-
PDF
- Split View
-
Views
-
Cite
Cite
Yaojun Dun, Yi Shi, Hongwei Guo, Yanxiang Liu, Xiangyang Qian, Xiaogang Sun, Cuntao Yu, The surgical management of type IA endoleak after thoracic endovascular aortic repair, Interactive CardioVascular and Thoracic Surgery, Volume 31, Issue 3, September 2020, Pages 346–353, https://doi.org/10.1093/icvts/ivaa124
Close - Share Icon Share
Abstract
Our goal was to investigate the surgical strategy for type Ia endoleak after thoracic endovascular aortic repair (TEVAR) by reporting our experiences.
From November 2012 to September 2019, a total of 23 patients received surgical management for type Ia endoleak after TEVAR.
The operations included total arch replacement with the frozen elephant trunk technique in 15 patients, direct closure of the endoleak in 2 patients, hybrid aortic arch repair in 4 patients, arch debranching with TEVAR in 1 patient and left common carotid artery to left subclavian artery bypass with TEVAR in 1 patient. Among 21 patients with cardiopulmonary bypass (CPB), the mean CPB and aortic cross-clamp times were 146.7 ± 42.2 and 81.0 ± 43.3 min, respectively. The selective cerebral perfusion time was 18.8 ± 8.2 min in 17 patients with hypothermic circulatory arrest. The in-hospital mortality was 8.7% (2/23). Type Ia endoleak was sealed successfully after surgery in 95.5% (21/22) of patients. The follow-up data were available for all 21 survivors. The median follow-up period was 18 months (range 1–84 months). During the follow-up period, a total of 8 patients died or had aortic events, including 5 deaths and 6 aortic events.
Different surgical strategies could be selected to treat patients with type Ia endoleak after TEVAR, with acceptable early and late outcomes.
INTRODUCTION
Thoracic endovascular aortic repair (TEVAR) has become the therapy of choice for many descending thoracic aortic diseases [1]. TEVAR has improved the morbidity, mortality and long-term prognosis of these diseases. TEVAR is, however, also associated with some complications such as retrograde type A aortic dissection, stroke, paraplegia and endoleak [2, 3]. Of these complications, endoleak is the most common complication after TEVAR, with an incidence of 23.3–32.9% [2, 4]. Endoleak is also the most common indication (44.8%) for secondary aortic interventions after TEVAR [5]. Type I is the most predominant and devastating type of endoleak [6], which not only leads to late reoperation but also represents an independent risk factor for early and late deaths [7, 8]. Because of the involvement of the distal arch, a type Ia endoleak remains a challenge for surgeons. Some investigators advocated the endovascular method to treat type Ia endoleak [5], but this method is not suitable for many patients because they have an insufficient proximal landing zone (PLZ). Therefore, many patients with type Ia endoleak after TEVAR required surgical treatment [9]. However, there are no guidelines or consensus for surgical treatment of type Ia endoleak thus far. Studies regarding the open repair of type Ia endoleak after TEVAR are limited. Our goal was to summarize surgical experiences with type Ia endoleak after TEVAR in our centre and to analyse the early and late outcomes.
PATIENTS AND METHODS
Patients population
From November 2012 to September 2019, we treated 23 patients who had a type Ia endoleak after TEVAR with surgery. The presence of a type Ia endoleak was identified by computed tomography (CT); 3 patients also had aortic angiography. Figure 1 provides CT information for 3 patients. The study was approved by the institutional review board of Fuwai Hospital. A waiver of informed consent was granted due to the design of the study.
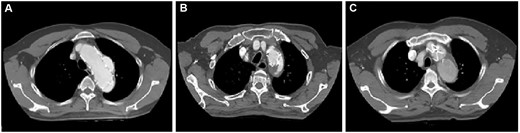
(A–C) Preoperative computed tomography scans of 3 patients with type Ia endoleak.
Operative techniques
In our centre, we recommended a timely operation once type Ia endoleak was identified. We adopted 5 different surgical approaches to treat type Ia endoleak after TEVAR. The procedures included total arch replacement (TAR) with the frozen elephant trunk (FET) technique, direct closure of the endoleak, hybrid aortic arch repair, arch debranching with TEVAR and left subclavian artery (LSA)–left common carotid artery (LCA) bypass with TEVAR. TAR with the FET technique was the preferred procedure, with satisfactory results in sealing the endoleak. This technique was suitable for patients with concurrent ascending or proximal arch diseases. For older patients who could not tolerate hypothermic circulatory arrest (HCA), we could use hybrid aortic arch repair instead of TAR with the FET procedure. If the endoleak was limited and located in the anterior wall or in the greater curvature of the arch, we could select direct closure of the endoleak. For older patients with prohibitive surgical risk, if they did not have concurrent ascending aorta or arch lesions (diameter of ascending or proximal arch <40 mm), arch debranching with TEVAR and LSA–LCA bypass with TEVAR could also be selected. The selection criteria were not absolute, and the final decision depended on the surgeon’s preference. The hybrid procedure (hybrid aortic arch repair, arch debranching with TEVAR and LSA–LCA bypass with TEVAR) was performed in a hybrid operating room with TEVAR performed at the same setting.
Total arch replacement with the frozen elephant trunk procedure
This technique has been described previously [10]. It was performed under HCA and selective antegrade cerebral perfusion (SCP). The right femoral artery, right axillary artery and right atrium were cannulated to initiate cardiopulmonary bypass (CPB). Once the rectal temperature reached 22–25°C, SCP was initiated. During SCP, the proximal bare spring of the TEVAR stent was removed by wire scissors to facilitate subsequent anastomosis and prevent aortic injuries. Chimney grafts were removed from patients with previous chimney graft implants (CGI). Then an FET (Cronus, MicroPort Endovascular Shanghai Co, Ltd, Shanghai, China) was implanted into the descending aorta. A four-branched vascular prosthesis (Terumo, Vascutek Limited, Renfrewshire, UK) was anastomosed with the FET, residual TEVAR stent and descending aorta. Then the SCP ended. The LCA was anastomosed to 1 branch of the vascular prosthesis and rewarming was started. Subsequently, the proximal end of the vascular prosthesis was anastomosed to the ascending aorta, and the other 2 branches were anastomosed to the LSA and the innominate artery, respectively.
Direct closure of the endoleak
The operation was performed under HCA and SCP. During HCA, the aortic arch was incised longitudinally, and the endoleak was identified carefully by direct inspection. Then the endoleak was closed thoroughly with 4–0 Prolene interrupted mattress sutures. The aortic arch incision was sutured with a running suture.
Hybrid aortic arch repair
This operation was performed with the use of CPB, and the lowest rectal temperature was set at ∼28°C. The cannulation site was the same as that for TAR. After the ascending aorta was transected, a four-branched vascular prosthesis (Terumo, Vascutek Limited, Renfrewshire, UK) was anastomosed to the proximal aortic stump. The aortic arch was transected between the innominate artery and the LCA. Then the distal end of the vascular prosthesis was anastomosed to the aortic arch stump. Next, the LCA, the LSA and the innominate artery were anastomosed to the branches of the vascular prosthesis, respectively. The endograft was retrogradely deployed with the PLZ in Dacron zone 0, which could cover the endoleak site and the arch lesions. The stent graft was oversized by 10–15%.
Arch debranching with thoracic endovascular aortic repair
The operation was performed off-pump. After a median sternotomy, the aortic arch, the innominate artery, the LCA and the LSA were mobilized and isolated. An 8-mm vascular prosthesis was anastomosed to a bifurcated graft with 8- and 10-mm branches (Terumo, Vascutek Limited) to construct a trifurcated graft. A side-biting clamp was put on the ascending aorta, and the proximal end of the graft was anastomosed to the ascending aorta in a side-to-end fashion. The 3 branches were anastomosed to the innominate artery, the LCA and the LSA in an end-to-end fashion, respectively. Then 3 brachiocephalic vessels were ligated at their origins. The endograft was subsequently deployed just distally to the debranching graft to cover the endoleak site.
Left subclavian artery–left common carotid artery bypass with thoracic endovascular aortic repair
The operation was performed through a left supraclavicular incision. The LCA and the LSA were carefully mobilized. Then the LSA was clamped and transected. The residual LSA stump was ligated. The LSA was anastomosed to an 8-mm graft (Terumo, Vascutek Limited) in an end-to-end fashion. The other side of the graft was anastomosed side-to-end with the LCA. Then the stent graft was retrogradely deployed to seal the endoleak site.
RESULTS
Patient characteristics
The mean age of the patients was 55.4 ± 9.8 years; 17 (73.9%) patients were men. The demographic details are shown in Table 1. Of the 23 patients, only 5 patients had an initial TEVAR in our hospital. The indications for the initial TEVAR were type B aortic dissection (TBAD) in 19 patients, descending thoracic aortic aneurysm in 2 patients and penetrating aortic ulcer in 2 patients. Nine patients received additional procedures during the initial TEVAR, including CGI, supra-aortic vessels bypass, LSA spring coil occlusion and LSA fenestration. No patient developed type Ia endoleak at the time of the initial TEVAR. The median interval between the initial TEVAR and endoleak was 72 months (range 1–156 months). All the initial TEVAR details are summarized in Table 2.
| Characteristics . | All patients (n = 23) . |
|---|---|
| Age (years) | 55.4 ± 9.8 (31–70) |
| Male gender | 17 (73.9) |
| Smoker | 11 (47.8) |
| Hypertension | 22 (95.7) |
| Coronary artery disease | 6 (26.1) |
| Abdominal aortic aneurysm | 3 (13.0) |
| Aortic valve regurgitation ≥ moderate | 4 (17.4) |
| Symptoms | |
| Chest pain or back pain | 10 (43.5) |
| Haemoptysis | 2 (8.7) |
| Dyspnoea | 2 (8.7) |
| Fever | 1 (4.3) |
| Fatigue | 1 (4.3) |
| Asymptomatic | 8 (34.8) |
| Characteristics . | All patients (n = 23) . |
|---|---|
| Age (years) | 55.4 ± 9.8 (31–70) |
| Male gender | 17 (73.9) |
| Smoker | 11 (47.8) |
| Hypertension | 22 (95.7) |
| Coronary artery disease | 6 (26.1) |
| Abdominal aortic aneurysm | 3 (13.0) |
| Aortic valve regurgitation ≥ moderate | 4 (17.4) |
| Symptoms | |
| Chest pain or back pain | 10 (43.5) |
| Haemoptysis | 2 (8.7) |
| Dyspnoea | 2 (8.7) |
| Fever | 1 (4.3) |
| Fatigue | 1 (4.3) |
| Asymptomatic | 8 (34.8) |
Data are presented as mean ± SD (range) or n (%).
SD: standard deviation.
| Characteristics . | All patients (n = 23) . |
|---|---|
| Age (years) | 55.4 ± 9.8 (31–70) |
| Male gender | 17 (73.9) |
| Smoker | 11 (47.8) |
| Hypertension | 22 (95.7) |
| Coronary artery disease | 6 (26.1) |
| Abdominal aortic aneurysm | 3 (13.0) |
| Aortic valve regurgitation ≥ moderate | 4 (17.4) |
| Symptoms | |
| Chest pain or back pain | 10 (43.5) |
| Haemoptysis | 2 (8.7) |
| Dyspnoea | 2 (8.7) |
| Fever | 1 (4.3) |
| Fatigue | 1 (4.3) |
| Asymptomatic | 8 (34.8) |
| Characteristics . | All patients (n = 23) . |
|---|---|
| Age (years) | 55.4 ± 9.8 (31–70) |
| Male gender | 17 (73.9) |
| Smoker | 11 (47.8) |
| Hypertension | 22 (95.7) |
| Coronary artery disease | 6 (26.1) |
| Abdominal aortic aneurysm | 3 (13.0) |
| Aortic valve regurgitation ≥ moderate | 4 (17.4) |
| Symptoms | |
| Chest pain or back pain | 10 (43.5) |
| Haemoptysis | 2 (8.7) |
| Dyspnoea | 2 (8.7) |
| Fever | 1 (4.3) |
| Fatigue | 1 (4.3) |
| Asymptomatic | 8 (34.8) |
Data are presented as mean ± SD (range) or n (%).
SD: standard deviation.
| Characteristics . | All patients (n = 23) . |
|---|---|
| TEVAR indications | |
| Acute type B aortic dissection | 17 (72.9) |
| Chronic type B aortic dissection | 2 (8.7) |
| Descending thoracic aortic aneurysm | 2 (8.7) |
| Penetrating aortic ulcer | 2 (8.7) |
| Proximal landing zone | |
| Zone 0 | 1 (4.3) |
| Zone 1 | 4 (17.4) |
| Zone 2 | 10 (43.5) |
| Zone 3 | 8 (34.8) |
| LSA coverage | 15 (65.2) |
| Additional procedures during TEVAR | |
| Chimney graft implantation | 3 (13.0) |
| LSA | 2 (8.7) |
| LCA | 2 (8.7) |
| Innominate artery | 1 (4.3) |
| Supra-aortic vessels bypass | 3 (13.0) |
| LSA–LCA bypass | 2 (8.7) |
| LCA–RCA bypass | 2 (8.7) |
| LSA spring coil occlusion | 3 (13.0) |
| LSA fenestration | 1 (4.3) |
| Time interval from TEVAR to endoleak (months) | 72 (1–156) |
| 1 | 2 (8.7) |
| 1–12 | 4 (17.4) |
| ≥12 | 17 (72.9) |
| Characteristics . | All patients (n = 23) . |
|---|---|
| TEVAR indications | |
| Acute type B aortic dissection | 17 (72.9) |
| Chronic type B aortic dissection | 2 (8.7) |
| Descending thoracic aortic aneurysm | 2 (8.7) |
| Penetrating aortic ulcer | 2 (8.7) |
| Proximal landing zone | |
| Zone 0 | 1 (4.3) |
| Zone 1 | 4 (17.4) |
| Zone 2 | 10 (43.5) |
| Zone 3 | 8 (34.8) |
| LSA coverage | 15 (65.2) |
| Additional procedures during TEVAR | |
| Chimney graft implantation | 3 (13.0) |
| LSA | 2 (8.7) |
| LCA | 2 (8.7) |
| Innominate artery | 1 (4.3) |
| Supra-aortic vessels bypass | 3 (13.0) |
| LSA–LCA bypass | 2 (8.7) |
| LCA–RCA bypass | 2 (8.7) |
| LSA spring coil occlusion | 3 (13.0) |
| LSA fenestration | 1 (4.3) |
| Time interval from TEVAR to endoleak (months) | 72 (1–156) |
| 1 | 2 (8.7) |
| 1–12 | 4 (17.4) |
| ≥12 | 17 (72.9) |
Data are presented as n (%) or median (range).
LCA: left common carotid artery; LSA: left subclavian artery; RCA: right common carotid artery; TEVAR: thoracic endovascular aortic repair.
| Characteristics . | All patients (n = 23) . |
|---|---|
| TEVAR indications | |
| Acute type B aortic dissection | 17 (72.9) |
| Chronic type B aortic dissection | 2 (8.7) |
| Descending thoracic aortic aneurysm | 2 (8.7) |
| Penetrating aortic ulcer | 2 (8.7) |
| Proximal landing zone | |
| Zone 0 | 1 (4.3) |
| Zone 1 | 4 (17.4) |
| Zone 2 | 10 (43.5) |
| Zone 3 | 8 (34.8) |
| LSA coverage | 15 (65.2) |
| Additional procedures during TEVAR | |
| Chimney graft implantation | 3 (13.0) |
| LSA | 2 (8.7) |
| LCA | 2 (8.7) |
| Innominate artery | 1 (4.3) |
| Supra-aortic vessels bypass | 3 (13.0) |
| LSA–LCA bypass | 2 (8.7) |
| LCA–RCA bypass | 2 (8.7) |
| LSA spring coil occlusion | 3 (13.0) |
| LSA fenestration | 1 (4.3) |
| Time interval from TEVAR to endoleak (months) | 72 (1–156) |
| 1 | 2 (8.7) |
| 1–12 | 4 (17.4) |
| ≥12 | 17 (72.9) |
| Characteristics . | All patients (n = 23) . |
|---|---|
| TEVAR indications | |
| Acute type B aortic dissection | 17 (72.9) |
| Chronic type B aortic dissection | 2 (8.7) |
| Descending thoracic aortic aneurysm | 2 (8.7) |
| Penetrating aortic ulcer | 2 (8.7) |
| Proximal landing zone | |
| Zone 0 | 1 (4.3) |
| Zone 1 | 4 (17.4) |
| Zone 2 | 10 (43.5) |
| Zone 3 | 8 (34.8) |
| LSA coverage | 15 (65.2) |
| Additional procedures during TEVAR | |
| Chimney graft implantation | 3 (13.0) |
| LSA | 2 (8.7) |
| LCA | 2 (8.7) |
| Innominate artery | 1 (4.3) |
| Supra-aortic vessels bypass | 3 (13.0) |
| LSA–LCA bypass | 2 (8.7) |
| LCA–RCA bypass | 2 (8.7) |
| LSA spring coil occlusion | 3 (13.0) |
| LSA fenestration | 1 (4.3) |
| Time interval from TEVAR to endoleak (months) | 72 (1–156) |
| 1 | 2 (8.7) |
| 1–12 | 4 (17.4) |
| ≥12 | 17 (72.9) |
Data are presented as n (%) or median (range).
LCA: left common carotid artery; LSA: left subclavian artery; RCA: right common carotid artery; TEVAR: thoracic endovascular aortic repair.
Operative data
Except for 1 patient who refused surgery initially and who was monitored for 44 months before the final operation, all other patients received timely operations after the endoleak was observed. No patient had a previous sternotomy. We performed TAR with the FET technique in 15 patients; direct closure of the endoleak in 2 patients; hybrid aortic arch repair in 4 patients; arch debranching with TEVAR in 1 patient; and LSA–LCA bypass with TEVAR in 1 patient. Twelve patients also had additional procedures, including 1 patient who had an additional TEVAR with the FET procedure. A large intimal tear was located distally to the previous stent on the preoperative CT of this patient. He had an additional TEVAR to cover the tear. For 21 patients with CPB, the mean CPB and aortic cross-clamp times were 146.7 ± 42.2 and 81.0 ± 43.3 min, respectively. HCA was used in 17 patients, with a mean SCP time of 18.8 ± 8.2 min. The operative details are summarized in Table 3.
| Variables . | All patients (n = 23) . |
|---|---|
| Total arch replacement + frozen elephant trunk technique | 15 (65.2) |
| Direct closure of the endoleak | 2 (8.7) |
| Hybrid aortic arch repair | 4 (17.4) |
| Arch debranching + TEVAR | 1 (4.3) |
| LSA–left common carotid artery bypass + TEVAR | 1 (4.3) |
| Additional procedures | 12 (52.2) |
| Aortic valve replacement | 2 (8.7) |
| Bentall procedure | 3 (13.0) |
| David procedure | 1 (4.3) |
| Coronary artery bypass grafting | 6 (26.1) |
| Ascending right femoral artery bypass | 2 (8.7) |
| TEVARa | 1 (4.3) |
| Cardiopulmonary bypass | 21 (91.3) |
| Cardiopulmonary bypass time (min) | 146.7 ± 42.2 (78–251) |
| Aortic cross-clamp time (min) | 81.0 ± 43.3 (24–188) |
| Hypothermic circulatory arrest | 17 (73.9) |
| Selective antegrade cerebral perfusion time (min) | 18.8 ± 8.2 (7–35) |
| Residual endoleak (n = 22) | 1 (4.5) |
| Ventilation time (h) | 19 (6–394) |
| >48 h | 4 (17.4) |
| Intensive care unit duration (days) | 5.5 ± 4.4 (1–17) |
| Postoperative in-hospital duration (days) | 12.6 ± 5.8 (5–27) |
| Major adverse events | 4 (17.4) |
| In-hospital mortality | 2 (8.7) |
| Reoperation for bleeding | 2 (8.7) |
| Extracardiac membrane oxygenation support | 2 (8.7) |
| Renal failure needing haemodialysis | 2 (8.7) |
| Stroke | 1 (4.3) |
| Pericardial effusion needing surgical intervention | 1 (4.3) |
| Variables . | All patients (n = 23) . |
|---|---|
| Total arch replacement + frozen elephant trunk technique | 15 (65.2) |
| Direct closure of the endoleak | 2 (8.7) |
| Hybrid aortic arch repair | 4 (17.4) |
| Arch debranching + TEVAR | 1 (4.3) |
| LSA–left common carotid artery bypass + TEVAR | 1 (4.3) |
| Additional procedures | 12 (52.2) |
| Aortic valve replacement | 2 (8.7) |
| Bentall procedure | 3 (13.0) |
| David procedure | 1 (4.3) |
| Coronary artery bypass grafting | 6 (26.1) |
| Ascending right femoral artery bypass | 2 (8.7) |
| TEVARa | 1 (4.3) |
| Cardiopulmonary bypass | 21 (91.3) |
| Cardiopulmonary bypass time (min) | 146.7 ± 42.2 (78–251) |
| Aortic cross-clamp time (min) | 81.0 ± 43.3 (24–188) |
| Hypothermic circulatory arrest | 17 (73.9) |
| Selective antegrade cerebral perfusion time (min) | 18.8 ± 8.2 (7–35) |
| Residual endoleak (n = 22) | 1 (4.5) |
| Ventilation time (h) | 19 (6–394) |
| >48 h | 4 (17.4) |
| Intensive care unit duration (days) | 5.5 ± 4.4 (1–17) |
| Postoperative in-hospital duration (days) | 12.6 ± 5.8 (5–27) |
| Major adverse events | 4 (17.4) |
| In-hospital mortality | 2 (8.7) |
| Reoperation for bleeding | 2 (8.7) |
| Extracardiac membrane oxygenation support | 2 (8.7) |
| Renal failure needing haemodialysis | 2 (8.7) |
| Stroke | 1 (4.3) |
| Pericardial effusion needing surgical intervention | 1 (4.3) |
Data are presented as mean ± SD (range), n (%) or median (range).
TEVAR was performed in combination with the frozen elephant trunk technique.
LSA: left subclavian artery; SD: standard deviation; TEVAR: thoracic endovascular aortic repair.
| Variables . | All patients (n = 23) . |
|---|---|
| Total arch replacement + frozen elephant trunk technique | 15 (65.2) |
| Direct closure of the endoleak | 2 (8.7) |
| Hybrid aortic arch repair | 4 (17.4) |
| Arch debranching + TEVAR | 1 (4.3) |
| LSA–left common carotid artery bypass + TEVAR | 1 (4.3) |
| Additional procedures | 12 (52.2) |
| Aortic valve replacement | 2 (8.7) |
| Bentall procedure | 3 (13.0) |
| David procedure | 1 (4.3) |
| Coronary artery bypass grafting | 6 (26.1) |
| Ascending right femoral artery bypass | 2 (8.7) |
| TEVARa | 1 (4.3) |
| Cardiopulmonary bypass | 21 (91.3) |
| Cardiopulmonary bypass time (min) | 146.7 ± 42.2 (78–251) |
| Aortic cross-clamp time (min) | 81.0 ± 43.3 (24–188) |
| Hypothermic circulatory arrest | 17 (73.9) |
| Selective antegrade cerebral perfusion time (min) | 18.8 ± 8.2 (7–35) |
| Residual endoleak (n = 22) | 1 (4.5) |
| Ventilation time (h) | 19 (6–394) |
| >48 h | 4 (17.4) |
| Intensive care unit duration (days) | 5.5 ± 4.4 (1–17) |
| Postoperative in-hospital duration (days) | 12.6 ± 5.8 (5–27) |
| Major adverse events | 4 (17.4) |
| In-hospital mortality | 2 (8.7) |
| Reoperation for bleeding | 2 (8.7) |
| Extracardiac membrane oxygenation support | 2 (8.7) |
| Renal failure needing haemodialysis | 2 (8.7) |
| Stroke | 1 (4.3) |
| Pericardial effusion needing surgical intervention | 1 (4.3) |
| Variables . | All patients (n = 23) . |
|---|---|
| Total arch replacement + frozen elephant trunk technique | 15 (65.2) |
| Direct closure of the endoleak | 2 (8.7) |
| Hybrid aortic arch repair | 4 (17.4) |
| Arch debranching + TEVAR | 1 (4.3) |
| LSA–left common carotid artery bypass + TEVAR | 1 (4.3) |
| Additional procedures | 12 (52.2) |
| Aortic valve replacement | 2 (8.7) |
| Bentall procedure | 3 (13.0) |
| David procedure | 1 (4.3) |
| Coronary artery bypass grafting | 6 (26.1) |
| Ascending right femoral artery bypass | 2 (8.7) |
| TEVARa | 1 (4.3) |
| Cardiopulmonary bypass | 21 (91.3) |
| Cardiopulmonary bypass time (min) | 146.7 ± 42.2 (78–251) |
| Aortic cross-clamp time (min) | 81.0 ± 43.3 (24–188) |
| Hypothermic circulatory arrest | 17 (73.9) |
| Selective antegrade cerebral perfusion time (min) | 18.8 ± 8.2 (7–35) |
| Residual endoleak (n = 22) | 1 (4.5) |
| Ventilation time (h) | 19 (6–394) |
| >48 h | 4 (17.4) |
| Intensive care unit duration (days) | 5.5 ± 4.4 (1–17) |
| Postoperative in-hospital duration (days) | 12.6 ± 5.8 (5–27) |
| Major adverse events | 4 (17.4) |
| In-hospital mortality | 2 (8.7) |
| Reoperation for bleeding | 2 (8.7) |
| Extracardiac membrane oxygenation support | 2 (8.7) |
| Renal failure needing haemodialysis | 2 (8.7) |
| Stroke | 1 (4.3) |
| Pericardial effusion needing surgical intervention | 1 (4.3) |
Data are presented as mean ± SD (range), n (%) or median (range).
TEVAR was performed in combination with the frozen elephant trunk technique.
LSA: left subclavian artery; SD: standard deviation; TEVAR: thoracic endovascular aortic repair.
Morbidity and mortality
The median duration of mechanical ventilation was 19 h (range 6–394 h). Only 4 patients required prolonged mechanical ventilation (>48 h). Two patients died in the hospital (8.7%). Case 1 was a 60-year-old man. He had an LSA CGI during the initial TEVAR. We performed a hybrid aortic arch repair. The operation was successful, but he had a descending thoracic aortic aneurysm rupture on the 2nd postoperative day. Then he had a reoperation via a left thoracotomy for bleeding. We sutured the ruptured descending thoracic aneurysm using 3–0 Prolene. Then we used a Dacron patch to wrap the aneurysm. The reoperation was performed under HCA. The patient had extracorporeal membrane oxygenation support after the operation. He gradually developed multiple organ failure, renal failure needing haemodialysis and died on the 5th postoperative day. Case 2 was a 56-year-old woman. She underwent TAR with the FET technique and coronary artery bypass grafting. After the operation, she had a renal failure needing haemodialysis, pulmonary dysfunction necessitating extracorporeal membrane oxygenation and gastrointestinal bleeding. She also had a severe perioperative stroke. She died on the 17th postoperative day. One patient was operated on for moderate pericardial effusion, and 1 patient was reoperated on for bleeding. No patient had cerebrospinal fluid drainage, and no patient developed paraplegia postoperatively. Except for the 2 patients who died, all other patients were discharged from the hospital. The postoperative details are shown in Table 3.
Postoperative computed tomography
Except for 1 death (Case 1), all patients had postoperative CT examinations. The type Ia endoleak had been sealed successfully in 95.5% (21/22) of the patients. The patient who had arch debranching with TEVAR had a small residual endoleak after the operation. Figure 2 shows the postoperative CT scans of the patients treated with the 4 different techniques.
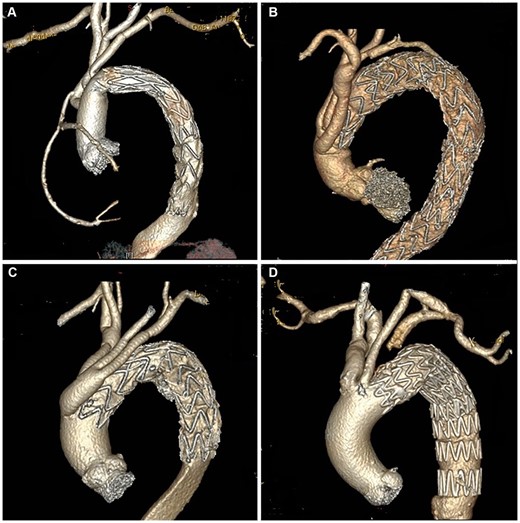
Postoperative computed tomography scans of patients treated with 4 different techniques. (A) Total arch replacement with the frozen elephant trunk technique; (B) hybrid aortic arch repair; (C) arch debranching with thoracic endovascular aortic repair (TEVAR); and (D) left subclavian artery–left common carotid artery bypass with TEVAR.
Among the 19 patients with previous TBAD, 2 patients presented with complete thrombosis (obliteration) of the false lumen (FL) before the operation; FL was patent in the other 17 patients. After excluding 1 death (Case 1), we analysed the postoperative aortic remodelling for the remaining 16 patients. At the proximal part of the stent, complete thrombosis of the FL occurred in 87.5% (14/16) of patients after the operation, whereas partial thrombosis of the FL occurred in 12.5% (2/16). At the distal part of the stent, the diaphragmatic hiatus and the renal arteries, the FL was obliterated in 81.3% (13/16), 37.5% (6/16) and 6.25% (1/16) of patients; partial thrombosis of FL was observed in 18.5% (3/16), 50.0% (8/16) and 6.25% (1/16) of patients; the FL was patent in 0, 12.5% (2/16) and 87.5% (14/16) of patients, respectively. Figure 3 shows complete thrombosis and partial thrombosis of the FL on the CT scan.
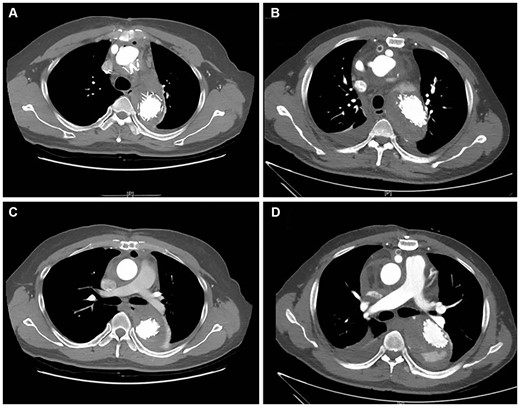
Postoperative complete thrombosis and partial thrombosis of the false lumen on computed tomography scans. (A) Complete thrombosis at the proximal part of the stent; (B) partial thrombosis at the proximal part of the stent; (C) complete thrombosis at the distal part of the stent; and (D) partial thrombosis at the distal part of the stent.
Follow-up data
The follow-up data were available for all 21 survivors. The median follow-up time was 18 months (range 1–84 months). Aortic events were defined as new aortic dissection, ruptured dissection, secondary aortic operation and aortic-related death. During the follow-up period, 8 patients died or had aortic events, including 5 deaths and 6 aortic events. The primary disease was TBAD for all 8 patients. One patient died of a ruptured aortic dissection; partial thrombosis of the FL before discharge occurred in this patient and the FL was progressively enlarged on CT re-examination (Fig. 4A). Another patient died of a ruptured abdominal aortic aneurysm. This patient presented with an abdominal aortic aneurysm (diameter = 53 mm) before the operation (Fig. 4B); we had recommended elective abdominal aortic aneurysm repair. Four patients had secondary thoraco-abdominal aortic aneurysm repairs for downstream FL dilatation (Fig. 4C). Among the 4 patients, 1 patient had pulmonary infection, paraplegia and renal failure needing haemodialysis after surgery and died 2 months after the operation. Another 2 patients died of thrombocytopaenia and hepatocellular carcinoma, respectively. The details of the deaths and aortic events are shown in Table 4.
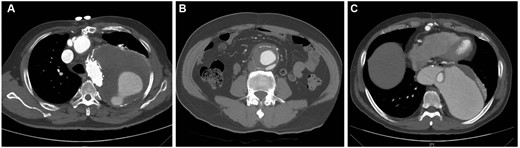
Computed tomography (CT) scans of 3 patients with aortic events. (A) The last CT scan shows a progressively enlarged false lumen; the patient died of ruptured aortic dissection 45 months after the CT examination. (B) CT scan obtained before the operation shows an abdominal aortic aneurysm (diameter = 53 mm); the patient died of a ruptured abdominal aortic aneurysm 12 months after the operation. (C) The CT scan shows progressive dilatation of the false lumen; the patient had a secondary thoraco-abdominal aortic aneurysm repair 4 months after the initial operation.
| Number . | Gender . | Age (years) . | Operative procedures . | Follow-up time (months) . | Cause of death . | Aortic events . |
|---|---|---|---|---|---|---|
| 1 | Male | 59 | TAR + FET + CABG | 12 | Ruptured AAA | Ruptured AAA |
| 2 | Male | 44 | TAR + FET | 48 | NA | TAAR 7 months later |
| 3 | Male | 61 | TAR + FET + CABG | 28 | Ruptured aortic dissection | Ruptured aortic dissection |
| 4 | Male | 60 | Arch debranching + TEVAR | 3 | Hepatocellular carcinoma | NA |
| 5 | Male | 67 | AVR + hybrid aortic arch repair + CABG | 1 | Thrombocytopaenia | NA |
| 6 | Male | 42 | TAR + FET | 21 | NA | TAAR 12 months later |
| 7 | Male | 55 | TAR + FET | 6 | Complications after TAAR | TAAR 4 months later |
| 8 | Male | 31 | TAR + FET + ascending -right femoral artery bypass | 11 | NA | TAAR 11 months later |
| Number . | Gender . | Age (years) . | Operative procedures . | Follow-up time (months) . | Cause of death . | Aortic events . |
|---|---|---|---|---|---|---|
| 1 | Male | 59 | TAR + FET + CABG | 12 | Ruptured AAA | Ruptured AAA |
| 2 | Male | 44 | TAR + FET | 48 | NA | TAAR 7 months later |
| 3 | Male | 61 | TAR + FET + CABG | 28 | Ruptured aortic dissection | Ruptured aortic dissection |
| 4 | Male | 60 | Arch debranching + TEVAR | 3 | Hepatocellular carcinoma | NA |
| 5 | Male | 67 | AVR + hybrid aortic arch repair + CABG | 1 | Thrombocytopaenia | NA |
| 6 | Male | 42 | TAR + FET | 21 | NA | TAAR 12 months later |
| 7 | Male | 55 | TAR + FET | 6 | Complications after TAAR | TAAR 4 months later |
| 8 | Male | 31 | TAR + FET + ascending -right femoral artery bypass | 11 | NA | TAAR 11 months later |
AAA: abdominal aortic aneurysm; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; FET: frozen elephant trunk; NA: not available; TAAR: thoraco-abdominal aortic aneurysm repair; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
| Number . | Gender . | Age (years) . | Operative procedures . | Follow-up time (months) . | Cause of death . | Aortic events . |
|---|---|---|---|---|---|---|
| 1 | Male | 59 | TAR + FET + CABG | 12 | Ruptured AAA | Ruptured AAA |
| 2 | Male | 44 | TAR + FET | 48 | NA | TAAR 7 months later |
| 3 | Male | 61 | TAR + FET + CABG | 28 | Ruptured aortic dissection | Ruptured aortic dissection |
| 4 | Male | 60 | Arch debranching + TEVAR | 3 | Hepatocellular carcinoma | NA |
| 5 | Male | 67 | AVR + hybrid aortic arch repair + CABG | 1 | Thrombocytopaenia | NA |
| 6 | Male | 42 | TAR + FET | 21 | NA | TAAR 12 months later |
| 7 | Male | 55 | TAR + FET | 6 | Complications after TAAR | TAAR 4 months later |
| 8 | Male | 31 | TAR + FET + ascending -right femoral artery bypass | 11 | NA | TAAR 11 months later |
| Number . | Gender . | Age (years) . | Operative procedures . | Follow-up time (months) . | Cause of death . | Aortic events . |
|---|---|---|---|---|---|---|
| 1 | Male | 59 | TAR + FET + CABG | 12 | Ruptured AAA | Ruptured AAA |
| 2 | Male | 44 | TAR + FET | 48 | NA | TAAR 7 months later |
| 3 | Male | 61 | TAR + FET + CABG | 28 | Ruptured aortic dissection | Ruptured aortic dissection |
| 4 | Male | 60 | Arch debranching + TEVAR | 3 | Hepatocellular carcinoma | NA |
| 5 | Male | 67 | AVR + hybrid aortic arch repair + CABG | 1 | Thrombocytopaenia | NA |
| 6 | Male | 42 | TAR + FET | 21 | NA | TAAR 12 months later |
| 7 | Male | 55 | TAR + FET | 6 | Complications after TAAR | TAAR 4 months later |
| 8 | Male | 31 | TAR + FET + ascending -right femoral artery bypass | 11 | NA | TAAR 11 months later |
AAA: abdominal aortic aneurysm; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; FET: frozen elephant trunk; NA: not available; TAAR: thoraco-abdominal aortic aneurysm repair; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
DISCUSSION
Endoleak is the most common complication after TEVAR [2, 4], and the most common and severe type is type Ia endoleak [6]. Type Ia endoleak arises from insufficient sealing at the proximal ends of the stent graft. It can happen immediately after TEVAR, a few days to several months post-TEVAR or during the extended follow-up period [6, 11, 12].
We only collected the clinical data of patients who developed type Ia endoleak; we did not have data for the entire TEVAR cohort. Therefore, we could only conclude the risk factors for type Ia endoleak after TEVAR from the results of previous studies [13–15]. Kanaoka et al. [13] concluded that risk factors for early type I endoleak after TEVAR for an aneurysm included PLZ 0–2, LSA coverage, large proximal neck and stent graft diameters, excessive oversizing and the use of the chimney technique. The bird-beak configuration was also identified as a risk factor [14, 15]. The use of stent grafts to treat TBAD by coverage of the LSA or placement of a chimney graft is a significant risk factor for a subsequent type Ia endoleak. In our study, the LSA was covered by TEVAR stents in 15 patients, and 3 patients had a CGI procedure during the initial TEVAR. Indeed, a type Ia endoleak represents an intractable problem. The best way to treat this problem is to avoid it in the first place. Cardiovascular surgeons should take the indications for TEVAR seriously and strive to avoid a type Ia endoleak during the index TEVAR. For patients with a high risk of a type Ia endoleak, TEVAR should be discouraged and open repair should be considered.
A type Ia endoleak is a risk factor for poor aortic remodelling [12] and for early and late mortality [7, 8]. Therefore, once a type Ia endoleak is confirmed, timely treatment is needed to eliminate the leakage and prevent high sac pressure, aneurysm dilation and aneurysm rupture [11]. Indeed, the majority of immediate type Ia endoleaks or small endoleaks following TEVAR could resolve spontaneously over time, without additional intervention needed [4, 11, 12]. For larger type Ia endoleaks, active treatment should be applied. However, it remains an intractable condition to repair due to the complexity of the aortic arch anatomy in patients with previous TEVAR. Several different techniques had been proposed to treat this intractable problem.
Type Ia endoleak could be solved by interventional methods. For many patients with type Ia endoleak, additional cuff or endograft implantation or balloon angioplasty could be performed to eliminate the endoleak [4]. However, the PLZ was often inadequate for redo TEVAR with an additional endograft. In this situation, the chimney technique could be applied in combination with re-TEVAR to extend the PLZ [16]. Transcatheter embolization with the liquid embolic agent Onyx (Micro Therapeutics, Inc., Irvine, CA, USA) (an ethylene vinyl alcohol copolymer) [17] and transthoracic embolization with metal coils and glue [18] were used to treat type Ia endoleak in some case reports. But the long-term outcome of these techniques remains uncertain. Indeed, in many patients with type Ia endoleak, a purely endovascular approach is not technically feasible, and hybrid surgery or open surgery is necessary.
When combining open surgical and endovascular techniques, the hybrid approach seems to simplify and shorten the repair. For patients with an inadequate PLZ, if we planned to perform redo TEVAR, additional supra-aortic vessels bypass or aortic arch debranching could be performed to extend the PLZ. Supra-aortic vessels bypass with TEVAR was a relatively simplified operation, with less surgical trauma. But it was also associated with the risk of graft infection, graft occlusion and anastomotic pseudoaneurysms during the follow-up [19, 20]. Aortic arch debranching with TEVAR could be performed off-pump in some seriously ill patients. But the long-term outcome of this procedure is still in doubt. The preceding 2 procedures are better suited for older patients who could not tolerate extensive surgery because of their poor condition. Patients still had a risk of proximal endoleak after surgery with the 2 techniques. Hybrid aortic arch repair entails replacement of the Dacron graft in the ascending aorta, debranching of the supra-aortic vessels and a TEVAR landing in the Dacron graft. It could be used to treat concurrent diseases of the ascending aorta or arch. With this technique, the primary stent with its proximal barbs or springs was not manipulated or incorporated in any anastomosis. Therefore, it was very convenient for arch repair. This approach also avoids HCA and shortens CPB duration, which could decrease postoperative complications. In this study, 6 patients had hybrid operations. Of these, there was 1 early death and 2 late deaths. The relatively poor outcome could be explained by the poor condition of these patients.
Conventional open surgery gets more support in current clinical practice. The FET technique [11] is widely used to treat type Ia endoleak. TAR with the FET technique is also the preferred procedure in this group. For patients with type Ia endoleak, the previous stent often conformed poorly to the arch circumferentially and was not amenable to direct sewing of the stent to the aortic wall. Distal anastomosis by incorporating the existing stent and aortic tissue would also carry the risk of suture leakage due to the fragile polytetrafluoroethylene membrane of the previous stent. Therefore, we always implanted a new FET stent into the descending aorta when treating type Ia endoleak. The FET stent used in our centre had an extra centimetre of Dacron graft proximally and distally. It could facilitate the distal anastomosis. It could also facilitate the proximal anastomosis when performing secondary thoraco-abdominal aortic aneurysm repair, or it could serve as a new PLZ for TEVAR. For patients with previous TBAD, the FET technique could also promote downstream aortic remodelling. Once the FET was implanted in the previous stent in the descending aorta, the remaining stent would expand, which would promote thrombosis in the FL of the distal aorta. Moreover, this technique could also repair ascending or arch pathologies simultaneously. In our study, we treated 15 patients with this technique, with a mortality of 6.7% (1/15). In addition, we used direct closure of the endoleak in 2 patients. This technique has not been reported previously. It allowed us to close the endoleak site under direct inspection. However, this technique is suited only for patients with limited endoleak located in the anterior wall or the greater curvature of the arch. Once it has been repaired successfully, the late prognosis is optimal.
A total of 82% of patients in this series underwent an initial TEVAR for TBAD. There were 8 aortic events or late deaths during the follow-up period. All 8 patients had previous TBAD. Therefore, patients should receive careful and regular CT follow-up after surgery, especially those with TBAD. Once progressive FL dilation was noted, a timely reoperation was recommended to decrease late mortality.
Limitations
Our study has some inevitable limitations. It is limited by its retrospective and observational nature, and it represents the surgical experience of our centre only. The sample size is relatively small, and the follow-up time is short. Further study is needed to investigate the optimal surgical strategy for treatment of type Ia endoleak after TEVAR and to evaluate the long-term outcomes of different approaches.
CONCLUSIONS
Our experience indicated that TAR with the FET procedure, direct closure of the endoleak, hybrid aortic arch repair, arch debranching with TEVAR and LSA–LCA bypass with TEVAR could be used to manage type Ia endoleak after TEVAR. Different approaches should be selected after a comprehensive evaluation of the patients. Acceptable early and late outcomes could be obtained with these techniques.
Conflict of interest: none declared.
Author contributions
Yaojun Dun: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Writing—original draft; Writing—review & editing. Yi Shi: Methodology. Hongwei Guo: Formal analysis. Yanxiang Liu: Data curation. Xiangyang Qian: Methodology; Resources; Supervision. Xiaogang Sun: Conceptualization; Methodology; Supervision; Writing—review & editing. Cuntao Yu: Methodology; Resources; Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks George J. Arnaoutakis, Patrizio Castelli and David Spielvogel for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- CGI
Chimney graft implants
- CPB
Cardiopulmonary bypass
- CT
Computed tomography
- FET
Frozen elephant trunk
- FL
False lumen
- HCA
Hypothermic circulatory arrest
- LCA
Left common carotid artery
- LSA
Left subclavian artery
- PLZ
Proximal landing zone
- SCP
Selective antegrade cerebral perfusion
- TAR
Total arch replacement
- TBAD
Type B aortic dissection
- TEVAR
Thoracic endovascular aortic repair





