94/6/0) | 0/0/11 (0/0/100) | <0.001 |
| IBD stent side (R/l/Bil) | 18/15/2 (51/43/6) | 8/3/0 (75/25/0) | 0.32 |
| Bridge stent (SE/BE) | 21/14 (60/40) | 1/10 (9/91) | 0.003 |
| Bridge stent diameter (mm) | 10.0 (10.0–11.0) | 8.0 (7.0–10.0) | 0.004 |
| Bridge stent cannulation access (axilla/femoral) | 5/30 (14/86) | 8/3 (73/27) | 0.001 |
| Contralateral IIA embolization (no/yes/prior) | 22/12/1 (63/34/3) | 9/0/2 (82/0/18) | 0.009 |
| Concomitant AAA stenting | 31 (89) | 4 (36) | 0.001 |
| Technical successa | 33 (94) | 11 (100) | 1.00 |
| Surgical complication, n | |||
| Retroperitoneal haematoma | 0 | 0 | |
| Stroke | 0 | 0 | |
| Buttock claudication | 0 | 0 | |
| Sexual claudication | 0 | 0 | |
| 30-Day mortality | 0 | 0 | |
BE: balloon-expandable; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device; EVAR: endovascular aortic repair; IMA: inferior mesenteric artery; SE: self-expandable.
aTechnical success was defined as the uneventful implantation of a stent graft with accompanying branch patency and without any endoleak by the end of the intervention period.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Perioperative characteristics, n (%) | |||
| Previous EVAR stenting | 4 (11) | 5 (46) | 0.025 |
| IMA (patent/stenosis/occlusion) | 28/3/4 (80/9/11) | 4/2/5 (36/18/46) | 0.011 |
| IBD manufacture, Cook/Gore/Medtronic | 33/2/0 (94/6/0) | 0/0/11 (0/0/100) | <0.001 |
| IBD stent side (R/l/Bil) | 18/15/2 (51/43/6) | 8/3/0 (75/25/0) | 0.32 |
| Bridge stent (SE/BE) | 21/14 (60/40) | 1/10 (9/91) | 0.003 |
| Bridge stent diameter (mm) | 10.0 (10.0–11.0) | 8.0 (7.0–10.0) | 0.004 |
| Bridge stent cannulation access (axilla/femoral) | 5/30 (14/86) | 8/3 (73/27) | 0.001 |
| Contralateral IIA embolization (no/yes/prior) | 22/12/1 (63/34/3) | 9/0/2 (82/0/18) | 0.009 |
| Concomitant AAA stenting | 31 (89) | 4 (36) | 0.001 |
| Technical successa | 33 (94) | 11 (100) | 1.00 |
| Surgical complication, n | |||
| Retroperitoneal haematoma | 0 | 0 | |
| Stroke | 0 | 0 | |
| Buttock claudication | 0 | 0 | |
| Sexual claudication | 0 | 0 | |
| 30-Day mortality | 0 | 0 | |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | P-value . |
|---|---|---|---|
| Perioperative characteristics, n (%) | |||
| Previous EVAR stenting | 4 (11) | 5 (46) | 0.025 |
| IMA (patent/stenosis/occlusion) | 28/3/4 (80/9/11) | 4/2/5 (36/18/46) | 0.011 |
| IBD manufacture, Cook/Gore/Medtronic | 33/2/0 (94/6/0) | 0/0/11 (0/0/100) | <0.001 |
| IBD stent side (R/l/Bil) | 18/15/2 (51/43/6) | 8/3/0 (75/25/0) | 0.32 |
| Bridge stent (SE/BE) | 21/14 (60/40) | 1/10 (9/91) | 0.003 |
| Bridge stent diameter (mm) | 10.0 (10.0–11.0) | 8.0 (7.0–10.0) | 0.004 |
| Bridge stent cannulation access (axilla/femoral) | 5/30 (14/86) | 8/3 (73/27) | 0.001 |
| Contralateral IIA embolization (no/yes/prior) | 22/12/1 (63/34/3) | 9/0/2 (82/0/18) | 0.009 |
| Concomitant AAA stenting | 31 (89) | 4 (36) | 0.001 |
| Technical successa | 33 (94) | 11 (100) | 1.00 |
| Surgical complication, n | |||
| Retroperitoneal haematoma | 0 | 0 | |
| Stroke | 0 | 0 | |
| Buttock claudication | 0 | 0 | |
| Sexual claudication | 0 | 0 | |
| 30-Day mortality | 0 | 0 | |
BE: balloon-expandable; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device; EVAR: endovascular aortic repair; IMA: inferior mesenteric artery; SE: self-expandable.
aTechnical success was defined as the uneventful implantation of a stent graft with accompanying branch patency and without any endoleak by the end of the intervention period.
The median follow-up time was 2.3 years for the cIBD group and 2.0 years for the cmIBD group. During follow-up, 1 type 1, 5 type 2 and 2 type 3 endoleak cases were recorded in the cIBD group, and no endoleak cases were identified in the cmIBD group (Table 4); however, this difference between the groups was not significant. Furthermore, 4 bridge stent graft occlusions were identified in the cIBD group, and 1 was identified in the cmIBD group, also without significant difference (Table 4). In the cIBD group, 1 patient developed stent graft mycotic infection and underwent reoperation. Three other patients in the cIBD group underwent reintervention because of type 3 endoleak, EIA stent graft total occlusion and the IBD main body floating separately, respectively (Table 4). The IBD patency rate was 87% (31/35) during 2.3 years of follow-up in the cIBD group and 91% (10/11) during 2.0 years for the cmIBD group. The primary, secondary patency, the freedom from reintervention, all-cause mortality and aneurysm-related mortality rate at 5 years in both groups were without significant difference (Fig. 3A–E).
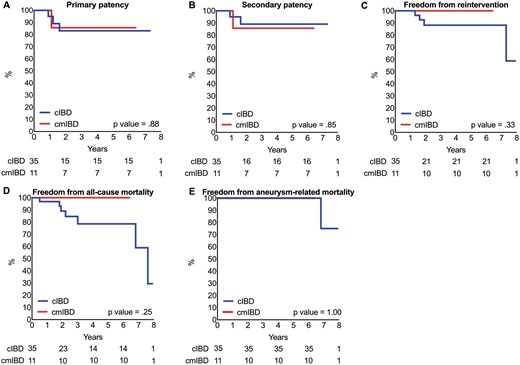
Kaplan–Meier analysis. (A) Primary patency rate. (B) Secondary patency rate. (C) Freedom from reintervention rate. (D) Freedom from all-cause mortality rate. (E) Freedom from aneurysm-related mortality rate.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | Relative risk (95% CI) . | P-value . |
|---|---|---|---|---|
| Follow-up duration (years), median (range) | 2.3 (1.7–4.8) | 2.0 (1.2–3.4) | 0.34 | |
| Endoleak, n (%) | ||||
| Type 1 | 1 (3) | 0 | 1.00 | |
| Type 2 | 5 (14) | 0 | 0.57 | |
| Type 3 | 2 (6) | 0 | 1.00 | |
| Total | 8 (23) | 0 | 0.17 | |
| Bridge stent occlusion n (%) | 4 (11) | 1 (9) | 1.3 (0.16–10.10) | 1.00 |
| Mycotic infection n (%) | 1 (3) | 0 | 1.00 | |
| Reintervention n (%) | 4 (11) | 0 | 0.56 | |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | Relative risk (95% CI) . | P-value . |
|---|---|---|---|---|
| Follow-up duration (years), median (range) | 2.3 (1.7–4.8) | 2.0 (1.2–3.4) | 0.34 | |
| Endoleak, n (%) | ||||
| Type 1 | 1 (3) | 0 | 1.00 | |
| Type 2 | 5 (14) | 0 | 0.57 | |
| Type 3 | 2 (6) | 0 | 1.00 | |
| Total | 8 (23) | 0 | 0.17 | |
| Bridge stent occlusion n (%) | 4 (11) | 1 (9) | 1.3 (0.16–10.10) | 1.00 |
| Mycotic infection n (%) | 1 (3) | 0 | 1.00 | |
| Reintervention n (%) | 4 (11) | 0 | 0.56 | |
cIBD: commercial iliac branch device; CI: confidence interval; cmIBD: custom-made iliac bifurcation device.
| . | cIBD (n = 35) . | cmIBD (n = 11) . | Relative risk (95% CI) . | P-value . |
|---|---|---|---|---|
| Follow-up duration (years), median (range) | 2.3 (1.7–4.8) | 2.0 (1.2–3.4) | 0.34 | |
| Endoleak, n (%) | ||||
| Type 1 | 1 (3) | 0 | 1.00 | |
| Type 2 | 5 (14) | 0 | 0.57 | |
| Type 3 | 2 (6) | 0 | 1.00 | |
| Total | 8 (23) | 0 | 0.17 | |
| Bridge stent occlusion n (%) | 4 (11) | 1 (9) | 1.3 (0.16–10.10) | 1.00 |
| Mycotic infection n (%) | 1 (3) | 0 | 1.00 | |
| Reintervention n (%) | 4 (11) | 0 | 0.56 | |
| . | cIBD (n = 35) . | cmIBD (n = 11) . | Relative risk (95% CI) . | P-value . |
|---|---|---|---|---|
| Follow-up duration (years), median (range) | 2.3 (1.7–4.8) | 2.0 (1.2–3.4) | 0.34 | |
| Endoleak, n (%) | ||||
| Type 1 | 1 (3) | 0 | 1.00 | |
| Type 2 | 5 (14) | 0 | 0.57 | |
| Type 3 | 2 (6) | 0 | 1.00 | |
| Total | 8 (23) | 0 | 0.17 | |
| Bridge stent occlusion n (%) | 4 (11) | 1 (9) | 1.3 (0.16–10.10) | 1.00 |
| Mycotic infection n (%) | 1 (3) | 0 | 1.00 | |
| Reintervention n (%) | 4 (11) | 0 | 0.56 | |
cIBD: commercial iliac branch device; CI: confidence interval; cmIBD: custom-made iliac bifurcation device.
In aneurysm remodelling, 32 legs in the cIBD group and 8 in the cmIBD group were fully followed up for longer than 1 year. No obvious postoperative AAA size decrease (compared with preoperative size) was observed in either group (Table 5). For CIAAs, postoperative diameter and volume obviously decreased from preoperative size in both groups (diameter: 3.9 to 3.6 cm in the cIBD group, P = 0.002; 4.3 to 3.5 cm in the cmIBD group, P = 0.012; volume: 29.0 to 25.0 ml in the cIBD group, P = 0.070; 32.0 to 27.4 ml in the cmIBD group, P = 0.012). The change ratios of diameter and volume were similar in the between-group comparison (Table 5).
| . | Preoperative . | Postoperative . | P-value (pre versus post) . | Change ratio (post − pre)/pre . | P-value (cIBD versus cmIBD) . |
|---|---|---|---|---|---|
| AAA (cIBD n = 30 vs cmIBD n = 8) | |||||
| Diameter (cm), median (range) | |||||
| cIBD | 4.6 (3.5–5.5) | 4.3 (3.6–5.6) | 0.85 | 0.02 (−0.08 to 0.05) | 0.77 |
| cmIBD | 3.4 (2.9–5.1) | 3.6 (3.1–5.2) | 0.48 | 0.03 (−0.08 to 0.08) | |
| Volume (ml) | |||||
| cIBD | 100.5 (59.8–148.0) | 89.8 (59.2–156.5) | 0.44 | 0.07 (−0.07 to 0.15) | 1.00 |
| cmIBD | 56.4 (33.5–123.9) | 67.9 (31.9–118.2) | 0.33 | 0.03 (−0.07 to 0.17) | |
| CIA aneurysm (by IBD side, cIBD n = 32 vs cmIBD n = 8) | |||||
| Diameter (cm) | |||||
| cIBD | 3.9 (3.4–4.2) | 3.6 (3.2–4.0) | 0.002 | −0.04 (−0.11 to 0.02) | 0.13 |
| cmIBD | 4.3 (3.6–4.5) | 3.5 (2.6–4.2) | 0.012 | −0.11 (−0.22 to −0.03) | |
| Volume (ml) | |||||
| cIBD | 29.0 (21.8–39.7) | 25.0 (18.4–32.7) | 0.070 | −0.05 (−0.18 to 0.07) | 0.31 |
| cmIBD | 32.0 (19.3–44.8) | 27.4 (12.8–42.6) | 0.012 | −0.10 (−0.19 to −0.02) | |
| . | Preoperative . | Postoperative . | P-value (pre versus post) . | Change ratio (post − pre)/pre . | P-value (cIBD versus cmIBD) . |
|---|---|---|---|---|---|
| AAA (cIBD n = 30 vs cmIBD n = 8) | |||||
| Diameter (cm), median (range) | |||||
| cIBD | 4.6 (3.5–5.5) | 4.3 (3.6–5.6) | 0.85 | 0.02 (−0.08 to 0.05) | 0.77 |
| cmIBD | 3.4 (2.9–5.1) | 3.6 (3.1–5.2) | 0.48 | 0.03 (−0.08 to 0.08) | |
| Volume (ml) | |||||
| cIBD | 100.5 (59.8–148.0) | 89.8 (59.2–156.5) | 0.44 | 0.07 (−0.07 to 0.15) | 1.00 |
| cmIBD | 56.4 (33.5–123.9) | 67.9 (31.9–118.2) | 0.33 | 0.03 (−0.07 to 0.17) | |
| CIA aneurysm (by IBD side, cIBD n = 32 vs cmIBD n = 8) | |||||
| Diameter (cm) | |||||
| cIBD | 3.9 (3.4–4.2) | 3.6 (3.2–4.0) | 0.002 | −0.04 (−0.11 to 0.02) | 0.13 |
| cmIBD | 4.3 (3.6–4.5) | 3.5 (2.6–4.2) | 0.012 | −0.11 (−0.22 to −0.03) | |
| Volume (ml) | |||||
| cIBD | 29.0 (21.8–39.7) | 25.0 (18.4–32.7) | 0.070 | −0.05 (−0.18 to 0.07) | 0.31 |
| cmIBD | 32.0 (19.3–44.8) | 27.4 (12.8–42.6) | 0.012 | −0.10 (−0.19 to −0.02) | |
AAA: abdominal aortic aneurysm; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device.
| . | Preoperative . | Postoperative . | P-value (pre versus post) . | Change ratio (post − pre)/pre . | P-value (cIBD versus cmIBD) . |
|---|---|---|---|---|---|
| AAA (cIBD n = 30 vs cmIBD n = 8) | |||||
| Diameter (cm), median (range) | |||||
| cIBD | 4.6 (3.5–5.5) | 4.3 (3.6–5.6) | 0.85 | 0.02 (−0.08 to 0.05) | 0.77 |
| cmIBD | 3.4 (2.9–5.1) | 3.6 (3.1–5.2) | 0.48 | 0.03 (−0.08 to 0.08) | |
| Volume (ml) | |||||
| cIBD | 100.5 (59.8–148.0) | 89.8 (59.2–156.5) | 0.44 | 0.07 (−0.07 to 0.15) | 1.00 |
| cmIBD | 56.4 (33.5–123.9) | 67.9 (31.9–118.2) | 0.33 | 0.03 (−0.07 to 0.17) | |
| CIA aneurysm (by IBD side, cIBD n = 32 vs cmIBD n = 8) | |||||
| Diameter (cm) | |||||
| cIBD | 3.9 (3.4–4.2) | 3.6 (3.2–4.0) | 0.002 | −0.04 (−0.11 to 0.02) | 0.13 |
| cmIBD | 4.3 (3.6–4.5) | 3.5 (2.6–4.2) | 0.012 | −0.11 (−0.22 to −0.03) | |
| Volume (ml) | |||||
| cIBD | 29.0 (21.8–39.7) | 25.0 (18.4–32.7) | 0.070 | −0.05 (−0.18 to 0.07) | 0.31 |
| cmIBD | 32.0 (19.3–44.8) | 27.4 (12.8–42.6) | 0.012 | −0.10 (−0.19 to −0.02) | |
| . | Preoperative . | Postoperative . | P-value (pre versus post) . | Change ratio (post − pre)/pre . | P-value (cIBD versus cmIBD) . |
|---|---|---|---|---|---|
| AAA (cIBD n = 30 vs cmIBD n = 8) | |||||
| Diameter (cm), median (range) | |||||
| cIBD | 4.6 (3.5–5.5) | 4.3 (3.6–5.6) | 0.85 | 0.02 (−0.08 to 0.05) | 0.77 |
| cmIBD | 3.4 (2.9–5.1) | 3.6 (3.1–5.2) | 0.48 | 0.03 (−0.08 to 0.08) | |
| Volume (ml) | |||||
| cIBD | 100.5 (59.8–148.0) | 89.8 (59.2–156.5) | 0.44 | 0.07 (−0.07 to 0.15) | 1.00 |
| cmIBD | 56.4 (33.5–123.9) | 67.9 (31.9–118.2) | 0.33 | 0.03 (−0.07 to 0.17) | |
| CIA aneurysm (by IBD side, cIBD n = 32 vs cmIBD n = 8) | |||||
| Diameter (cm) | |||||
| cIBD | 3.9 (3.4–4.2) | 3.6 (3.2–4.0) | 0.002 | −0.04 (−0.11 to 0.02) | 0.13 |
| cmIBD | 4.3 (3.6–4.5) | 3.5 (2.6–4.2) | 0.012 | −0.11 (−0.22 to −0.03) | |
| Volume (ml) | |||||
| cIBD | 29.0 (21.8–39.7) | 25.0 (18.4–32.7) | 0.070 | −0.05 (−0.18 to 0.07) | 0.31 |
| cmIBD | 32.0 (19.3–44.8) | 27.4 (12.8–42.6) | 0.012 | −0.10 (−0.19 to −0.02) | |
AAA: abdominal aortic aneurysm; cIBD: commercial iliac branch device; cmIBD: custom-made iliac bifurcation device.
DISCUSSION
Evidence is growing that a cIBD can be used as an independent treatment or as an EVAR adjunct for treatment of aortic aneurysms with extensive iliac involvement, ensuring both the safety and durability of repairs for up to 5 years. The durability of iliac repair was estimated to be around 85.4% patency and 81.3% freedom from reintervention rates at 5 years [5, 10, 11]. These results support the use of a cIBD as first-line therapy for patients with iliac aneurysm and suitable anatomy. Although both endovascular therapy and open surgery are safe and effective methods with similar postoperative mortality and long-term patency, endovascular treatment can reduce transfusion and the duration of hospital stay [12–14]. In our study, the primary and secondary patency rates of the cIBD group were 81% and 87% at 5 years, respectively. The freedom from reintervention rate was 88% at 5 years, which corresponded with the review standards and indicated that the treatment was not inferior to others. However, the major disadvantage of cIBDs is technical feasibility related to anatomical restraints. The anatomical characteristics for cIBD application are that CIA length should be >40 mm in Cook and >55 mm in Gore devices and the diameter of the CIA adjacent to the branch should be >16 mm. Furthermore, the non-aneurysm EIA fixation segment distal to the aneurysm should be >20 mm in length and 8–11 mm in diameter. The IIA should be >10 mm in length, and its diameter should be acceptable for proper sealing. These limitations restrict the application of cIBDs in challenging anatomical cases. Although the Gore IBE can be applied in cases of 6.5 mm EIA and IIA diameters, in our cmIBD patients, the Gore cIBE could not be applied due to short CIA length and small bifurcation diameter.
Hence, the use of cmIBDs has become an alternative method to treat anatomy-limited iliac aneurysm. The concept is similar to that of the cIBD but is more suitable for challenging anatomy. The technique preserves internal iliac artery perfusion without prominent complication. The chimney technique is another choice; however, gutter leak and compression between bridging stents can cause more complications. Therefore, cmIBDs may simplify the procedure and reduce concern. Our results indicated that the cIBD and cmIBD groups had similar preoperative characteristics, including comorbidity severity, aneurysm anatomy and anatomic severity. Although a higher bilateral mean iliac TI score was identified in the cIBD group, average anatomic severity scores for AAAs were not significantly different. Regarding the iliac artery characteristics of the IBD side, CIA length and EIA and IIA sealing length were significantly larger and longer in the cIBD than in the cmIBD group. This implies that the landing criteria of cmIBD are more flexible than are those of cIBD. IIA cannulation by axillary access was used more frequently in cmIBD than in cIBD because of higher prevalence of prior abdominal aortic endografting in the cmIBD group. A sharp aortic stent graft bifurcation angle is more difficult to cross over by contralateral femoral access. Balloon-expandable stent usage was more frequent in cmIBD bridging. This study has further confirmed implantation accuracy and fixation security of bridge stents, particularly when the landing zone is located only at the ridge of the fenestrated hole of the main stent graft in the cmIBD group. Although a smaller bridge stent size may be tolerable in the cmIBD group, its stent occlusion rates were not significantly higher than those in the cIBD group. Only 1 bridge stent was occluded in the cmIBD group, but 4 were occluded in the cIBD group during the mean 2–3-year follow-up. However, none expressed any concomitant symptoms. The gradual occlusion of bridge stent graft in our current study allowed full compensation with well-developed collateral circulation [15, 16].
In the cmIBD group, no iliac endoleak was detected during the follow-up period, but a few endoleak cases were observed in the cIBD group. Although no significant difference was found, the results implied that the conformity of cmIBDs might be superior to that of cIBDs in challenging anatomy. A cIBD is of uniform size but is an anatomy-limited product that cannot be adapted to different anatomical characteristics. For CIA length segment selection, products with only 2 sizes are available in cIBDs of Zenith design: 45 and 61 mm (proximal edge to tip of side branch). This indicates that if CIA length is shorter than 45 mm, the cIBD proximal edge may extend into the aorta or distal CIA wall, restricting side branch full opening. Conversely, the main body of the cmIBDs was the iliac limb of a stent graft, which is available in several sizes. The proximal landing zone is required only to be 22 mm; therefore, cmIBDs may be suitable for a shorter CIA length of between 22 and 45 mm. Hence, for some CIAAs with a short neck, an iliac leg stent graft in cmIBDs can be fenestrated as the side branch hole, and the diameter can be adjusted according to preoperative CT images to ensure an adequately sized landing zone. In our cmIBD group, average CIA length was only 46.3 mm that is smaller than cIBD group. It implied that cmIBD is more adaptable for this shorter and difficult anatomy. For the IIA side branch of cIBDs, the size choice is only 8 mm in diameter. Hence, the large discrepancy between IIA diameter and the overlapping side branch of a cIBD is a point at risk of endoleak. It also restricts the size choice for an IIA bridging stent. A smaller IIA may induce bridge stent graft deployment folding, and a larger IIA may cause bridge stent graft floating. Either would increase the risk of type I or type III endoleak. For cmIBDs, the fenestration diameter of the side branch can be adjusted according to preoperative CT images. A hole can be created by electrocauterizing ~80–90% of the orifice of the IIA in diameter, which is enforced by a 0.014″ wire so that it is visible under fluorescence. Hence, bridge stent graft size can be selected appropriately, and types I and III endoleak can be avoided. Furthermore, after IIA stent graft deployment through the fenestration hole, the protruding section of the stent may then be dilated using a larger sized balloon to form a funnel-shaped conduit for blood flow into the IIA. This process is referred to as ‘flaring skill’ and is necessary to ensure intimate contact between the IIA stent graft and fenestration hole. It also reduces the risk of type 3 endoleak [17, 18]. Hence, it may also explain why no type 3 endoleak was recorded in the cmIBD group. In the study by Kandail et al., flaring was implemented within the renal stent and fenestrated stent graft connection. A flared renal stent can maintain sufficient renal perfusion but may increase thrombus formation at the renal ostia because of disturbance in the flow [17]. However, the thrombus may also seal the gap between the fenestration hole edge and the bridging stent graft. Hence, the flaring method is suggested for use with the cmIBD group.
In our study, aneurysm remodelling at 1-year follow-up revealed decreased diameter and volume of CIAAs in both the groups, and cmIBDs achieved aneurysm shrinkage ratios similar to those of cIBDs. Hence, cmIBDs offer an alternative method for situations in which cIBDs may not be suitable.
Limitations
This study was limited by its retrospective single-centre design, small sample size and short follow-up interval, particularly with the cmIBD group, patients of which were followed up for a mean of only 2 years after surgery. Although the P-value in many postoperative events did not indicate significant difference and confidence intervals are still pending, partially due to ‘zero’ events in the small cmIBD group, the cmIBD design may be a novel innovation for treating patients when a cIBD is not compatible. Long-term evidence for bridging stent occlusion, endoleak and aneurysm remodelling must be evaluated to support the findings of the present study.
CONCLUSION
For iliac aneurysm, a novel cmIBD was not inferior to cIBDs with regard to postoperative complications, bridge occlusion, endoleak, or short-term aneurysm remodelling. A cmIBD with an adaptable design offers a simple alternative method for iliac aneurysm exclusion in challenging anatomic situations where use of a cIBD is not suitable.
ACKNOWLEDGEMENTS
The authors thank Chun-Han Shih and Chun-Kai Shih for providing technical assistance.
Funding
This work was supported by National Science Council, Taiwan [MOST105-2314-B-010-048-MY3]; Taipei Medical University, Taiwan [TMU108-F-005, TMU108-AE1-B18]; and Wan Fang Hospital [109-wf-eva-02, 109-wf-eva-07, 109TMU-WFH-19].
Conflict of interest: none declared.
Author contributions
Chun-Yang Huang: Data curation; Formal analysis; Methodology; Software; Writing—original draft. Po-Lin Chen: Investigation; Validation. Hsin-Ying Lu: Investigation. Hung-Lung Hsu: Data curation; Investigation; Resources. Tzu-Ting Kuo: Investigation; Validation. I-Ming Chen: Investigation; Resources. Chiao-Po Hsu: Investigation; Validation. Chun-Che Shih: Conceptualization; Funding acquisition; Project administration; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Roman Gottardi, Stoyan Kostadinov Kondov, Mario Lescan and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
REFERENCES
Abbreviations
- AAA
Abdominal aortic aneurysm
- cIAA
Common iliac artery aneurysm
- cIBD
Commercial iliac branch device
- cmBID
Custom-made iliac bifurcation device
- CT
Computed tomography
- IAA
Iliac artery aneurysm
- TI
Tortuosity index





