-
PDF
- Split View
-
Views
-
Cite
Cite
Hui Zhuang, Fanggang Cai, Zhixian Wu, Tenghui Zhan, Hongyu Chen, Cheng Chen, Hanyue Zhang, Pingfan Guo, Salvage endovascular septectomy in patients with chronic abdominal aortic dissection after failed endovascular aneurysm repair, Interactive CardioVascular and Thoracic Surgery, Volume 29, Issue 4, October 2019, Pages 621–624, https://doi.org/10.1093/icvts/ivz128
Close - Share Icon Share
Abstract
This study aimed to investigate the efficacy and safety of salvage endovascular septectomy in patients with abdominal chronic aortic dissection (CAD) after endovascular aneurysm repair. A study cohort comprising 6 patients with chronic abdominal aortic dissection after failed endovascular aortic repair [mean age 62.5 (36–69) years] were enrolled to undergo salvage endovascular septectomy. The procedure involved entering the false lumen via the intrinsic visceral entry to perform a confined septectomy using a ‘Gigli wire’ to merge the true and false lumens. The outcomes were assessed by Digital angiography and computed tomography angiography. All 6 patients were successfully operated on; the diameters of the visceral abdominal aorta and the infrarenal abdominal aorta were similar at 1, 3, 6 and 12 months compared with the baseline; the patency of the visceral branch arteries was also stable at 1, 3, 6 and 12 months compared with the baseline; no occlusion of the visceral branch arteries was noted; no major vascular adverse events or deaths were observed. In this preliminary study, it was proven that salvage endovascular septectomy is a potentially advantageous technique that is safe and effective in the treatment of patients with CAD after failed endovascular aortic repair.
INTRODUCTION
Chronic aortic dissection (CAD) is a critical clinical condition with a high mortality and warrants sophisticated therapeutic techniques [1]. Although some advances have been achieved in endovascular aortic repair (EVAR), it remains ineffective in the treatment of some patients with complicated CAD [2]. Chronic abdominal aortic dissection (CAAD) with multiple visceral entries is refractory to interventional therapies [3]. There are several major obstacles: the difficulty in maintaining the patency of the visceral branch arteries while repairing the visceral entries; conventional stents barely cover the visceral entries; the stiffness and the difficulty in remodelling the chronically diseased septum impede the landing and expansion of aortic stents [4].
Some groups have employed aortic septectomy using a combination of the ‘Cheese wire/Gigli saw’ and EVAR to treat CAAD patients with visceral entry or multiple aneurysms [5, 6]. Endovascular septectomy merges the true and false lumens, improves the dynamical stability of false lumen, creates proximal landing zones for EVAR and has demonstrated promising therapeutic outcomes [7]. However, the feasibility of these techniques in CAAD patients after a failed EVAR has not been investigated. We selected a cohort of CAAD patients with failed EVAR to undergo salvage endovascular septectomy.
MATERIALS AND METHODS
Patients
Six male patients with a history of CAAD and failed EVAR were enrolled at the Department of Vascular Surgery, the First Affiliated Hospital, Fujian Medical University from June 2016 to February 2017. The median age was 62.5 (range 36–69) years. All patients experienced severe back pain refractory to medication after EVAR. Medical imaging confirmed that the conditions of 2 patients had progressed and that the diameters of false lumens were expanding at a rate of >5 mm per year. Based on medical assessment, all patients were ineligible for open surgical repair, and they were subjected to salvage endovascular septectomy.
Examination and measurement
Preoperative computed tomography angiography (CTA) was used to assess the thoracic and abdominal aorta and the iliac artery. The diameters of true and false lumens of the abdominal aorta at coeliac, mesenteric and renal levels were measured. The number and position of the entries in the septum were noted (Supplementary Material, Fig. S1). The degree of dissection was measured and the patency of the visceral branch arteries was assessed.
Operative procedure
Establishing the femoral access. Either a unilateral or a bilateral femoral access is feasible. We preferred the bilateral femoral access and an 8-Fr sheath was used. Two ProGlide systems were placed for subsequent use.
Visceral entry access. A 4-Fr catheter and a 0.035′ wire were introduced via a single sheath. These were retained in the true lumen to avoid penetrating other entries before reaching the targeted visceral entry under the guide of CTA images and fluoroscopy. When the catheter and wire had reached the visceral entry, they were adjusted to access the entry and retained in the false lumen (Fig. 1A).
Advancement of a snare via the false lumen. Another 4-Fr catheter and 0.035′ wire were advanced via another sheath through the distal entry in CAAD or the iliac artery into the false lumen. The morphology of the septum varied and, therefore, operators should carefully review the CTA images and repeat angiography when necessary to ensure that the most distal entry is selected. The snare should be placed into the false lumen and expanded completely.
Establishing a wire access. A 0.014′ wire was manoeuvred via the catheter in the true lumen and then pulled out by the snare to establish a through-and-through wire access (Fig. 1B). Two 6-Fr long sheaths were placed via both femoral access to approach the entry to provide firm support.
Septectomy. The wires were pulled at both sides and a Gigli saw was used to cut the septum to merge the true and false lumens (Fig. 1C). The distal cutting end was confined to 1 inch below the renal arterial level. Due to the high risk involved in this step, an assistant should sustain the long sheath at 1 cm below the cutting level to avoid over-cutting. Transcatheter angiography was performed whenever needed to monitor the cutting procedure.
EVAR. The landing zone of a second EVAR was at the post-septectomy abdominal aortic segment below the level of the renal artery (Fig. 1D). The diameter of the selected stents was oversized by 10–20% of the transaortic diameter.
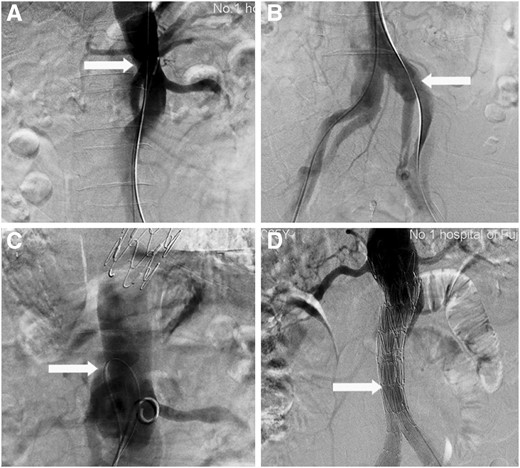
Septectomy using an intrinsic visceral entry, featured with confined cutting range. (A) Access of the visceral entry (write arrow) is completed. (B) The 0.014’ wire is captured by the snare and pulled out via the most distal entry (write arrow) and thereby establishing a through-and-through wire access. (C) The septum is cut using a Gigli saw (write arrow). (D) Endovascular aneurysm repair (write arrow) is performed using the landing zone at the post-cut abdominal aortic segment below the level of the renal artery.
Follow-up and postoperative assessment
The patients were followed up at 1, 3, 6 and 12 months. The primary end points were the CTA-measured diameters of the abdominal aorta at the levels of the coeliac trunk and the superior mesenteric artery, and below the renal artery. The patency of the visceral branch arteries was also observed. The patient’s symptoms were assessed using a visual analogue scale. Safety end points including death, aortic rupture, endoleak, stent displacement and stent thrombosis were observed.
Statistical analysis
The measured parameters are presented as the median (range), and the Wilcoxon signed-rank test was used to compare the pre- and postoperative variables. A P-value <0.05 was considered statistically significant.
RESULTS
Demographical and general data
The patients’ mean age was 62.6 (36–69) years and history of CAD lasting for a mean of 6 (range 3–24) years. All had a history of smoking, hypertension and pleural effusion. Two patients had renal dysfunction (Supplementary Material, Table S1).
Efficacy
Abdominal aortic diameters at the visceral levels
As shown in Supplementary Material, Table S2, the diameters of the abdominal aorta at the levels of the coeliac trunk and the superior mesenteric artery, and below the renal artery did not increase at 1, 3, 6 and 12 months compared with the baseline (Fig. 2).

The true and false lumens of the abdominal aorta are successfully merged and the diameters of the abdominal aorta at the levels of the coeliac trunk and the superior mesenteric artery, and below the renal artery are similar before (A) and after (B) the operation.
Blood supply of the visceral branch arteries
The blood supply of the majority of the visceral branch arteries was not altered significantly compared with the baseline. The blood supply of the right renal arteries of patient 1 and 5 were converted from through false lumen to the true lumen at 1 month postoperative and remained stable at 3, 6 and 12 months. No occlusion of the visceral branch arteries was noted. The blood supply of the visceral branch arteries of all patients is detailed in Supplementary Material, Table S3.
Improvement in symptoms
All 6 patients experienced back and waist pains before the operation. The VAS score was 9 (4–10). The pains were relieved after the operation. The VAS scores were 3 (1–5), 3 (1–4), 3 (1–4) and 3 (1–4) at 1, 3, 6 and 12 months, respectively (P < 0.027).
Safety
No major adverse events associated with the operation were noted. No complications including aortic rupture, endoleak, stent thrombosis, stent displacement, and readmission were noted during the follow-up. No patient death was reported. The duration of the postoperative hospital stay was 4 (3–6) days.
DISCUSSION
In this study, we used a 0.014-inch guidewire to access the intrinsic entry in the visceral region of aortic dissection, avoiding possible impairment of the aortic wall by using various needle puncture techniques; the Cheese wire and Gigli saw were both employed to ensure the regularity of the cutting, reducing the risk of lesioning in the tunica media or adventitia of the abdominal aortic wall; the distal cutting end was confined to 1 inch below the renal artery, avoiding dilatation of the infrarenal aorta. The favourable therapeutic outcomes seen in the cohort, including stable aortic diameters, no ischaemia of the visceral branch arteries, and the mild adverse side effects suggest that this may be a promising procedure for the treatment of selected patients.
However, salvage endovascular septectomy is still risky, and may result in iatrogenic occlusion of the branch vessels. Folding or collapsing of the septum may obstruct the aortic outflow.
CONCLUSIONS
Salvage endovascular septectomy is a potentially advantageous technique and is effective in treating patients with CAAD with multiple visceral entries.
ACKNOWLEDGEMENTS
The authors would like to thank Wenyu Lin for providing language-editing services, and Chen Lin and Xia Zhang for the support in this work.
Funding
This study was supported by the Guiding Projects of Fujian Provincial Science and Technology Department [2015Y5007, 2018Y0028], the Natural Science Fund of Fujian Province [2016J01583, 2016J01590 and 2017J01327], Science and Technology Innovation Joint Fund Project of Fujian Province [2016Y9063] and the China Postdoctoral Fund [2016M590597].
Conflict of interest: none declared.
REFERENCES
Author notes
Hui Zhuang, Fanggang Cai and ZhixianWu authors contributed equally to the writing of this work.




