-
PDF
- Split View
-
Views
-
Cite
Cite
Sameh M Said, Prabhjot Nijjar, Molly Klein, Ranjit John, Left bundle branch block revealing a primary small bowel carcinoid metastasizing to the interventricular septum, Interactive CardioVascular and Thoracic Surgery, Volume 31, Issue 3, September 2020, Pages 408–410, https://doi.org/10.1093/icvts/ivaa099
Close - Share Icon Share
Abstract
Carcinoid tumours of the heart occur most commonly as a result of metastatic disease and usually affect the right side of the heart. We report a case of a solitary carcinoid metastasis to the interventricular septum without hepatic involvement in a 74-year-old man.
CASE REPORT
The Institutional Review Board approval was waived due to the case report nature of the manuscript.
A 74-year-old man presented with sudden onset of amaurosis fugax. Electrocardiogram showed left bundle branch block. Echocardiogram showed a heterogeneous, hyperechoic mass in the interventricular septum (IVS) (Fig. 1A, Video 1). Left ventricular ejection fraction was 45%. With cardiac magnetic resonance imaging, the mass showed brisk perfusion and late gadolinium enhancement (Fig. 1B). There was also dyskinesia of the septum secondary to the left bundle branch block (Video 2). Positron emission tomography (PET) scan did not show abnormal uptake elsewhere (Fig. 1C). Twenty-four-hour urine 5-hydroxyindoleacetic acid was normal.
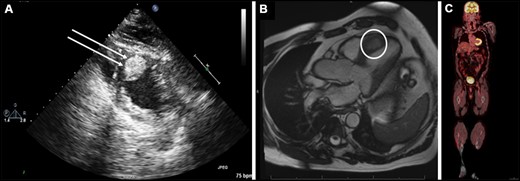
Preoperative (A) trans-thoracic echocardiogram showing a heterogeneous, hyperechoic mass (double arrows) in the mid-to-apical ventricular septum that is embedded in the myocardium, (B) cardiac magnetic resonance imaging showing a 3.2 cm × 1.6 cm ventricular septal mass (white circle) extending from the mid-to-apical septum and (C) preoperative positron emission tomography scan showing no evidence of uptake anywhere in the body.
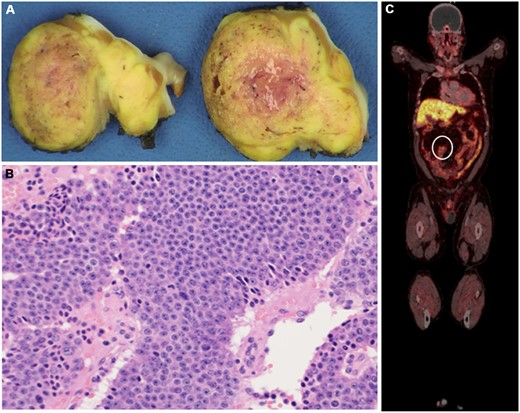
(A) Cut section of the resected mass showing 3.9 cm × 2.5 cm × 1.8 cm, partially encapsulated, yellow-tan mass and (B) histopathological examination of the resected mass showing epithelioid cells arranged in nested and organoid patterns, with eosinophilic secretions. The tumour cells had moderate amounts of eosinophilic cytoplasm and stippled chromatin; there were up to 5 mitotic figures/mm2. The Ki-67 proliferative index was up to 10%, with no necrosis. (C) Follow-up positron emission tomography scan with Gallium-68 dotatate showing the increased uptake in the small bowel and mesenteric lymph nodes (circle). These are specific for neuroendocrine tumours.
Preoperative trans-thoracic echocardiogram showing a heterogeneous, hyperechoic mass in the mid-to-apical ventricular septum (red arrow).
Preoperative cardiac magnetic resonance imaging showing mildly reduced left ventricular function with the interventricular septal mass extending from the mid anteroseptum to the apical septum (red arrow). The dyskinesis of the septum is consistent with the left bundle branch block.
Through a sternotomy and cardiopulmonary bypass, the left anterior descending coronary artery was identified. A left ventriculotomy was made and, using electrocautery, the mass was excised. The apical incision was closed in two layers. The postoperative course was uneventful, and the patient was discharged 5 days later.
Pathological examination of the specimen revealed a partially encapsulated, yellow-tan mass (4.0 cm × 3.6 cm × 2.3 cm) (Fig. 2A). It appears to extend out of its capsule, and composed of epithelioid cells arranged in nested and organoid patterns, with eosinophilic secretions (Fig. 2B). Immunostains (Cytokeratin AE1/AE3, Chromogranin, Synaptophysin) supported a diagnosis of neuroendocrine tumour (NET). Based on the 2018 World Health Organization classification, this tumour is a grade 2 NET also known as atypical carcinoid tumour [1].
During the follow-up period, a PET Gallium 68 dotatate was performed, which showed multiple enlarged mesenteric lymph nodes in addition to 3 foci of uptake in the small bowel; these were consistent with carcinoid (Fig. 2C). The patient continued to have no carcinoid manifestations and will not require resection of the primary tumour for now. He has been started on Somatostatin analog therapy.
DISCUSSION
Carcinoid tumours are NETs that most commonly arise from the gastrointestinal tract and the bronchial tree, with an annual incidence of 2/100 000 [2]. Carcinoid tumours involve the heart in up to 60% due to metastatic disease from primary carcinoid elsewhere [3].
Metastatic carcinoid tumour to the heart without hepatic involvement is extremely rare but has been reported [4]. In the current patient, the tumour was in a very unusual location and was not suspected preoperatively due to the absence of manifestations of carcinoid syndrome and there was no evidence of metastatic disease or primary NET elsewhere at that time. Involvement of the myocardium with carcinoid tumour and without right-sided valvular dysfunction is uncommon.
A multimodality approach to the diagnosis is mandatory in these unusual cases, and the search for a primary NET is needed after resection of the metastatic disease, as the primary tumour can be occult initially as in the current case and especially in the absence of carcinoid symptoms. Resection of solitary cardiac metastasis is warranted to help reaching the right diagnosis, which will help providing the right treatment.
In regard to resection of the current mass, few approaches may be considered: ‘left ventricular approach’ as was done in the current case, through an apical incision, ‘right ventriculotomy’, but this requires an incision through the right ventricular free wall and through the IVS and will need reconstruction of the IVS after resection, and finally, a ‘transaortic approach’, which is possible for tumours in the IVS that are close to the base of the heart. In some views, this tumour appeared to be close to the aortic valve; however, it would have required the apical incision to reach the inferior margin of the tumour. With these approaches in our mind, we decided to proceed with the left ventricular approach as it requires the least amount of time and reconstruction and as expected the tumour had a pseudocapsule that facilitated its resection without the need for reconstruction of the IVS. We are also very familiar with this approach from our experience with transapical septal myectomy in patients with hypertrophic cardiomyopathy [5].
CONCLUSION
In conclusion, although extremely rare, cardiac metastasis from a neuroendocrine carcinoma can be the initial presentation of the disease and can occur in the absence of liver metastasis. A multimodality approach is needed to help guide diagnosis and treatment.
Conflict of interest: Sameh M. Said is a consultant for Cryolife. The other authors declared no conflict of interest.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewers for their contribution to the peer review process of this article.




