-
PDF
- Split View
-
Views
-
Cite
Cite
Yuki Nakamura, Shunsuke Saito, Shigeru Miyagawa, Yasushi Yoshikawa, Hiroki Hata, Daisuke Yoshioka, Koichi Toda, Yoshiki Sawa, Perioperative ischaemic reperfusion injury and allograft function in the early post-transplantation period, Interactive CardioVascular and Thoracic Surgery, Volume 29, Issue 2, August 2019, Pages 230–236, https://doi.org/10.1093/icvts/ivz086
Close - Share Icon Share
Abstract
Ischaemic reperfusion injury (IRI) is an inevitable complication of heart transplantation (HTX) and is observed as a pathological finding in biopsies from transplanted allografts. The aim of this study was to evaluate the severity of IRI and determine the clinical outcomes of HTX in patients with severe IRI.
We enrolled 74 consecutive patients who had undergone HTX since 2007. Endomyocardial biopsy samples were obtained from the right ventricle of the transplanted heart. IRI was graded as ‘trivial’, ‘mild’, ‘moderate’ or ‘severe’ according to the extent of IRI-specific findings in the samples. The cohort was divided into a moderate-to-severe IRI group with 21 patients [IRI(+)] and a low-grade group with 53 patients [IRI(−)].
The frequency of mechanical circulatory support and duration of catecholamine dependence in the early postoperative period were significantly higher in the IRI(+) group compared to the IRI(−) group. However, overall survival after HTX and mid-term cardiac allograft function were not significantly different between the groups. Among perioperative factors, cardiac ischaemic time was significantly different between the groups [IRI(−) vs IRI(+), 199 ± 38 min vs 239 ± 39 min; P < 0.001]. Incremental increases in cardiac ischaemic time were correlated with increases in IRI severity. Serum troponin T levels 3 h after donor heart reperfusion was significantly correlated with cardiac ischaemic time (r = 0.418, P = 0.0007).
IRI is associated with a complicated clinical course in the early post-HTX period due to temporary deterioration of allograft function. This may be attributable to myocardial stunning caused by long donor heart ischaemic time during HTX.
INTRODUCTION
Ischaemic reperfusion injury (IRI) is an unavoidable complication during heart transplantation (HTX) and is observed as a pathological finding in transplanted allograft biopsy samples several weeks after HTX [1–4]. IRI leads to irreversible cell death by apoptosis and necrosis through oxidative stress, inflammation and intracellular Ca2+ overload [5, 6]. It is also known that reperfusion itself can lead to life-threatening ventricular arrhythmia and, ultimately, sudden mortality [7]. However, the clinical impact of allograft IRI of differing severity on early and long-term outcomes after HTX has not been sufficiently investigated. A previous study systematically reported the specific histological features that can be used to distinguish IRI from other pathologies, such as acute allograft rejection [8]. Therefore, the aim of this study was to evaluate the severity of IRI and determine the clinical outcomes of HTX in patients with more severe IRI.
MATERIALS AND METHODS
Patients and study design
This study was approved by the institutional medical ethics committee and was conducted in accordance with the Declaration of Helsinki. A consecutive series of 74 adult patients who underwent HTX at our institution between 2007 and 2017, and provided informed consent, were enrolled in the study. Three patients who underwent both heart and lung transplantation were excluded from this study. We also excluded 1 patient who could not undergo endomyocardial biopsy due to a poor general condition after HTX.
Surgical procedure
Donor heart procurement was performed using the standard techniques, and the grafts were protected with the infusion of 2 l of cold (4–8°C) Celsior (Waters Medical Systems, Rochester, MN, USA) solution into the aortic root when the donor heart was retrieved and stored in a cold saline slush during transport. Patients underwent HTX using a modified bicaval technique as previously described [9]. Intravenous methylprednisolone sodium (500 mg) was administered immediately before reperfusion of the donor heart. Inhaled nitric oxide was routinely initiated intraoperatively and weaned off as soon as possible, usually within a few days. Prednisolone, mycophenolate mofetil and tacrolimus were administered as post-HTX immunosuppressive therapy in accordance with our hospital protocol. In our cohort, there were no patients who received anti-thymocyte globulin therapy after HTX.
Endomyocardial biopsy
Endomyocardial biopsy samples (usually 3 or 4 pieces from each procedure) were obtained from the right ventricle of the transplanted heart using a conventional technique. At our institute, surveillance endomyocardial biopsies during the first post-HTX year were performed routinely according to the following schedule: weekly for the first 4 weeks, biweekly for 4 weeks, monthly for 6 months and 1 year after HTX. From the second post-HTX year, endomyocardial biopsy was performed once a year. Endomyocardial biopsy samples obtained 3 and 4 weeks after HTX were used for histological analysis to evaluate IRI severity. The biopsy samples were fixed in buffered formalin, embedded in paraffin, serially sectioned at 3–4 μm and stained with haematoxylin and eosin and Masson trichrome. Acute cellular rejection was graded according to the International Society for Heart and Lung Transplantation criteria, regardless of the presence or absence of coagulative myocyte necrosis within the same sample. Two biopsy samples from patients diagnosed with acute cellular rejection were excluded from evaluation of IRI.
Diagnosis of ischaemic reperfusion injury
The pathological findings of IRI were defined as coagulative myocyte necrosis (Fig. 1A), infiltration of polymorphonuclear inflammatory cells (Fig. 1B) and de novo fibrosis (Fig. 1C). The presence of coagulative myocyte necrosis, with or without contraction bands, is the most definitive sign of IRI. Inflammation was classified by cell type (i.e. polymorphonuclear leucocytes, macrophages, lymphocytes, plasma cells and eosinophils). Inflammation consisting of polymorphonuclear leucocytes and macrophages was regarded as indicative of IRI. Rough fibrosis was considered as a sign of early healing of IRI. The findings in the samples were graded semiquantitatively: grade 0, trivial IRI (scant evidence of IRI); grade 1, mild IRI (evidence of IRI limited to the subendocardial myocardium); grade 2, moderate IRI (evidence of IRI, limited to single foci throughout the subendocardial myocardium); grade 3, severe IRI (evidence of multifocal IRI). The IRI grade used in the data represents the most extensive injury noted across all biopsies from a particular patient. The patients were divided into those who had moderate-to-severe IRI (grades 2 and 3) [IRI(+) group, n = 21] and those who had trivial-to-mild IRI (grades 0 and 1) [IRI(−) group, n = 53]. The primary end points of this study were all-cause mortality and major adverse events after HTX, such as acute cellular rejection.
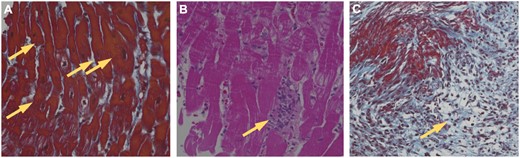
Histological evaluation of ischaemic reperfusion injury using endomyocardial biopsy samples of the right ventricle. Specific pathological findings of IRI include (A) coagulative myocyte necrosis (yellow arrows), (B) polymorphonuclear inflammation (yellow arrow) and (C) de novo fibrosis (yellow arrow).
Postoperative myocardial enzyme analysis
Blood samples were obtained from recipients at 3 h after donor heart reperfusion. All samples were centrifuged and the extracted sera were stored at −80° C until the analysis. Blood serum levels of troponin T (TnT) in each patient were measured by subcontracting enzyme immunoassays (SRL Corporation, Osaka, Japan).
Statistical analysis
Preoperative, intraoperative and postoperative variables were compared between the 2 groups using the Wilcoxon’s rank sum test or one-way analysis of variance with Tukey’s honestly significant difference test for continuous variables and the Fisher’s exact test for categorical variables. Cardiac enzyme levels were compared using the Wilcoxon’s rank sum test. We also assessed the correlation between serum TnT and the perioperative factors using the Pearson product-moment correlation coefficient. A P-value <0.05 was considered significant. The approximate normality of the distributions of each continuous variable was assessed using the Shapiro–Wilk test. Based on these results, the continuous variables are presented as means ± standard deviations and medians (25th–75th percentiles). Survival analyses were performed using the Kaplan–Meier method. The Cox proportional hazards model was used to determine a covariate’s impact on the patient hazards. Statistical analyses were performed using JMP Pro version 11.2.0 (SAS Institute, Cary, NC, USA).
RESULTS
Perioperative characteristics
The baseline characteristics of the recipients and donors, and intraoperative factors in the 2 groups are listed in Tables 1 and 2. Among the recipient factors, there were no significant differences between the groups. Regarding the echocardiographic parameters of the donor hearts, the left ventricle ejection fraction in the IRI(+) group was significantly higher than that in the IRI(−) group (P = 0.032). Regarding intraoperative factors, cardiac ischaemic time (CIT) in the IRI(+) group was significantly longer than in the IRI(−) group (P < 0.001).
| Baseline characteristics . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 40 ± 14 | 39 ± 12 | 0.990 |
| Sex (male) | 37 (69.8) | 13 (61.9) | 0.586 |
| BMI (kg/m2) | 20.3 ± 2.8 | 21.0 ± 4.3 | 0.615 |
| Diagnosis | |||
| ICM | 8 (15.1) | 2 (9.5) | 0.714 |
| DCM | 30 (56.6) | 11 (52.4) | |
| Others | 15 (28.3) | 8 (38.1) | |
| Preoperative haemodynamics | |||
| Preoperative inotropes | 4 (7.8) | 2 (10.0) | 0.785 |
| LVAD implantation | 49 (92.5) | 21 (100) | 0.573 |
| Mean duration of LVAD support (days) | 1008 (679–1189) | 895 (695–1146) | 0.670 |
| Preoperative laboratory data | |||
| T-bil (mg/dl) | 0.70 (0.50–1.15) | 0.65 (0.40–1.10) | 0.700 |
| BUN (mg/dl) | 15.0 (12.0–20.5) | 13.7 (11.0–22.8) | 0.629 |
| Cr (mg/dl) | 0.82 (0.71–1.12) | 0.78 (0.65–1.08) | 0.450 |
| BNP (pg/ml) | 163.7 (59.3–271.8) | 110.9 (62.4–219.4) | 0.369 |
| WBC (×103/ml) | 6.2 ± 1.5 | 6.6 ± 2.1 | 0.701 |
| Hb (g/dl) | 12.2 ± 1.5 | 11.6 ± 2.1 | 0.390 |
| Plt (×104/μl) | 17.9 (14.4–25.1) | 20.3 (15.0–25.7) | 0.520 |
| Baseline characteristics . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 40 ± 14 | 39 ± 12 | 0.990 |
| Sex (male) | 37 (69.8) | 13 (61.9) | 0.586 |
| BMI (kg/m2) | 20.3 ± 2.8 | 21.0 ± 4.3 | 0.615 |
| Diagnosis | |||
| ICM | 8 (15.1) | 2 (9.5) | 0.714 |
| DCM | 30 (56.6) | 11 (52.4) | |
| Others | 15 (28.3) | 8 (38.1) | |
| Preoperative haemodynamics | |||
| Preoperative inotropes | 4 (7.8) | 2 (10.0) | 0.785 |
| LVAD implantation | 49 (92.5) | 21 (100) | 0.573 |
| Mean duration of LVAD support (days) | 1008 (679–1189) | 895 (695–1146) | 0.670 |
| Preoperative laboratory data | |||
| T-bil (mg/dl) | 0.70 (0.50–1.15) | 0.65 (0.40–1.10) | 0.700 |
| BUN (mg/dl) | 15.0 (12.0–20.5) | 13.7 (11.0–22.8) | 0.629 |
| Cr (mg/dl) | 0.82 (0.71–1.12) | 0.78 (0.65–1.08) | 0.450 |
| BNP (pg/ml) | 163.7 (59.3–271.8) | 110.9 (62.4–219.4) | 0.369 |
| WBC (×103/ml) | 6.2 ± 1.5 | 6.6 ± 2.1 | 0.701 |
| Hb (g/dl) | 12.2 ± 1.5 | 11.6 ± 2.1 | 0.390 |
| Plt (×104/μl) | 17.9 (14.4–25.1) | 20.3 (15.0–25.7) | 0.520 |
Data are presented as the mean ± standard deviation or medians (25th–75th percentiles) for continuous variables and counts (%) for categorical variables.
BMI: body mass index; BNP: brain natriuretic peptide; BUN: blood urea nitrogen; Cr: creatinine; DCM: dilated cardiomyopathy; Hb: haemoglobin; ICM: ischaemic cardiomyopathy; IRI: ischaemic reperfusion injury; LVAD: left ventricular assist device; Plt: platelet; T-bil: total bilirubin; WBC: white blood cell.
| Baseline characteristics . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 40 ± 14 | 39 ± 12 | 0.990 |
| Sex (male) | 37 (69.8) | 13 (61.9) | 0.586 |
| BMI (kg/m2) | 20.3 ± 2.8 | 21.0 ± 4.3 | 0.615 |
| Diagnosis | |||
| ICM | 8 (15.1) | 2 (9.5) | 0.714 |
| DCM | 30 (56.6) | 11 (52.4) | |
| Others | 15 (28.3) | 8 (38.1) | |
| Preoperative haemodynamics | |||
| Preoperative inotropes | 4 (7.8) | 2 (10.0) | 0.785 |
| LVAD implantation | 49 (92.5) | 21 (100) | 0.573 |
| Mean duration of LVAD support (days) | 1008 (679–1189) | 895 (695–1146) | 0.670 |
| Preoperative laboratory data | |||
| T-bil (mg/dl) | 0.70 (0.50–1.15) | 0.65 (0.40–1.10) | 0.700 |
| BUN (mg/dl) | 15.0 (12.0–20.5) | 13.7 (11.0–22.8) | 0.629 |
| Cr (mg/dl) | 0.82 (0.71–1.12) | 0.78 (0.65–1.08) | 0.450 |
| BNP (pg/ml) | 163.7 (59.3–271.8) | 110.9 (62.4–219.4) | 0.369 |
| WBC (×103/ml) | 6.2 ± 1.5 | 6.6 ± 2.1 | 0.701 |
| Hb (g/dl) | 12.2 ± 1.5 | 11.6 ± 2.1 | 0.390 |
| Plt (×104/μl) | 17.9 (14.4–25.1) | 20.3 (15.0–25.7) | 0.520 |
| Baseline characteristics . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 40 ± 14 | 39 ± 12 | 0.990 |
| Sex (male) | 37 (69.8) | 13 (61.9) | 0.586 |
| BMI (kg/m2) | 20.3 ± 2.8 | 21.0 ± 4.3 | 0.615 |
| Diagnosis | |||
| ICM | 8 (15.1) | 2 (9.5) | 0.714 |
| DCM | 30 (56.6) | 11 (52.4) | |
| Others | 15 (28.3) | 8 (38.1) | |
| Preoperative haemodynamics | |||
| Preoperative inotropes | 4 (7.8) | 2 (10.0) | 0.785 |
| LVAD implantation | 49 (92.5) | 21 (100) | 0.573 |
| Mean duration of LVAD support (days) | 1008 (679–1189) | 895 (695–1146) | 0.670 |
| Preoperative laboratory data | |||
| T-bil (mg/dl) | 0.70 (0.50–1.15) | 0.65 (0.40–1.10) | 0.700 |
| BUN (mg/dl) | 15.0 (12.0–20.5) | 13.7 (11.0–22.8) | 0.629 |
| Cr (mg/dl) | 0.82 (0.71–1.12) | 0.78 (0.65–1.08) | 0.450 |
| BNP (pg/ml) | 163.7 (59.3–271.8) | 110.9 (62.4–219.4) | 0.369 |
| WBC (×103/ml) | 6.2 ± 1.5 | 6.6 ± 2.1 | 0.701 |
| Hb (g/dl) | 12.2 ± 1.5 | 11.6 ± 2.1 | 0.390 |
| Plt (×104/μl) | 17.9 (14.4–25.1) | 20.3 (15.0–25.7) | 0.520 |
Data are presented as the mean ± standard deviation or medians (25th–75th percentiles) for continuous variables and counts (%) for categorical variables.
BMI: body mass index; BNP: brain natriuretic peptide; BUN: blood urea nitrogen; Cr: creatinine; DCM: dilated cardiomyopathy; Hb: haemoglobin; ICM: ischaemic cardiomyopathy; IRI: ischaemic reperfusion injury; LVAD: left ventricular assist device; Plt: platelet; T-bil: total bilirubin; WBC: white blood cell.
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 42 ± 13 | 45 ± 12 | 0.192 |
| Sex (male) | 34 (64.2) | 14 (66.7) | 0.594 |
| Cardiac arrest | 27 (50.9) | 11 (52.4) | 1.000 |
| Brain haemorrhage | 31 (64.6) | 13 (65.0) | 1.000 |
| Inotrope usage | 22 (42.3) | 10 (47.6) | 0.796 |
| Time between donor brain death and organ retrieval (h) | 49 (40–81) | 62 (48–105) | 0.157 |
| Echocardiogram | |||
| LV ejection fraction (%) | 65.5 ± 8.7 | 68.9 ± 5.6 | 0.032 |
| LVDd (mm) | 45.5 ± 5.2 | 44.9 ± 5.0 | 0.337 |
| LVDs (mm) | 29.2 ± 4.5 | 26.7 ± 5.3 | 0.070 |
| PWD (mm) | 10 (8.9–12.0) | 11 (10–11.8) | 0.263 |
| IVSD (mm) | 9.6 (8.0–11.0) | 10.0 (8.2–11.0) | 0.527 |
| Intraoperative factors | |||
| CPB time (min) | 174 (157–202) | 233 (197–275) | 0.0002 |
| Clamp time (min) | 103 (93–120) | 112 (101–134) | 0.162 |
| Ischaemic time (min) | 199 ± 38 | 239 ± 39 | <0.001 |
| Donor–recipient sex mismatch | 11 (20.8) | 4 (19.0) | 1.000 |
| Weight mismatch >±20% | 25 (47.2) | 8 (38.1) | 0.422 |
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 42 ± 13 | 45 ± 12 | 0.192 |
| Sex (male) | 34 (64.2) | 14 (66.7) | 0.594 |
| Cardiac arrest | 27 (50.9) | 11 (52.4) | 1.000 |
| Brain haemorrhage | 31 (64.6) | 13 (65.0) | 1.000 |
| Inotrope usage | 22 (42.3) | 10 (47.6) | 0.796 |
| Time between donor brain death and organ retrieval (h) | 49 (40–81) | 62 (48–105) | 0.157 |
| Echocardiogram | |||
| LV ejection fraction (%) | 65.5 ± 8.7 | 68.9 ± 5.6 | 0.032 |
| LVDd (mm) | 45.5 ± 5.2 | 44.9 ± 5.0 | 0.337 |
| LVDs (mm) | 29.2 ± 4.5 | 26.7 ± 5.3 | 0.070 |
| PWD (mm) | 10 (8.9–12.0) | 11 (10–11.8) | 0.263 |
| IVSD (mm) | 9.6 (8.0–11.0) | 10.0 (8.2–11.0) | 0.527 |
| Intraoperative factors | |||
| CPB time (min) | 174 (157–202) | 233 (197–275) | 0.0002 |
| Clamp time (min) | 103 (93–120) | 112 (101–134) | 0.162 |
| Ischaemic time (min) | 199 ± 38 | 239 ± 39 | <0.001 |
| Donor–recipient sex mismatch | 11 (20.8) | 4 (19.0) | 1.000 |
| Weight mismatch >±20% | 25 (47.2) | 8 (38.1) | 0.422 |
Data are presented as the mean ± standard deviation or medians (25th–75th percentiles) for continuous variables and counts (%) for binary categorical variables.
CPB: cardiopulmonary bypass; IRI: ischaemic reperfusion injury; IVSD: interventricular septum diameter; LV: left ventricle; LVDd: left ventricular diameter at end-diastole; LVDs: left ventricular diameter at end-systole; PWD: posterior left ventricular wall diameter.
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 42 ± 13 | 45 ± 12 | 0.192 |
| Sex (male) | 34 (64.2) | 14 (66.7) | 0.594 |
| Cardiac arrest | 27 (50.9) | 11 (52.4) | 1.000 |
| Brain haemorrhage | 31 (64.6) | 13 (65.0) | 1.000 |
| Inotrope usage | 22 (42.3) | 10 (47.6) | 0.796 |
| Time between donor brain death and organ retrieval (h) | 49 (40–81) | 62 (48–105) | 0.157 |
| Echocardiogram | |||
| LV ejection fraction (%) | 65.5 ± 8.7 | 68.9 ± 5.6 | 0.032 |
| LVDd (mm) | 45.5 ± 5.2 | 44.9 ± 5.0 | 0.337 |
| LVDs (mm) | 29.2 ± 4.5 | 26.7 ± 5.3 | 0.070 |
| PWD (mm) | 10 (8.9–12.0) | 11 (10–11.8) | 0.263 |
| IVSD (mm) | 9.6 (8.0–11.0) | 10.0 (8.2–11.0) | 0.527 |
| Intraoperative factors | |||
| CPB time (min) | 174 (157–202) | 233 (197–275) | 0.0002 |
| Clamp time (min) | 103 (93–120) | 112 (101–134) | 0.162 |
| Ischaemic time (min) | 199 ± 38 | 239 ± 39 | <0.001 |
| Donor–recipient sex mismatch | 11 (20.8) | 4 (19.0) | 1.000 |
| Weight mismatch >±20% | 25 (47.2) | 8 (38.1) | 0.422 |
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Age (years) | 42 ± 13 | 45 ± 12 | 0.192 |
| Sex (male) | 34 (64.2) | 14 (66.7) | 0.594 |
| Cardiac arrest | 27 (50.9) | 11 (52.4) | 1.000 |
| Brain haemorrhage | 31 (64.6) | 13 (65.0) | 1.000 |
| Inotrope usage | 22 (42.3) | 10 (47.6) | 0.796 |
| Time between donor brain death and organ retrieval (h) | 49 (40–81) | 62 (48–105) | 0.157 |
| Echocardiogram | |||
| LV ejection fraction (%) | 65.5 ± 8.7 | 68.9 ± 5.6 | 0.032 |
| LVDd (mm) | 45.5 ± 5.2 | 44.9 ± 5.0 | 0.337 |
| LVDs (mm) | 29.2 ± 4.5 | 26.7 ± 5.3 | 0.070 |
| PWD (mm) | 10 (8.9–12.0) | 11 (10–11.8) | 0.263 |
| IVSD (mm) | 9.6 (8.0–11.0) | 10.0 (8.2–11.0) | 0.527 |
| Intraoperative factors | |||
| CPB time (min) | 174 (157–202) | 233 (197–275) | 0.0002 |
| Clamp time (min) | 103 (93–120) | 112 (101–134) | 0.162 |
| Ischaemic time (min) | 199 ± 38 | 239 ± 39 | <0.001 |
| Donor–recipient sex mismatch | 11 (20.8) | 4 (19.0) | 1.000 |
| Weight mismatch >±20% | 25 (47.2) | 8 (38.1) | 0.422 |
Data are presented as the mean ± standard deviation or medians (25th–75th percentiles) for continuous variables and counts (%) for binary categorical variables.
CPB: cardiopulmonary bypass; IRI: ischaemic reperfusion injury; IVSD: interventricular septum diameter; LV: left ventricle; LVDd: left ventricular diameter at end-diastole; LVDs: left ventricular diameter at end-systole; PWD: posterior left ventricular wall diameter.
Clinical outcomes
Early outcomes after HTX are shown in Table 3. The frequency of mechanical circulatory support, such as percutaneous cardiopulmonary support and intra-aortic balloon pumping, in the IRI(+) group was significantly higher than that in the IRI(−) group (42.9% vs 13.2%, P = 0.007). The catecholamine-dependent period after HTX was significantly longer in the IRI(+) group than in the IRI(−) group (P = 0.0009). The durations of postoperative intensive care unit (ICU) stays and intubation time in the IRI(+) group were also significantly longer than those in the IRI(−) group [ICU stay, 12 (4–54) days vs 5 (4–6) days, P = 0.006; intubation time, 83 (39–497) h vs 28 (22–52) h, respectively, P < 0.001]. Regarding mid-term outcomes, the overall survival showed no significant difference between the groups (Fig. 2A). Additionally, according to the Cox regression analysis, there were no significant risk factors that were predictive of overall survival post-HTX (Table 4). Freedom from acute cellular rejection was also equivalent between the groups (Fig. 2B). None of the patients developed cardiac allograft vasculopathies of any type (mild, moderate or severe), which were classified as CAV1, CAV2 or CAV3, in our cohort. The cardiac index on right heart catheterization of the allografts was not significantly different between the groups (Fig. 3A). Furthermore, there was no significant difference in the allograft pulmonary capillary wedge pressure between the groups (Fig. 3B).
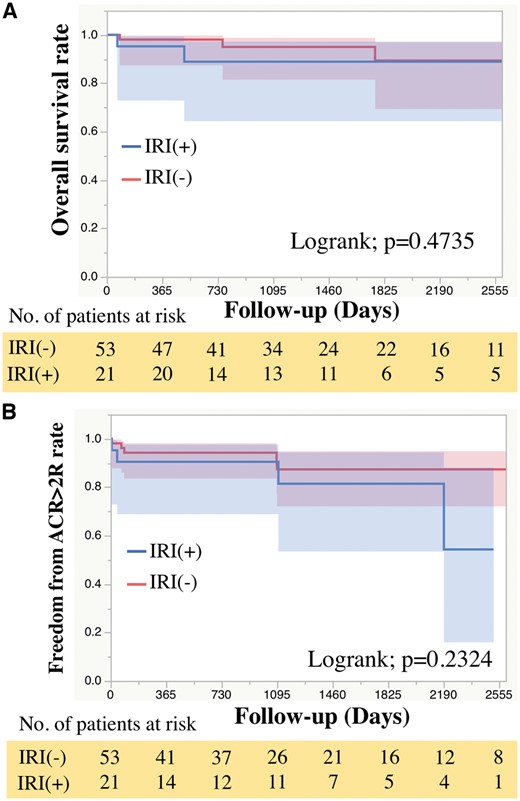
Mid-term outcomes after heart transplantation. The Kaplan–Meier curve showing (A) freedom from all-cause mortality and (B) freedom from ACR ≥2R. ACR: acute cellular rejection; IRI: ischaemic reperfusion injury.
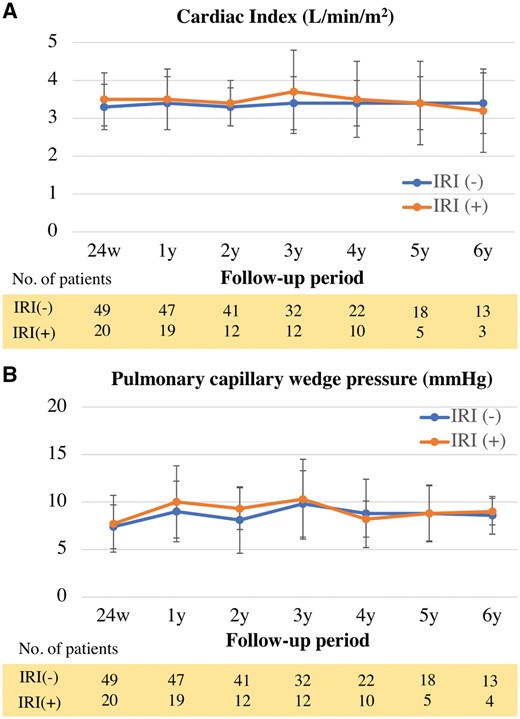
Cardiac allograft function in mid-term after heart transplantation (HTX). (A) Cardiac index of allograft after HTX. (B) Pulmonary capillary wedge pressure of allograft after HTX. IRI: ischaemic reperfusion injury.
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Hospital death | 1 (1.9) | 2 (9.5) | 0.192 |
| ICU stay (days) | 5 (4–6) | 12 (4–54) | 0.006 |
| Intubation time (h) | 28 (22–52) | 83 (39–497) | <0.0001 |
| MCS | 7 (13.2) | 9 (42.9) | 0.010 |
| Catecholamine-dependent period (days) | 5 (4–7) | 11 (5–19) | 0.0009 |
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Hospital death | 1 (1.9) | 2 (9.5) | 0.192 |
| ICU stay (days) | 5 (4–6) | 12 (4–54) | 0.006 |
| Intubation time (h) | 28 (22–52) | 83 (39–497) | <0.0001 |
| MCS | 7 (13.2) | 9 (42.9) | 0.010 |
| Catecholamine-dependent period (days) | 5 (4–7) | 11 (5–19) | 0.0009 |
Data are presented as medians (25th–75th percentiles) for continuous variables and counts (%) for binary categorical variables.
ICU: intensive care unit; IRI: ischaemic reperfusion injury; MCS: mechanical circulatory support.
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Hospital death | 1 (1.9) | 2 (9.5) | 0.192 |
| ICU stay (days) | 5 (4–6) | 12 (4–54) | 0.006 |
| Intubation time (h) | 28 (22–52) | 83 (39–497) | <0.0001 |
| MCS | 7 (13.2) | 9 (42.9) | 0.010 |
| Catecholamine-dependent period (days) | 5 (4–7) | 11 (5–19) | 0.0009 |
| . | IRI(−) (n = 53) . | IRI(+) (n = 21) . | P-value . |
|---|---|---|---|
| Hospital death | 1 (1.9) | 2 (9.5) | 0.192 |
| ICU stay (days) | 5 (4–6) | 12 (4–54) | 0.006 |
| Intubation time (h) | 28 (22–52) | 83 (39–497) | <0.0001 |
| MCS | 7 (13.2) | 9 (42.9) | 0.010 |
| Catecholamine-dependent period (days) | 5 (4–7) | 11 (5–19) | 0.0009 |
Data are presented as medians (25th–75th percentiles) for continuous variables and counts (%) for binary categorical variables.
ICU: intensive care unit; IRI: ischaemic reperfusion injury; MCS: mechanical circulatory support.
Univariate Cox regression analysis for post-HTX survival using perioperative values
| . | Univariate . | |
|---|---|---|
| . | P-value . | HR (95% CI) . |
| Severity of IRI | 0.505 | 1.88 (0.25–11.45) |
| Serum troponin T level at 3 h after reperfusion of the donor heart | 0.300 | 1.66 (0.60–4.03) |
| Cardiac ischaemic time | 0.759 | 1.00 (0.98–1.02) |
| CPB time | 0.167 | 1.01 (0.99–1.01) |
| . | Univariate . | |
|---|---|---|
| . | P-value . | HR (95% CI) . |
| Severity of IRI | 0.505 | 1.88 (0.25–11.45) |
| Serum troponin T level at 3 h after reperfusion of the donor heart | 0.300 | 1.66 (0.60–4.03) |
| Cardiac ischaemic time | 0.759 | 1.00 (0.98–1.02) |
| CPB time | 0.167 | 1.01 (0.99–1.01) |
CI: confidence interval; HR: hazard ratio; CPB: cardiopulmonary bypass; HTX: heart transplantation; IRI: ischaemic reperfusion injury.
Univariate Cox regression analysis for post-HTX survival using perioperative values
| . | Univariate . | |
|---|---|---|
| . | P-value . | HR (95% CI) . |
| Severity of IRI | 0.505 | 1.88 (0.25–11.45) |
| Serum troponin T level at 3 h after reperfusion of the donor heart | 0.300 | 1.66 (0.60–4.03) |
| Cardiac ischaemic time | 0.759 | 1.00 (0.98–1.02) |
| CPB time | 0.167 | 1.01 (0.99–1.01) |
| . | Univariate . | |
|---|---|---|
| . | P-value . | HR (95% CI) . |
| Severity of IRI | 0.505 | 1.88 (0.25–11.45) |
| Serum troponin T level at 3 h after reperfusion of the donor heart | 0.300 | 1.66 (0.60–4.03) |
| Cardiac ischaemic time | 0.759 | 1.00 (0.98–1.02) |
| CPB time | 0.167 | 1.01 (0.99–1.01) |
CI: confidence interval; HR: hazard ratio; CPB: cardiopulmonary bypass; HTX: heart transplantation; IRI: ischaemic reperfusion injury.
The myocardial enzyme levels in samples taken 3 h after reperfusion of the donor heart are shown in Fig. 4A. The serum level of TnT was significantly higher in the IRI(+) group than in the IRI(−) group (2.46 ± 1.30 ng/ml vs 1.62 ± 0.79 ng/ml, P = 0.002) (Fig. 4A).
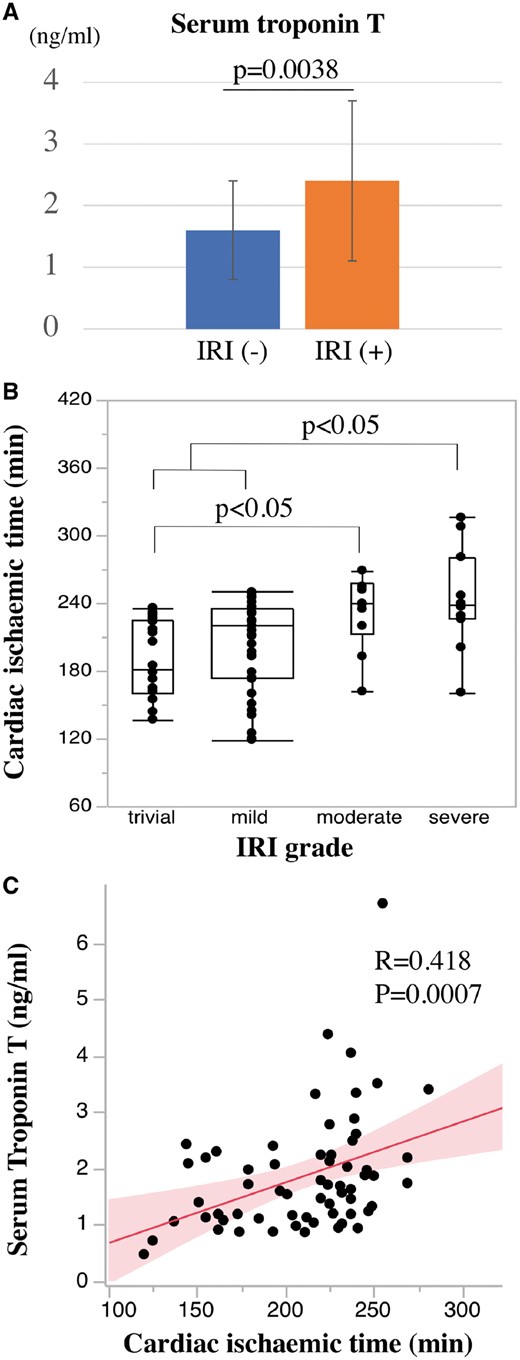
Relationship between IRI and cardiac ischaemic time (CIT). (A) Serum levels of troponin T 3 h after reperfusion of the donor heart. (B) CIT in each IRI grade. (C) The correlation of CIT and serum troponin T level 3 h after reperfusion of the donor heart. IRI: ischaemic reperfusion injury.
Risk factor analysis
Among perioperative risk factors, the CIT in patients with severe IRI was significantly longer than in those with trivial and mild IRI (P < 0.05; Fig. 3B). The CIT in patients with moderate IRI was also significantly longer than in those with trivial IRI (P < 0.05; Fig. 4B). Additionally, the levels of serum TnT 3 h after reperfusion of the donor heart showed a significant correlation with CIT (r = 0.418, P = 0.0007) (Fig. 4C).
DISCUSSION
The present study demonstrates the clinical impact of IRI according to severity, which was diagnosed by conventional histological criteria after examining endomyocardial biopsy samples obtained during the early post-transplant period. The patients with moderate-to-severe allograft IRI required more intensive haemodynamic support compared with those with trivial-to-mild IRI. De Santo et al. [10] had reported that perioperative myocardial injury evaluated by leakage of cardiac troponin I at 24 h after HTX was associated with a complicated clinical course, including early graft dysfunction. Their results, which are similar to our early outcomes, mostly demonstrate the impact of allograft IRI. However, previous studies had reported that elevated levels of serum TnT are related to severe histological acute rejection [11, 12]. Therefore, the leakage of myocardial enzymes may not always reflect post-HTX IRI. We believed that IRI after HTX would be more accurately evaluated by a histological approach using biopsy samples than by leakage of myocardial enzymes, as this conventional method can contribute to distinguishing IRI from acute rejection. This distinction is important to determine the optimal strategy for managing allograft function in the early post-HTX period.
While moderate-to-severe IRI impaired allograft function in the early post-HTX period, the mid-term outcomes were satisfactory regardless of IRI severity. This result indicates that myocardial damage due to IRI may be mainly reversible changes in myocytes. Several different forms of myocardial IRI are recognized, including reperfusion-induced arrhythmias, myocardial stunning, microvascular obstruction and lethal myocardial reperfusion injury, of which only the first 2 are reversible [5]. Braunwald and Kloner [13] were the first to define myocardial stunning as an ischaemic insult of insufficient severity or duration to produce myocardial necrosis that interferes with myocardial function for prolonged periods, even after reperfusion. Imamura et al. [14] reported that decreased left atrium function due to atrial stunning recovered in a time-dependent manner within 1 month of HTX. Considering the observed time-dependent reversibility of allograft function in our study, myocardial stunning due to exposure to several hours of ischaemia may be a major cause of impaired haemodynamics. Moreover, operative mortality was not increased in patients with moderate-to-severe IRI, while the early postoperative clinical course was complicated in these patients. This suggests that intensive haemodynamic management, comprising aggressive mechanical circulatory support and relatively long-term administration of inotropes in the early period after HTX, may be more crucial in patients with moderate-to-severe IRI. An intensive approach may help avoid early mortality due to IRI-associated temporary graft dysfunction.
Our analysis showed that CIT was the sole risk factor for moderate-to-severe IRI after HTX and that IRI severity was positively correlated with CIT. There is consistent evidence that the longer the graft ischaemia, the lower the chances of post-HTX survival [15–17]. Considering that IRI is time-dependent, CIT during preservation with current methods should be kept as short as possible to avoid a complicated clinical course early after HTX, even though CIT was within 4 h. Improvement of donor heart preservation may be required to attenuate IRI severity and avoid early allograft dysfunction. Moreover, several studies have reported that longer ischaemic times are associated with a significant increase in the incidence of primary graft dysfunction [18, 19]. Together with these findings, severe IRI may be one of the mechanisms underlying primary graft dysfunction after HTX.
In our study, attenuating IRI in allografts because of cardiac ischaemia is important for avoiding graft dysfunction in the early period after HTX. The current standard of care for managing recipient allografts from harvest to implantation is static cold storage with a heart-preserving solution. Supplementation of the heart preservation solutions with anti-ischaemic agents was reported to provide certain benefits, in terms of myocardial IRI alleviation. Underlying mechanisms behind myocardial protection mainly included inhibition of apoptosis [20–22], inhibition of the inflammatory response [23, 24] and vasodilation [23, 25]. Preconditioning of the donor heart [26] and the recipient [27] is reported to be another technique that can be used for cardioprotection in HTX. The normothermic ex vivo perfusion technique is an alternative preservation system for transporting donor hearts. The Organ Care System (TransMedics, Andover, MA, USA) was developed as a mobile perfusion module to maintain the donor heart in a functional, physiologic state using warm, oxygenated, nutrient-enriched donor blood, which enables high-risk extended-criteria donor hearts to be preserved [28]. The PROCEED II trial, a prospective multi-institutional randomized study, reported non-inferior intermediate outcomes of donor hearts perfused using the Organ Care System compared with those preserved with standard cold storage, even though total ischaemia time with the Organ Care System was significantly longer than those in cold storage [29]. This new method of donor heart preservation might be expected to potentially attenuate the IRI of the allograft; however, further investigation is required.
Limitations
Our study has several limitations. First, it was conducted at a single centre, which accounts for the small number of patients. This may hide the effect of some risk factors that have been recognized in registries and cardiac transplant research databases. Second, the biopsy samples could not determine the extent of IRI in the whole heart, especially in the left ventricle. However, to classify IRI severity as accurately as possible, multiple biopsy samples were evaluated at 2 time points in each patient. Finally, we could not examine whether an extended CIT impacts early mortality or long-term allograft function because most recipients in this study underwent HTX within 4 h.
CONCLUSION
In conclusion, severe IRI is associated with a complicated clinical course in the early post-HTX period due to temporary deterioration of allograft function. However, post-HTX intensive haemodynamic management may be important for patients with severe IRI to avoid early mortality after HTX. This clinical result may be attributed to myocardial stunning, the severity of which is proportional to donor heart CIT, even if the CIT seems to be appropriate. Improving donor heart preservation methods may protect against IRI and help avoid temporary allograft dysfunction in the early post-HTX period.
Conflict of interest: none declared.





