-
PDF
- Split View
-
Views
-
Cite
Cite
Yanxiang Liu, Shenghua Liang, Bowen Zhang, Yunfeng Li, Lucheng Wang, Yaojun Dun, Zujun Chen, Yi Shi, Hongwei Guo, Xiaogang Sun, Early outcomes of hybrid type II arch repair versus total arch replacement with frozen elephant trunk in acute DeBakey type I aortic dissection: a propensity score-matched analysis, Interactive CardioVascular and Thoracic Surgery, Volume 31, Issue 4, October 2020, Pages 565–572, https://doi.org/10.1093/icvts/ivaa121
Close - Share Icon Share
Abstract
The aim was to evaluate the short-term outcomes of hybrid type II arch repair (HAR) and total arch replacement with frozen elephant trunk (TAR with FET) for acute DeBakey type I aortic dissection patients.
From January 2017 to June 2019, the clinical data of acute DeBakey type I aortic dissection patients in a single centre were retrospectively reviewed; there were 92 cases of HAR and 268 cases of TAR with FET, with 56 pairs by propensity score matching.
After matching, the composite end points including 30-day mortality, stroke, paraplegia, renal failure, hepatic failure, reintubation or tracheotomy and low cardiac output syndrome were comparable (21.4%, 12/56 in the HAR group vs 21.4%, 12/56 in the TAR with FET group, P = 1.000). The rate of acute kidney injury (AKI) was significantly lower in the HAR group (58.9%, 33/56 vs 80.4%, 45/56, P = 0.031). The distribution of AKI stage according to the Kidney Disease Improving Global Outcomes criteria was different (P = 0.039), with more patients suffering from high-grade AKI in the TAR with FET group. Multivariable logistic analysis showed that the procedure type (HAR or TAR with FET) was not an independent predictor of composite adverse events or stroke. HAR was identified as a protective factor against AKI (odds ratio 0.485, 95% confidence interval 0.287–0.822; P = 0.007).
In the treatment of acute DeBakey type I aortic dissection, no significant differences were found in early outcomes between the 2 groups, but HAR was associated with a significantly lower incidence of AKI.
INTRODUCTION
At present, total arch replacement with frozen elephant trunk (TAR with FET) has become a routine surgical procedure to treat acute DeBakey type I aortic dissection (AIAD), since Sun et al. [1] introduced this surgery in China in 2006. The FET was used to expand the true lumen, promote thrombosis of the residual false lumen and simplify the second-phase operation for the descending aorta.
However, long-term hypothermic circulatory arrest is inevitable and regarded as one cause of mortality and morbidity [2]. In patients thought to be at prohibitively high risk of TAR with FET, an alternative therapy is much desired. Milewski et al. [3] first described the classification of hybrid procedures in 2010, and divided them into 3 types. Hybrid type II arch repair (HAR) combined open ascending aortic replacement with arch vessel debranching and endovascular total arch exclusion without circulation arrest in DeBakey type I aortic dissection.
Theoretically, avoiding lower body circulatory arrest could provide better organ protection. However, the protective effect of this hybrid procedure remains unclear. This study is designed to compare the short-term outcomes of HAR and TAR with FET by the use of propensity score matching.
PATIENTS AND METHODS
Patients
Between January 2017 and June 2019, a total of 360 consecutive patients with AIAD (within 14 days from the onset of symptoms to the operation) underwent total aortic arch repair at Fuwai Hospital, despite the location of the primary intimal tear. Patients were divided into 2 groups according to the surgical procedure: 92 patients in the HAR group and 268 patients in the TAR with FET group.
In our institute, TAR with FET is considered the first choice. We also selected hybrid approaches for high-risk patients based on the patient’s age, preoperative status, frailty and extent of aortic dissection treatment.
Operative techniques
Total arch replacement with frozen elephant trunk group
The surgical technique of TAR with FET (Fig. 1A), sometimes called Sun’s procedure, has been previously described in detail [4]. Sun’s procedure was performed through a standard median sternotomy under cardiopulmonary bypass (CPB) and selective cerebral perfusion through the right axillary artery. The right axillary artery was our preferred inflow site for CPB. The innominate artery and femoral artery were sometimes used based on each patient’s status. During the cooling phase, aortic root procedures were performed if necessary. Circulatory arrest was instituted when the nasopharyngeal temperature reached 24°C. Meanwhile, antegrade selective cerebral perfusion was started at a rate of ∼5–8 ml/(kg · min). The aortic arch was transected between the left common carotid and left subclavian arteries to avoid recurrent laryngeal nerve injury. Then the stented elephant trunk (Cronus, MicroPort Endovascular Shanghai Co, Ltd, Shanghai, China) was inserted into the true lumen of the descending aorta under direct vision. Subsequently, the distal end of a tetrafurcate prosthetic graft (Terumo, Vascutek Limited, Renfrewshire, Scotland, UK) was anastomosed to the descending aorta incorporating a stented elephant trunk using the open anastomosis technique. When the anastomosis was completed, perfusion of the lower body was resumed through the perfusion limb of the four-branched graft and the CPB flow was gradually returned. The last step was epiaortic vessel reconstruction and proximal arch anastomosis.
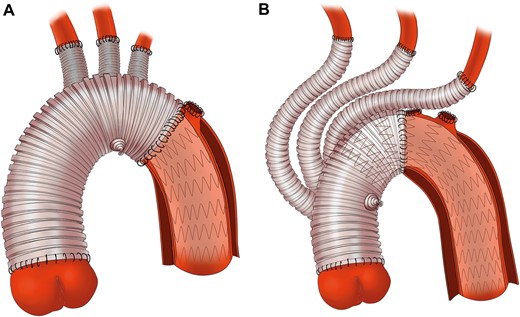
Surgical details of total arch replacement with frozen elephant trunk and hybrid type II arch repair. (A) In total arch replacement with frozen elephant trunk group, aortic arch was transected between the left carotid artery and left subclavian artery and replaced by a four-branched prosthetic graft with the frozen elephant trunk implanted into the descending aorta. (B) In hybrid type II arch repair group, aortic arch was cross cut between the innominate artery and left carotid artery, and ascending aorta was replaced with a four-branched prosthetic graft whose trunk served as the proximal landing zone for the stent graft.
Hybrid type II arch repair group
Surgery was performed in the hybrid operating room. The surgical procedure has been previously described in detail (Fig. 1B) [5, 6]. Briefly, the right axillary and femoral arteries were both used as cannulation sites for this procedure. After cross-clamping, the ascending aorta was replaced with a four-branched prosthesis graft (Terumo, Vascutek Limited) with the proximal end of the graft anastomosed to the aortic root. When the nasopharyngeal temperature reached 28°C, 1 clamp was set on the innominate artery and another clamp was set on the arch between the innominate artery and left carotid artery. The aortic arch was transected proximal to the left common carotid artery. The distal end of the graft was then sutured end to end to the aortic arch. With air expelled in the ascending aorta, the arch was unclamped to restore coronary perfusion. The arch vessels were sequentially anastomosed with the left common artery, left subclavian artery and anonymous artery. The endograft was delivered in a retrograde fashion through the incision of the original femoral cannulation. Its proximal end was anchored to the prosthetic graft to complete the arch repair. The stent graft was oversized by 10–20%. Four kinds of commercial stents were used: Zenith (Cook Medical Inc., Bloomington, IN, USA), Relay (Bolton Medical, Sunrise, FL, USA), Talent and Valiant (Medtronic Inc., Santa Rosa, CA, USA) and Hercules (MicroPort Medical Co, Ltd, Shanghai, China).
Definitions and study end points
Cardiac malperfusion was defined as ST-segment elevation on electrocardiography, abnormal ventricular wall motion on echocardiography or a significantly increased value of serum cardiac troponin I. Other malperfusions were also recorded, including cerebral (alterations in mental status), visceral (abdominal pain, distention or a transaminase level higher than 100 IU/l), renal (serum creatinine level higher than 200 µmol/l) and limb (absence of pulse with limb compromise) malperfusion.
The primary end points were defined as composite adverse events including 30-day mortality, stroke, paraplegia, renal failure, hepatic failure, reintubation or tracheotomy and low cardiac output syndrome. Stroke was defined as new brain injury that was clinically or radiographically evident after the procedure. Paraplegia was defined as lower limb strength less than or equal to grade 3 (able to resist gravity but not resistance). Renal failure referred to the need for haemodialysis during hospitalization. Hepatic failure was defined as a postoperative aminotransferase level exceeding 1000 IU/l. Low cardiac output syndrome referred to the need for an intra-aortic balloon pump.
The secondary end point was acute kidney injury (AKI) ascertained and categorized according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria with slight modifications. Postoperative AKI was defined when postoperative serum creatinine (SCr) levels increased by >50% of the baseline or if there was an increase of 26.5 μmol/l (0.3 mg/dl) at 48 h postoperatively. Preoperative SCr values nearest to the time of surgery were used as the baseline SCr levels. The highest SCr values within 48 h after surgery were collected. Urine output was not taken into consideration due to the difficulty and inaccuracy when collected retrospectively. AKI was staged for severity according to the criteria presented in Table 1.
| Stage . | SCr increase . |
|---|---|
| 0 | <1.5 times baseline and <0.3 mg/dl (26.5 μmol/l) increase (no AKI) |
| 1 | 1.5–1.9 times baseline or ≥0.3 mg/dl (26.5 μmol/l) increase |
| 2 | 2.0–2.9 times baseline |
| 3 | ≥3 times baseline or increase in SCr to ≥4.0 mg/dl (353.6 μmol/l) or initiation of renal replacement therapy |
| Stage . | SCr increase . |
|---|---|
| 0 | <1.5 times baseline and <0.3 mg/dl (26.5 μmol/l) increase (no AKI) |
| 1 | 1.5–1.9 times baseline or ≥0.3 mg/dl (26.5 μmol/l) increase |
| 2 | 2.0–2.9 times baseline |
| 3 | ≥3 times baseline or increase in SCr to ≥4.0 mg/dl (353.6 μmol/l) or initiation of renal replacement therapy |
AKI: acute kidney injury; KDIGO: Kidney Disease Improving Global Outcomes; SCr: serum creatinine.
| Stage . | SCr increase . |
|---|---|
| 0 | <1.5 times baseline and <0.3 mg/dl (26.5 μmol/l) increase (no AKI) |
| 1 | 1.5–1.9 times baseline or ≥0.3 mg/dl (26.5 μmol/l) increase |
| 2 | 2.0–2.9 times baseline |
| 3 | ≥3 times baseline or increase in SCr to ≥4.0 mg/dl (353.6 μmol/l) or initiation of renal replacement therapy |
| Stage . | SCr increase . |
|---|---|
| 0 | <1.5 times baseline and <0.3 mg/dl (26.5 μmol/l) increase (no AKI) |
| 1 | 1.5–1.9 times baseline or ≥0.3 mg/dl (26.5 μmol/l) increase |
| 2 | 2.0–2.9 times baseline |
| 3 | ≥3 times baseline or increase in SCr to ≥4.0 mg/dl (353.6 μmol/l) or initiation of renal replacement therapy |
AKI: acute kidney injury; KDIGO: Kidney Disease Improving Global Outcomes; SCr: serum creatinine.
Statistical analysis
Continuous data are presented as the mean ± standard deviation and were analysed with Student’s t-test or the Mann–Whitney U-test, as appropriate. Categorical variables are reported as counts and percentages and were compared using the Pearson χ2 test or Fisher’s exact test (expected counts <1 or at least 2 expected counts <5). The stage of AKI was an ordinal categorical variable that was compared with the Mann–Whitney U-test.
We performed propensity score matching using a 1:1 nearest-available matching algorithm with a ±0.15 calliper and no replacement for the adjustment of significantly different patient characteristics. The analysis yielded 56 pairs of propensity score-matched observations. The propensity score was obtained by the use of a multivariable logistic regression model with hybrid approaches as the variables and the 19 baseline characteristics [age, sex, body mass index (BMI), hypertension, coronary artery disease, diabetes, chronic obstructive pulmonary disease, cerebrovascular accident, myocardial infarction, redo sternotomy, chronic kidney disease, New York Heart Association class ≥3, cardiac/cerebral/visceral/renal/limb malperfusion, left ventricular ejection fraction, median or massive aortic regurgitation] as covariates. The matching model achieved good discriminatory power (C-statistic = 0.912). We compared standardized mean differences for all covariates after matching. We also compared preoperative, intraoperative and postoperative data using the McNemar test for categorical variables and paired t-tests or Wilcoxon signed-rank tests for continuous variables.
Multivariable logistic regression analysis was applied to identify the predictors of composite adverse events, stroke and AKI after the HAR and TAR with FET procedures. It was also used to analyse whether HAR was a protective factor in regard to composite adverse events, stroke and AKI. Group, 19 preoperative variables, the Bentall procedure and coronary artery bypass grafting were included in the multivariable logistic models, and a forward variable selection approach was then performed. To avoid multicollinearity, the CPB time and cross-clamp time were excluded from the models.
For all analysis, a P-value of <0.05 was considered statistically significant, and all statistical tests were two-sided. R version 3.6.3 (The R Foundation for Statistical Computing) was used for propensity score matching and standardized mean difference calculations. Other statistics were analysed using SPSS version 25 (IBM, Armonk, NY, USA).
RESULTS
Patient characteristics
On an unmatched basis, patients subjected to HAR were significantly older than patients undergoing TAR with FET (61.4 ± 7.7 vs 46.0 ± 9.2 years, P < 0.001), and the percentage of males was significantly lower in the HAR group (64.1% vs 79.9%, P = 0.002). The HAR (versus TAR with FET) group also included a higher proportion of patients with chronic obstructive pulmonary disease and a lower proportion of patients with median or massive aortic regurgitation. No group differences were observed in terms of other preoperative variables.
After propensity score matching, the patient demographic characteristics and the clinical risk factors in the 2 groups appeared well balanced (Table 2).
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | |||||
|---|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | SMD . | |
| Age (years) | 61.4 ± 7.7 | 46.0 ± 9.2 | <0.001 | 57.7 ± 7.2 | 57.1 ± 6.9 | 0.385 | 0.084 |
| Male gender | 59 (64.1) | 214 (79.9) | 0.002 | 35 (62.5) | 34 (60.7) | 1.000 | 0.036 |
| BMI (kg/m2) | 26.0 ± 3.9 | 26.3 ± 4.0 | 0.510 | 25.7 ± 3.6 | 25.9 ± 3.5 | 0.757 | 0.053 |
| Hypertension | 81 (88.0) | 240 (89.6) | 0.688 | 49 (87.5) | 46 (82.1) | 0.549 | 0.148 |
| CAD | 19 (20.7) | 49 (18.3) | 0.617 | 11 (19.6) | 14 (25.0) | 0.678 | 0.128 |
| Diabetes | 5 (5.4) | 8 (3.0) | 0.446 | 3 (5.4) | 4 (7.1) | 1.000 | 0.073 |
| COPD | 3 (3.3) | 0 (0.0) | 0.016 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Cerebrovascular accident | 8 (8.7) | 14 (5.2) | 0.230 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Myocardial infarction | 1 (1.1) | 1 (0.4) | 0.446 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Redo sternotomy | 4 (4.3) | 8 (3.0) | 0.771 | 2 (3.6) | 3 (5.4) | 1.000 | 0.086 |
| CKD | 1 (1.1) | 3 (1.1) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| NYHA ≥3 | 4 (4.3) | 10 (3.7) | 1.000 | 1 (1.8) | 2 (3.6) | 1.000 | 0.110 |
| Organ malperfusion | |||||||
| Cardiac | 7 (7.6) | 15 (5.6) | 0.487 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Cerebral | 3 (3.3) | 12 (4.5) | 0.840 | 2 (3.6) | 2 (3.6) | 1.000 | <0.001 |
| Visceral | 5 (5.4) | 19 (7.1) | 0.583 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Renal | 1 (1.1) | 13 (4.9) | 0.194 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Limb | 0 (0.0) | 9 (3.4) | 0.164 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Ejection fraction (%) | 60.1 ± 5.1 | 60.7 ± 3.9 | 0.617 | 60.6 ± 4.4 | 60.9 ± 4.5 | 0.604 | 0.060 |
| Median or massive AR | 14 (15.2) | 70 (26.1) | 0.033 | 10 (17.9) | 10 (17.9) | 1.000 | <0.001 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | |||||
|---|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | SMD . | |
| Age (years) | 61.4 ± 7.7 | 46.0 ± 9.2 | <0.001 | 57.7 ± 7.2 | 57.1 ± 6.9 | 0.385 | 0.084 |
| Male gender | 59 (64.1) | 214 (79.9) | 0.002 | 35 (62.5) | 34 (60.7) | 1.000 | 0.036 |
| BMI (kg/m2) | 26.0 ± 3.9 | 26.3 ± 4.0 | 0.510 | 25.7 ± 3.6 | 25.9 ± 3.5 | 0.757 | 0.053 |
| Hypertension | 81 (88.0) | 240 (89.6) | 0.688 | 49 (87.5) | 46 (82.1) | 0.549 | 0.148 |
| CAD | 19 (20.7) | 49 (18.3) | 0.617 | 11 (19.6) | 14 (25.0) | 0.678 | 0.128 |
| Diabetes | 5 (5.4) | 8 (3.0) | 0.446 | 3 (5.4) | 4 (7.1) | 1.000 | 0.073 |
| COPD | 3 (3.3) | 0 (0.0) | 0.016 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Cerebrovascular accident | 8 (8.7) | 14 (5.2) | 0.230 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Myocardial infarction | 1 (1.1) | 1 (0.4) | 0.446 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Redo sternotomy | 4 (4.3) | 8 (3.0) | 0.771 | 2 (3.6) | 3 (5.4) | 1.000 | 0.086 |
| CKD | 1 (1.1) | 3 (1.1) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| NYHA ≥3 | 4 (4.3) | 10 (3.7) | 1.000 | 1 (1.8) | 2 (3.6) | 1.000 | 0.110 |
| Organ malperfusion | |||||||
| Cardiac | 7 (7.6) | 15 (5.6) | 0.487 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Cerebral | 3 (3.3) | 12 (4.5) | 0.840 | 2 (3.6) | 2 (3.6) | 1.000 | <0.001 |
| Visceral | 5 (5.4) | 19 (7.1) | 0.583 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Renal | 1 (1.1) | 13 (4.9) | 0.194 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Limb | 0 (0.0) | 9 (3.4) | 0.164 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Ejection fraction (%) | 60.1 ± 5.1 | 60.7 ± 3.9 | 0.617 | 60.6 ± 4.4 | 60.9 ± 4.5 | 0.604 | 0.060 |
| Median or massive AR | 14 (15.2) | 70 (26.1) | 0.033 | 10 (17.9) | 10 (17.9) | 1.000 | <0.001 |
Values are expressed as mean ± SD or n (%).
AR: aortic regurgitation; BMI: body mass index; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HAR: hybrid type II arch repair; NYHA: New York Heart Association; SD: standard deviation; SMD: standardized mean difference; TAR with FET: total arch replacement with frozen elephant trunk.
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | |||||
|---|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | SMD . | |
| Age (years) | 61.4 ± 7.7 | 46.0 ± 9.2 | <0.001 | 57.7 ± 7.2 | 57.1 ± 6.9 | 0.385 | 0.084 |
| Male gender | 59 (64.1) | 214 (79.9) | 0.002 | 35 (62.5) | 34 (60.7) | 1.000 | 0.036 |
| BMI (kg/m2) | 26.0 ± 3.9 | 26.3 ± 4.0 | 0.510 | 25.7 ± 3.6 | 25.9 ± 3.5 | 0.757 | 0.053 |
| Hypertension | 81 (88.0) | 240 (89.6) | 0.688 | 49 (87.5) | 46 (82.1) | 0.549 | 0.148 |
| CAD | 19 (20.7) | 49 (18.3) | 0.617 | 11 (19.6) | 14 (25.0) | 0.678 | 0.128 |
| Diabetes | 5 (5.4) | 8 (3.0) | 0.446 | 3 (5.4) | 4 (7.1) | 1.000 | 0.073 |
| COPD | 3 (3.3) | 0 (0.0) | 0.016 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Cerebrovascular accident | 8 (8.7) | 14 (5.2) | 0.230 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Myocardial infarction | 1 (1.1) | 1 (0.4) | 0.446 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Redo sternotomy | 4 (4.3) | 8 (3.0) | 0.771 | 2 (3.6) | 3 (5.4) | 1.000 | 0.086 |
| CKD | 1 (1.1) | 3 (1.1) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| NYHA ≥3 | 4 (4.3) | 10 (3.7) | 1.000 | 1 (1.8) | 2 (3.6) | 1.000 | 0.110 |
| Organ malperfusion | |||||||
| Cardiac | 7 (7.6) | 15 (5.6) | 0.487 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Cerebral | 3 (3.3) | 12 (4.5) | 0.840 | 2 (3.6) | 2 (3.6) | 1.000 | <0.001 |
| Visceral | 5 (5.4) | 19 (7.1) | 0.583 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Renal | 1 (1.1) | 13 (4.9) | 0.194 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Limb | 0 (0.0) | 9 (3.4) | 0.164 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Ejection fraction (%) | 60.1 ± 5.1 | 60.7 ± 3.9 | 0.617 | 60.6 ± 4.4 | 60.9 ± 4.5 | 0.604 | 0.060 |
| Median or massive AR | 14 (15.2) | 70 (26.1) | 0.033 | 10 (17.9) | 10 (17.9) | 1.000 | <0.001 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | |||||
|---|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | SMD . | |
| Age (years) | 61.4 ± 7.7 | 46.0 ± 9.2 | <0.001 | 57.7 ± 7.2 | 57.1 ± 6.9 | 0.385 | 0.084 |
| Male gender | 59 (64.1) | 214 (79.9) | 0.002 | 35 (62.5) | 34 (60.7) | 1.000 | 0.036 |
| BMI (kg/m2) | 26.0 ± 3.9 | 26.3 ± 4.0 | 0.510 | 25.7 ± 3.6 | 25.9 ± 3.5 | 0.757 | 0.053 |
| Hypertension | 81 (88.0) | 240 (89.6) | 0.688 | 49 (87.5) | 46 (82.1) | 0.549 | 0.148 |
| CAD | 19 (20.7) | 49 (18.3) | 0.617 | 11 (19.6) | 14 (25.0) | 0.678 | 0.128 |
| Diabetes | 5 (5.4) | 8 (3.0) | 0.446 | 3 (5.4) | 4 (7.1) | 1.000 | 0.073 |
| COPD | 3 (3.3) | 0 (0.0) | 0.016 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Cerebrovascular accident | 8 (8.7) | 14 (5.2) | 0.230 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Myocardial infarction | 1 (1.1) | 1 (0.4) | 0.446 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Redo sternotomy | 4 (4.3) | 8 (3.0) | 0.771 | 2 (3.6) | 3 (5.4) | 1.000 | 0.086 |
| CKD | 1 (1.1) | 3 (1.1) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| NYHA ≥3 | 4 (4.3) | 10 (3.7) | 1.000 | 1 (1.8) | 2 (3.6) | 1.000 | 0.110 |
| Organ malperfusion | |||||||
| Cardiac | 7 (7.6) | 15 (5.6) | 0.487 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Cerebral | 3 (3.3) | 12 (4.5) | 0.840 | 2 (3.6) | 2 (3.6) | 1.000 | <0.001 |
| Visceral | 5 (5.4) | 19 (7.1) | 0.583 | 4 (7.1) | 3 (5.4) | 1.000 | 0.073 |
| Renal | 1 (1.1) | 13 (4.9) | 0.194 | 1 (1.8) | 1 (1.8) | 1.000 | <0.001 |
| Limb | 0 (0.0) | 9 (3.4) | 0.164 | 0 (0.0) | 0 (0.0) | 1.000 | <0.001 |
| Ejection fraction (%) | 60.1 ± 5.1 | 60.7 ± 3.9 | 0.617 | 60.6 ± 4.4 | 60.9 ± 4.5 | 0.604 | 0.060 |
| Median or massive AR | 14 (15.2) | 70 (26.1) | 0.033 | 10 (17.9) | 10 (17.9) | 1.000 | <0.001 |
Values are expressed as mean ± SD or n (%).
AR: aortic regurgitation; BMI: body mass index; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HAR: hybrid type II arch repair; NYHA: New York Heart Association; SD: standard deviation; SMD: standardized mean difference; TAR with FET: total arch replacement with frozen elephant trunk.
Operative data
In the unmatched cohort, the Bentall procedure and extra-anatomical bypass were performed in higher proportions in the TAR with FET group than in the HAR group (26.5% vs 9.8%, P = 0.001; 6.3% vs 0%, P = 0.029, respectively). The CPB time and cross-clamp time were significantly shorter in the HAR group than in the TAR with FET group (153.3 ± 60.3 vs 184.7 ± 68.8 min, P < 0.001; 97.2 ± 97.6 vs 112.6 ± 41.2 min, P < 0.001, respectively).
In the propensity-matched cohort, no significant differences were found in combined surgical procedures between the 2 groups. However, the CPB time and cross-clamp time were shorter in the HAR group than in the TAR with FET group (P < 0.001). The lowest nasopharyngeal temperature and the lowest bladder temperature in the HAR group were significantly higher than those in the TAR with FET group before and after matching. The operative data are summarized for comparison in Table 3.
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Combined surgery | ||||||
| Bentall | 9 (9.8) | 71 (26.5) | 0.001 | 6 (10.7) | 10 (17.9) | 0.289 |
| Sinus reconstruction | 37 (40.2) | 99 (36.9) | 0.576 | 22 (39.3) | 22 (39.3) | 1.000 |
| CABG | 17 (18.5) | 51 (19.0) | 0.907 | 8 (14.3) | 12 (21.4) | 0.454 |
| Extra-anatomical bypass | 0 (0.0) | 17 (6.3) | 0.029 | 0 (0) | 3 (5.4) | 0.250 |
| Wheat’s | 5 (5.4) | 6 (2.2) | 0.236 | 4 (7.1) | 2 (3.6) | 0.688 |
| Other (David, mitral, congenital) | 1 (1.1) | 11 (4.1) | 0.292 | 1 (1.8) | 2 (3.6) | 1.000 |
| CPB time (min) | 153.3 ± 60.3 | 184.7 ± 68.8 | <0.001 | 149.1 ± 60.6 | 202.2 ± 91.3 | <0.001 |
| Cross-clamp time (min) | 97.2 ± 97.6 | 112.6 ± 41.2 | <0.001 | 99.1 ± 122.0 | 125.8 ± 38.6 | <0.001 |
| Circulatory arrest time (min) | 0 | 16.7 ± 3.4 | <0.001 | 0 | 16.3 ± 2.6 | <0.001 |
| Lowest nasopharyngeal temperature (°C) | 27.5 ± 1.1 | 24.6 ± 1.1 | <0.001 | 27.6 ± 0.8 | 24.5 ± 0.9 | <0.001 |
| Lowest bladder temperature (°C) | 28.7 ± 1.6 | 26.8 ± 1.6 | <0.001 | 28.8 ± 1.1 | 26.4 ± 1.4 | <0.001 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Combined surgery | ||||||
| Bentall | 9 (9.8) | 71 (26.5) | 0.001 | 6 (10.7) | 10 (17.9) | 0.289 |
| Sinus reconstruction | 37 (40.2) | 99 (36.9) | 0.576 | 22 (39.3) | 22 (39.3) | 1.000 |
| CABG | 17 (18.5) | 51 (19.0) | 0.907 | 8 (14.3) | 12 (21.4) | 0.454 |
| Extra-anatomical bypass | 0 (0.0) | 17 (6.3) | 0.029 | 0 (0) | 3 (5.4) | 0.250 |
| Wheat’s | 5 (5.4) | 6 (2.2) | 0.236 | 4 (7.1) | 2 (3.6) | 0.688 |
| Other (David, mitral, congenital) | 1 (1.1) | 11 (4.1) | 0.292 | 1 (1.8) | 2 (3.6) | 1.000 |
| CPB time (min) | 153.3 ± 60.3 | 184.7 ± 68.8 | <0.001 | 149.1 ± 60.6 | 202.2 ± 91.3 | <0.001 |
| Cross-clamp time (min) | 97.2 ± 97.6 | 112.6 ± 41.2 | <0.001 | 99.1 ± 122.0 | 125.8 ± 38.6 | <0.001 |
| Circulatory arrest time (min) | 0 | 16.7 ± 3.4 | <0.001 | 0 | 16.3 ± 2.6 | <0.001 |
| Lowest nasopharyngeal temperature (°C) | 27.5 ± 1.1 | 24.6 ± 1.1 | <0.001 | 27.6 ± 0.8 | 24.5 ± 0.9 | <0.001 |
| Lowest bladder temperature (°C) | 28.7 ± 1.6 | 26.8 ± 1.6 | <0.001 | 28.8 ± 1.1 | 26.4 ± 1.4 | <0.001 |
Values are expressed as mean ± SD or n (%). CABG were performed in patients with severe CAD and in patients with coronary artery involvement that cannot be repaired. Extra-anatomical bypass from the ascending aorta to the femoral artery was created concomitantly: (i) to improve lower extremity perfusion or (ii) to facilitate visceral perfusion when the descending thoracic aorta was clamped in the secondary surgery of descending aorta.
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CPB: cardiopulmonary bypass; HAR: hybrid type II arch repair; SD: standard deviation; TAR with FET: total arch replacement with frozen elephant trunk.
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Combined surgery | ||||||
| Bentall | 9 (9.8) | 71 (26.5) | 0.001 | 6 (10.7) | 10 (17.9) | 0.289 |
| Sinus reconstruction | 37 (40.2) | 99 (36.9) | 0.576 | 22 (39.3) | 22 (39.3) | 1.000 |
| CABG | 17 (18.5) | 51 (19.0) | 0.907 | 8 (14.3) | 12 (21.4) | 0.454 |
| Extra-anatomical bypass | 0 (0.0) | 17 (6.3) | 0.029 | 0 (0) | 3 (5.4) | 0.250 |
| Wheat’s | 5 (5.4) | 6 (2.2) | 0.236 | 4 (7.1) | 2 (3.6) | 0.688 |
| Other (David, mitral, congenital) | 1 (1.1) | 11 (4.1) | 0.292 | 1 (1.8) | 2 (3.6) | 1.000 |
| CPB time (min) | 153.3 ± 60.3 | 184.7 ± 68.8 | <0.001 | 149.1 ± 60.6 | 202.2 ± 91.3 | <0.001 |
| Cross-clamp time (min) | 97.2 ± 97.6 | 112.6 ± 41.2 | <0.001 | 99.1 ± 122.0 | 125.8 ± 38.6 | <0.001 |
| Circulatory arrest time (min) | 0 | 16.7 ± 3.4 | <0.001 | 0 | 16.3 ± 2.6 | <0.001 |
| Lowest nasopharyngeal temperature (°C) | 27.5 ± 1.1 | 24.6 ± 1.1 | <0.001 | 27.6 ± 0.8 | 24.5 ± 0.9 | <0.001 |
| Lowest bladder temperature (°C) | 28.7 ± 1.6 | 26.8 ± 1.6 | <0.001 | 28.8 ± 1.1 | 26.4 ± 1.4 | <0.001 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Combined surgery | ||||||
| Bentall | 9 (9.8) | 71 (26.5) | 0.001 | 6 (10.7) | 10 (17.9) | 0.289 |
| Sinus reconstruction | 37 (40.2) | 99 (36.9) | 0.576 | 22 (39.3) | 22 (39.3) | 1.000 |
| CABG | 17 (18.5) | 51 (19.0) | 0.907 | 8 (14.3) | 12 (21.4) | 0.454 |
| Extra-anatomical bypass | 0 (0.0) | 17 (6.3) | 0.029 | 0 (0) | 3 (5.4) | 0.250 |
| Wheat’s | 5 (5.4) | 6 (2.2) | 0.236 | 4 (7.1) | 2 (3.6) | 0.688 |
| Other (David, mitral, congenital) | 1 (1.1) | 11 (4.1) | 0.292 | 1 (1.8) | 2 (3.6) | 1.000 |
| CPB time (min) | 153.3 ± 60.3 | 184.7 ± 68.8 | <0.001 | 149.1 ± 60.6 | 202.2 ± 91.3 | <0.001 |
| Cross-clamp time (min) | 97.2 ± 97.6 | 112.6 ± 41.2 | <0.001 | 99.1 ± 122.0 | 125.8 ± 38.6 | <0.001 |
| Circulatory arrest time (min) | 0 | 16.7 ± 3.4 | <0.001 | 0 | 16.3 ± 2.6 | <0.001 |
| Lowest nasopharyngeal temperature (°C) | 27.5 ± 1.1 | 24.6 ± 1.1 | <0.001 | 27.6 ± 0.8 | 24.5 ± 0.9 | <0.001 |
| Lowest bladder temperature (°C) | 28.7 ± 1.6 | 26.8 ± 1.6 | <0.001 | 28.8 ± 1.1 | 26.4 ± 1.4 | <0.001 |
Values are expressed as mean ± SD or n (%). CABG were performed in patients with severe CAD and in patients with coronary artery involvement that cannot be repaired. Extra-anatomical bypass from the ascending aorta to the femoral artery was created concomitantly: (i) to improve lower extremity perfusion or (ii) to facilitate visceral perfusion when the descending thoracic aorta was clamped in the secondary surgery of descending aorta.
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CPB: cardiopulmonary bypass; HAR: hybrid type II arch repair; SD: standard deviation; TAR with FET: total arch replacement with frozen elephant trunk.
Early outcomes
The early outcomes of the unmatched and matched cohorts are listed in Table 4. Both before and after matching, there were no significant differences between the 2 groups in terms of composite adverse events, 30-day mortality, stroke, paraplegia, renal failure, hepatic failure, reintubation or tracheotomy or low cardiac output syndrome.
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Composite adverse events | 21 (22.8) | 56 (20.9) | 0.697 | 12 (21.4) | 12 (21.4) | 1.000 |
| 30-Day mortality | 6 (6.5) | 15 (5.6) | 0.744 | 4 (7.1) | 4 (7.1) | 1.000 |
| Stroke | 4 (4.3) | 9 (3.4) | 0.908 | 4 (7.1) | 2 (3.6) | 0.688 |
| Paraplegia | 4 (4.3) | 13 (4.9) | 1.000 | 3 (5.4) | 4 (7.1) | 1.000 |
| Renal failure | 10 (10.9) | 30 (11.2) | 0.932 | 4 (7.1) | 6 (10.7) | 0.754 |
| Hepatic failure | 4 (4.3) | 19 (7.1) | 0.354 | 2 (3.6) | 4 (7.1) | 0.688 |
| Reintubation or tracheotomy | 4 (4.3) | 7 (2.6) | 0.629 | 1 (1.8) | 3 (5.4) | 0.625 |
| Low cardiac output syndrome | 3 (3.3) | 5 (1.9) | 0.709 | 1 (1.8) | 0 (0.0) | 1.000 |
| AKI | 58 (63.0) | 208 (77.6) | 0.006 | 33 (58.9) | 45 (80.4) | 0.031 |
| Stage 0 | 34 (37.0) | 60 (22.4) | 0.012 | 23 (41.1) | 11 (19.6) | 0.039 |
| Stage 1 | 38 (41.3) | 123 (45.9) | 24 (42.9) | 27 (48.2) | ||
| Stage 2 | 6 (6.5) | 39 (14.6) | 2 (3.6) | 8 (14.3) | ||
| Stage 3 | 14 (15.2) | 46 (17.2) | 7 (12.5) | 10 (17.9) | ||
| Ventilation time (h) | 42.9 ± 46.0 | 40.8 ± 45.9 | 0.344 | 32.4 ± 40.1 | 44.1 ± 46.4 | 0.057 |
| Ventilation time >24 h | 42 (45.7) | 115 (42.9) | 0.647 | 18 (32.1) | 26 (46.4) | 0.201 |
| ICU time (h) | 130.4 ± 102.9 | 120.5 ± 104.3 | 0.223 | 102.5 ± 73.3 | 123.3 ± 83.9 | 0.073 |
| In-hospital time (d) | 14.1 ± 6.2 | 12.8 ± 5.6 | 0.009 | 13.0 ± 6.1 | 12.7 ± 4.8 | 0.632 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Composite adverse events | 21 (22.8) | 56 (20.9) | 0.697 | 12 (21.4) | 12 (21.4) | 1.000 |
| 30-Day mortality | 6 (6.5) | 15 (5.6) | 0.744 | 4 (7.1) | 4 (7.1) | 1.000 |
| Stroke | 4 (4.3) | 9 (3.4) | 0.908 | 4 (7.1) | 2 (3.6) | 0.688 |
| Paraplegia | 4 (4.3) | 13 (4.9) | 1.000 | 3 (5.4) | 4 (7.1) | 1.000 |
| Renal failure | 10 (10.9) | 30 (11.2) | 0.932 | 4 (7.1) | 6 (10.7) | 0.754 |
| Hepatic failure | 4 (4.3) | 19 (7.1) | 0.354 | 2 (3.6) | 4 (7.1) | 0.688 |
| Reintubation or tracheotomy | 4 (4.3) | 7 (2.6) | 0.629 | 1 (1.8) | 3 (5.4) | 0.625 |
| Low cardiac output syndrome | 3 (3.3) | 5 (1.9) | 0.709 | 1 (1.8) | 0 (0.0) | 1.000 |
| AKI | 58 (63.0) | 208 (77.6) | 0.006 | 33 (58.9) | 45 (80.4) | 0.031 |
| Stage 0 | 34 (37.0) | 60 (22.4) | 0.012 | 23 (41.1) | 11 (19.6) | 0.039 |
| Stage 1 | 38 (41.3) | 123 (45.9) | 24 (42.9) | 27 (48.2) | ||
| Stage 2 | 6 (6.5) | 39 (14.6) | 2 (3.6) | 8 (14.3) | ||
| Stage 3 | 14 (15.2) | 46 (17.2) | 7 (12.5) | 10 (17.9) | ||
| Ventilation time (h) | 42.9 ± 46.0 | 40.8 ± 45.9 | 0.344 | 32.4 ± 40.1 | 44.1 ± 46.4 | 0.057 |
| Ventilation time >24 h | 42 (45.7) | 115 (42.9) | 0.647 | 18 (32.1) | 26 (46.4) | 0.201 |
| ICU time (h) | 130.4 ± 102.9 | 120.5 ± 104.3 | 0.223 | 102.5 ± 73.3 | 123.3 ± 83.9 | 0.073 |
| In-hospital time (d) | 14.1 ± 6.2 | 12.8 ± 5.6 | 0.009 | 13.0 ± 6.1 | 12.7 ± 4.8 | 0.632 |
Values are expressed as mean ± SD or n (%).
AKI: acute kidney injury; HAR: hybrid type II arch repair; ICU: intensive care unit; SD: standard deviation; TAR with FET: total arch replacement with frozen elephant trunk.
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Composite adverse events | 21 (22.8) | 56 (20.9) | 0.697 | 12 (21.4) | 12 (21.4) | 1.000 |
| 30-Day mortality | 6 (6.5) | 15 (5.6) | 0.744 | 4 (7.1) | 4 (7.1) | 1.000 |
| Stroke | 4 (4.3) | 9 (3.4) | 0.908 | 4 (7.1) | 2 (3.6) | 0.688 |
| Paraplegia | 4 (4.3) | 13 (4.9) | 1.000 | 3 (5.4) | 4 (7.1) | 1.000 |
| Renal failure | 10 (10.9) | 30 (11.2) | 0.932 | 4 (7.1) | 6 (10.7) | 0.754 |
| Hepatic failure | 4 (4.3) | 19 (7.1) | 0.354 | 2 (3.6) | 4 (7.1) | 0.688 |
| Reintubation or tracheotomy | 4 (4.3) | 7 (2.6) | 0.629 | 1 (1.8) | 3 (5.4) | 0.625 |
| Low cardiac output syndrome | 3 (3.3) | 5 (1.9) | 0.709 | 1 (1.8) | 0 (0.0) | 1.000 |
| AKI | 58 (63.0) | 208 (77.6) | 0.006 | 33 (58.9) | 45 (80.4) | 0.031 |
| Stage 0 | 34 (37.0) | 60 (22.4) | 0.012 | 23 (41.1) | 11 (19.6) | 0.039 |
| Stage 1 | 38 (41.3) | 123 (45.9) | 24 (42.9) | 27 (48.2) | ||
| Stage 2 | 6 (6.5) | 39 (14.6) | 2 (3.6) | 8 (14.3) | ||
| Stage 3 | 14 (15.2) | 46 (17.2) | 7 (12.5) | 10 (17.9) | ||
| Ventilation time (h) | 42.9 ± 46.0 | 40.8 ± 45.9 | 0.344 | 32.4 ± 40.1 | 44.1 ± 46.4 | 0.057 |
| Ventilation time >24 h | 42 (45.7) | 115 (42.9) | 0.647 | 18 (32.1) | 26 (46.4) | 0.201 |
| ICU time (h) | 130.4 ± 102.9 | 120.5 ± 104.3 | 0.223 | 102.5 ± 73.3 | 123.3 ± 83.9 | 0.073 |
| In-hospital time (d) | 14.1 ± 6.2 | 12.8 ± 5.6 | 0.009 | 13.0 ± 6.1 | 12.7 ± 4.8 | 0.632 |
| Variables . | Total cohort (n = 360) . | Propensity-matched cohort (n = 112) . | ||||
|---|---|---|---|---|---|---|
| HAR (n = 92) . | TAR with FET (n = 268) . | P-value . | HAR (n = 56) . | TAR with FET (n = 56) . | P-value . | |
| Composite adverse events | 21 (22.8) | 56 (20.9) | 0.697 | 12 (21.4) | 12 (21.4) | 1.000 |
| 30-Day mortality | 6 (6.5) | 15 (5.6) | 0.744 | 4 (7.1) | 4 (7.1) | 1.000 |
| Stroke | 4 (4.3) | 9 (3.4) | 0.908 | 4 (7.1) | 2 (3.6) | 0.688 |
| Paraplegia | 4 (4.3) | 13 (4.9) | 1.000 | 3 (5.4) | 4 (7.1) | 1.000 |
| Renal failure | 10 (10.9) | 30 (11.2) | 0.932 | 4 (7.1) | 6 (10.7) | 0.754 |
| Hepatic failure | 4 (4.3) | 19 (7.1) | 0.354 | 2 (3.6) | 4 (7.1) | 0.688 |
| Reintubation or tracheotomy | 4 (4.3) | 7 (2.6) | 0.629 | 1 (1.8) | 3 (5.4) | 0.625 |
| Low cardiac output syndrome | 3 (3.3) | 5 (1.9) | 0.709 | 1 (1.8) | 0 (0.0) | 1.000 |
| AKI | 58 (63.0) | 208 (77.6) | 0.006 | 33 (58.9) | 45 (80.4) | 0.031 |
| Stage 0 | 34 (37.0) | 60 (22.4) | 0.012 | 23 (41.1) | 11 (19.6) | 0.039 |
| Stage 1 | 38 (41.3) | 123 (45.9) | 24 (42.9) | 27 (48.2) | ||
| Stage 2 | 6 (6.5) | 39 (14.6) | 2 (3.6) | 8 (14.3) | ||
| Stage 3 | 14 (15.2) | 46 (17.2) | 7 (12.5) | 10 (17.9) | ||
| Ventilation time (h) | 42.9 ± 46.0 | 40.8 ± 45.9 | 0.344 | 32.4 ± 40.1 | 44.1 ± 46.4 | 0.057 |
| Ventilation time >24 h | 42 (45.7) | 115 (42.9) | 0.647 | 18 (32.1) | 26 (46.4) | 0.201 |
| ICU time (h) | 130.4 ± 102.9 | 120.5 ± 104.3 | 0.223 | 102.5 ± 73.3 | 123.3 ± 83.9 | 0.073 |
| In-hospital time (d) | 14.1 ± 6.2 | 12.8 ± 5.6 | 0.009 | 13.0 ± 6.1 | 12.7 ± 4.8 | 0.632 |
Values are expressed as mean ± SD or n (%).
AKI: acute kidney injury; HAR: hybrid type II arch repair; ICU: intensive care unit; SD: standard deviation; TAR with FET: total arch replacement with frozen elephant trunk.
Both before and after matching, the HAR group experienced a significantly lower incidence of AKI than the TAR with FET group (unmatched: 63% vs 77.6%, P = 0.006; matched: 58.9% vs 80.4%, P = 0.031). According to the KDIGO criteria, the distribution of AKI stages was discrepant between the 2 groups (unmatched: P = 0.012; matched: P = 0.039). Fewer patients developed stage 2 and stage 3 AKI in the HAR group than in the TAR with FET group, regardless of the unmatched or matched cohort (Fig. 2).
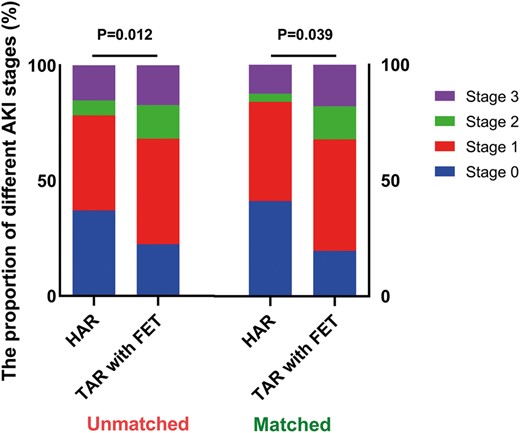
The proportion of postoperative AKI stages in the unmatched and matched cohort. There was a significant difference in the distribution of AKI stages between HAR and TAR with FET (unmatched: P = 0.012; matched: P = 0.039). More patients developed stage 0 (blue) and stage 1 (red) AKI, lower grade AKI, in the HAR group, regardless of the unmatched or matched cohort. AKI: acute kidney injury; HAR: hybrid type II arch repair; TAR with FET: total arch replacement with frozen elephant trunk.
Unmatched comparison revealed that the ventilation time, ventilation time >24 h and intensive care unit (ICU) time did not differ between the HAR and TAR with FET groups. The in-hospital time in the HAR group was longer than that in the TAR with FET group (14.1 ± 6.2 vs 12.8 ± 5.6 days, P = 0.009). In matched comparisons, there was no significant difference in the ventilation time, ventilation time >24 h, ICU time or in-hospital time between the 2 groups.
Regression analysis
The multivariable analysis for all 360 patients (Table 5) did not identify procedure type (HAR vs TAR with FET) as an independent predictor of composite adverse events or stroke.
Multivariable logistic analysis for risk factors associated with composite adverse events, stroke and AKI
| Factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Composite adverse events | |||
| Diabetes | 5.669 | 1.681–19.121 | 0.005 |
| Organ malperfusion | |||
| Cardiac | 7.987 | 2.806–22.733 | <0.001 |
| Cerebral | 4.176 | 1.311–13.302 | 0.016 |
| Visceral | 7.302 | 2.821–18.901 | <0.001 |
| Renal | 8.178 | 2.193–30.491 | 0.002 |
| Limb | 11.666 | 2.399–56.723 | 0.002 |
| CABG | 3.783 | 1.920–7.456 | <0.001 |
| Stroke | |||
| Cerebrovascular accident | 5.367 | 1.255–22.951 | 0.023 |
| Organ malperfusion | |||
| Cardiac | 5.367 | 1.255–22.951 | 0.023 |
| Cerebral | 7.495 | 1.398–40.171 | 0.019 |
| AKI | |||
| HAR | 0.485 | 0.287–0.822 | 0.007 |
| BMI | 1.097 | 1.026–1.173 | 0.007 |
| CABG | 2.369 | 1.135–4.941 | 0.022 |
| Factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Composite adverse events | |||
| Diabetes | 5.669 | 1.681–19.121 | 0.005 |
| Organ malperfusion | |||
| Cardiac | 7.987 | 2.806–22.733 | <0.001 |
| Cerebral | 4.176 | 1.311–13.302 | 0.016 |
| Visceral | 7.302 | 2.821–18.901 | <0.001 |
| Renal | 8.178 | 2.193–30.491 | 0.002 |
| Limb | 11.666 | 2.399–56.723 | 0.002 |
| CABG | 3.783 | 1.920–7.456 | <0.001 |
| Stroke | |||
| Cerebrovascular accident | 5.367 | 1.255–22.951 | 0.023 |
| Organ malperfusion | |||
| Cardiac | 5.367 | 1.255–22.951 | 0.023 |
| Cerebral | 7.495 | 1.398–40.171 | 0.019 |
| AKI | |||
| HAR | 0.485 | 0.287–0.822 | 0.007 |
| BMI | 1.097 | 1.026–1.173 | 0.007 |
| CABG | 2.369 | 1.135–4.941 | 0.022 |
AKI: acute kidney injury; BMI: body mass index; CABG: coronary artery bypass grafting; CI: confidence interval; HAR: hybrid type II arch repair; OR: odds ratio.
Multivariable logistic analysis for risk factors associated with composite adverse events, stroke and AKI
| Factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Composite adverse events | |||
| Diabetes | 5.669 | 1.681–19.121 | 0.005 |
| Organ malperfusion | |||
| Cardiac | 7.987 | 2.806–22.733 | <0.001 |
| Cerebral | 4.176 | 1.311–13.302 | 0.016 |
| Visceral | 7.302 | 2.821–18.901 | <0.001 |
| Renal | 8.178 | 2.193–30.491 | 0.002 |
| Limb | 11.666 | 2.399–56.723 | 0.002 |
| CABG | 3.783 | 1.920–7.456 | <0.001 |
| Stroke | |||
| Cerebrovascular accident | 5.367 | 1.255–22.951 | 0.023 |
| Organ malperfusion | |||
| Cardiac | 5.367 | 1.255–22.951 | 0.023 |
| Cerebral | 7.495 | 1.398–40.171 | 0.019 |
| AKI | |||
| HAR | 0.485 | 0.287–0.822 | 0.007 |
| BMI | 1.097 | 1.026–1.173 | 0.007 |
| CABG | 2.369 | 1.135–4.941 | 0.022 |
| Factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Composite adverse events | |||
| Diabetes | 5.669 | 1.681–19.121 | 0.005 |
| Organ malperfusion | |||
| Cardiac | 7.987 | 2.806–22.733 | <0.001 |
| Cerebral | 4.176 | 1.311–13.302 | 0.016 |
| Visceral | 7.302 | 2.821–18.901 | <0.001 |
| Renal | 8.178 | 2.193–30.491 | 0.002 |
| Limb | 11.666 | 2.399–56.723 | 0.002 |
| CABG | 3.783 | 1.920–7.456 | <0.001 |
| Stroke | |||
| Cerebrovascular accident | 5.367 | 1.255–22.951 | 0.023 |
| Organ malperfusion | |||
| Cardiac | 5.367 | 1.255–22.951 | 0.023 |
| Cerebral | 7.495 | 1.398–40.171 | 0.019 |
| AKI | |||
| HAR | 0.485 | 0.287–0.822 | 0.007 |
| BMI | 1.097 | 1.026–1.173 | 0.007 |
| CABG | 2.369 | 1.135–4.941 | 0.022 |
AKI: acute kidney injury; BMI: body mass index; CABG: coronary artery bypass grafting; CI: confidence interval; HAR: hybrid type II arch repair; OR: odds ratio.
The independent predictors of composite adverse events were diabetes [odds ratio (OR) 5.669, 95% confidence interval (CI) 1.681–19.121; P = 0.005], concomitant coronary artery bypass grafting (CABG) (OR 3.783, 95% CI 1.920–7.456; P < 0.001) and organ malperfusion, including cardiac (OR 7.987, 95% CI 2.806–22.733; P < 0.001), cerebral (OR 4.176, 95% CI 1.311–13.302; P = 0.016), visceral (OR 7.302, 95% CI 2.821–18.901; P < 0.001), renal (OR 8.178, 95% CI 2.193–30.491; P = 0.002) and limb (OR 11.666, 95% CI 2.399–56.723; P = 0.002) malperfusion.
Previous cerebrovascular accidents (OR 8.178, 95% CI 2.193–30.491; P = 0.002), cardiac malperfusion (OR 8.178, 95% CI 2.193–30.491; P = 0.002) and cerebral malperfusion (OR 8.178, 95% CI 2.193–30.491; P = 0.002) were independent predictors of stroke.
HAR was identified as a protective factor of AKI (OR 0.485, 95% CI 0.287–0.822; P = 0.007). BMI (OR 1.097, 95% CI 1.026–1.173; P = 0.007) and concomitant CABG (OR 2.369, 95% CI 1.135–4.941; P = 0.022) independently predicted AKI.
DISCUSSION
Due to the complexity, the application of TAR in AIAD is limited. As was reported, TAR was only performed in 12% of patients in the recent International Registry of Acute Aortic Dissection (IRAD) series [7]. In a large (N = 3265) retrospective study of open TAR cases, the in-hospital mortality rate at specialized centres was >10%, and the permanent neurological deficit rate was >6.7% [8]. To minimize the morbidity and mortality associated with this procedure, hybrid procedures have been developed to perform arch replacement by using endovascular technology.
Compared with 3 different types of hybrid approaches described by Milewski et al. [3], TAR with FET is similar to type III, except that frozen elephant stents are implanted in an antegrade fashion during open arch repair. Hypothermic circulatory arrest is still needed in the hybrid type III approach, and we think one of the real benefits of hybrid procedures is organ protection by avoiding hypothermic circulatory arrest. Therefore, the type III approach should be excluded from the hybrid group when comparing the 2 different procedures. In addition, a fair comparison between hybrid procedures and traditional TAR should include only those hybrid procedures that involve CPB and open arch vessel debranching via median sternotomy, in other words, type II hybrid repairs. Hence, in our study, we chose AIAD patients as study subjects in which ascending aortic replacement under CPB and debranching via sternotomy were imperative in the hybrid procedures to make the comparison fairer.
Whether the hybrid technique is superior to conventional open repair of aortic arch lesions remains a topic of debate. Most published articles reveal equivalent short-term outcomes in the hybrid group compared with the traditional arch replacement group [3, 9–12]. Iba et al. [9] reported that the hybrid procedure was superior in terms of early recovery from surgery. Hiraoka et al. [13] reported a greater incidence of stroke in the hybrid group. In fact, many studies are confusing, often involving different types of hybrid procedures (I, II and III) to compare with open arch replacement. Given the multiple partial and complete revascularization approaches and the heterogeneity of the patient population, it is challenging to reach a uniform conclusion. Zhang et al. [14] compared the type II hybrid procedure and FET for TAR in DeBakey type I dissection. Their analysis demonstrated that there was no significant difference in early mortality or the composite of complications between the hybrid group and the FET group. Another valuable study was a comparative study of zone 0 hybrid arch exclusion versus traditional open repair performed by Preventza et al. [15], revealing that adverse outcomes were not significantly different between the 2 groups and that the procedure type was not an independent predictor of permanent stroke. Our study echoes these findings: both propensity score-matched analysis and multivariable analysis showed that HAR could not reduce the incidence of composite adverse events or stroke. Moreover, the multivariable analysis revealed that composite adverse events and stroke were more associated with comorbidities and malperfusion syndromes, which were also identified as risk factors for AIAD patients by other studies [2, 16, 17]. The reason why HAR fails to demonstrate better short-term results than open surgery may be that it changes the mode of extracorporeal circulation, but it is difficult to reverse the outcome of high-risk patients especially those with severe organ function damage before surgery.
Our study found that HAR reduced the incidence of AKI and degraded it but did not reduce the incidence of dialysis. Zhang et al. [14] reported similar results. The rate of postoperative renal insufficiency was significantly lower in the hybrid group, but no significant difference was found in the rate of dialysis. We suppose it is also the true role of HAR and the actual effect of avoidance of hypothermic circulatory arrest to increase lower body perfusion and protect kidney function but not prevent renal failure.
Limitations
There were several limitations in this study. First, this was a single-centre retrospective study that only evaluated short-term results but not mid- to long-term outcomes. Second, the heterogeneity between the 2 groups may not have been well adjusted by propensity score matching. Nevertheless, to our knowledge, this study is one of the few comparative studies of hybrid total arch exclusion versus open TAR for AIAD, including the same aortic pathology and unified surgical procedure in each group. Third, aortic dissection occurs at a younger age in China than in Western countries, and patients have fewer complications associated with other diseases, which limits us to compare the 2 surgical procedures in older and more high-risk patients. Last, the sample size was small, especially after matching. More samples are needed.
In the treatment of AIAD, no significant differences were found in early outcomes between the 2 groups, but HAR was associated with a significantly lower incidence of AKI. At present, we favour an individualized approach based on a patient’s clinical factors when selecting the type of arch repair method. More high-quality trials with large samples and long follow-up periods are suggested.
Funding
This work was supported by the Beijing Municipal Science and Technology Commission [The Funding of Clinically Featured Application Research in Capital City, Z181100001718197].
Conflict of interest: none declared.
Author contributions
Yanxiang Liu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Writing—original draft; Writing—review & editing. Shenghua Liang: Data curation; Investigation; Methodology. Bowen Zhang: Data curation; Investigation; Methodology. Yunfeng Li: Data curation; Investigation; Methodology. Lucheng Wang: Data curation; Investigation; Methodology. Yaojun Dun: Data curation; Investigation; Methodology. Zujun Chen: Investigation; Resources; Supervision. Yi Shi: Conceptualization; Funding acquisition; Project administration; Resources. Hongwei Guo: Conceptualization; Funding acquisition; Project administration; Resources. Xiaogang Sun: Conceptualization; Funding acquisition; Project administration; Resources; Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- AIAD
Acute DeBakey type I aortic dissection
- AKI
Acute kidney injury
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- HAR
Hybrid type II arch repair
- ICU
Intensive care unit
- OR
Odds ratio
- SCr
Serum creatinine
- TAR with FET
Total arch replacement with frozen elephant trunk





