-
PDF
- Split View
-
Views
-
Cite
Cite
Vladimiro L Vida, Lorenza Zanotto, Lucia Zanotto, Chiara Tessari, Massimo A Padalino, Fabio Zanella, Demetrio Pittarello, Giovanni Stellin, Minimally invasive surgery for atrial septal defects: a 20-year experience at a single centre, Interactive CardioVascular and Thoracic Surgery, Volume 28, Issue 6, June 2019, Pages 961–967, https://doi.org/10.1093/icvts/ivz017
Close - Share Icon Share
Abstract
Our goal was to evaluate the results of our 20-year experience with minimally invasive surgical approaches for closing ostium secundum atrial septal defects, focusing on clinical results, patient satisfaction and cost-effectiveness.
We included 538 patients who underwent surgical ostium secundum atrial septal defects closure with minimally invasive approaches.
The minimally invasive approaches included right anterior minithoracotomy (n = 335, 62%), midline lower ministernotomy (135, 25%) and right lateral minithoracotomy (n = 68, 13%). Central cannulation was used in 374 patients (69%), whereas, more recently, a remote cardiopulmonary bypass with peripheral cannulation was used in 164 selected patients (31%). Median intensive care unit and postoperative hospitalization stays were 1 day [interquartile range (IQR) 1–1 day] and 5 days (IQR 5–6 days), respectively. Thirty-one patients had postoperative complications (5.8%); postcardiotomy syndrome was the most frequent complication (n = 20/538, 3.7%). Decreases in the length of postoperative hospitalization (P < 0.001) and in hospital costs (P = 0.009) were achieved over time. At a median follow-up of 12.1 years (IQR 0.6–14 years), all patients are in good clinical condition with no limitations to physical activity. The vast majority of patients (524/538 patients, 97%) were very satisfied with the result of the minimally invasive approaches (99/100 patients, 99% in the last 5 years).
Minimally invasive approaches for closing ostium secundum atrial septal defects proved safe and effective both in children and in adults with a very high satisfaction rate for the cosmetic result. A continuous evolution of our minimally invasive approaches, with a constant quest for less invasive procedures, led us to a miniaturization of the surgical accesses, reducing hospitalization time and hospital costs.
INTRODUCTION
Surgery for closing atrial septal defects has changed considerably during the last 2 decades [1–3]. A routine median sternotomy, which has been the conventional approach for many years, has been progressively abandoned in many centres in favour of minimally invasive approaches [4–6].
Since the middle 1990s, we routinely adopted minimally invasive approaches for treating simple congenital heart defects (CHDs), both in children and adults, with the aim of combining excellent functional and cosmetic results [7–9].
The aim of this study was to evaluate the results of our 20-year experience with minimally invasive approaches for closing ostium secundum atrial septal defects (ASD II) and to describe the evolution of our techniques by focusing on our clinical results, patient satisfaction and cost-effectiveness.
MATERIALS AND METHODS
Our review of the medical records, of the Clinical Investigation Committee of Padua-approved computerized hospital data and of the procedures followed were in accordance with the institutional guidelines for retrospective record review and protection of patient confidentiality. Individual consent was not obtained from patients enrolled in this study; the patients were not identified, and the chairperson of the ethics committee of our institution consented for their data to be sent for publication (protocol number 4482/AO/18).
We reviewed the hospital course and the follow-up of patients who underwent ASD II closure using minimally invasive techniques from 1 January 1996 to 30 August 2017. Patients who underwent surgical closure were discussed by our team and scheduled for surgery because the defect was not suitable for percutaneous intervention or because the family preferred surgical closure. During the same period, 253 children and adolescents underwent percutaneous ASD II closure.
At the beginning of our experience, we arbitrarily chose a different surgical approach based on the patient’s gender [a ministernotomy in males and a right anterior (RA) minithoracotomy in females], with the goal of obtaining patient satisfaction with the best cosmetic surgical result [5] (Fig. 1A, B). A ministernotomy approach was rarely used in patients with a body weight above 35 kg; an RA minithoracotomy incision was favoured [5, 6]. Beginning in 2013, we added the right lateral (RL) minithoracotomy (or subaxillary approach) to our minimally invasive surgical armamentarium [7–9] (Fig. 1C).
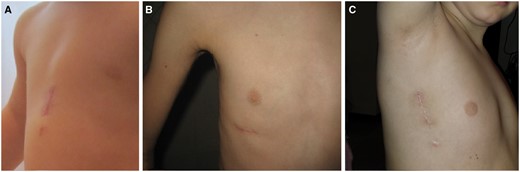
Postoperative images (1 month after surgery) showing the different minimally invasive surgical approaches used for ostium secundum atrial septal defect closure in our institution. (A) Ministernotomy; (B) right anterior minithoracotomy; and (C) right lateral minithoracotomy.
From 2007, the process of miniaturizing our incisions was facilitated by the use of the Bookwalter chest retraction system (Codman Surgical Instruments, GS Medical Ltd, Dublin, Ireland) in patients who had a ministernotomy; in patients with a body weight <15 kg who had an RA minithoracotomy to ease the access to the aorta and superior vena cava; and in patients who had central cannulation. Miniaturization of the incisions was also facilitated using remote cardiopulmonary bypass (CPB) by means of cannulation of the femoral vessels. The remote CPB was initially used in selected patients with a body weight above 30 kg; subsequently, it was also applied in smaller patients. Our more recent protocol includes the routine use of femoral arterial cannulation in patients with a body weight ≥15 kg and the use of femoral venous cannulation in patients with a body weight ≥7 kg) [10] (Supplementary Material, Figs S1 and S2). The percutaneous cannulation of the internal jugular vein, which was used in patients with a body weight ≥15 kg until 2016, has more recently been reserved for more complex CHD only. A central aortic/bicaval cannulation was used in the remaining patients.
Surgical techniques and intra- and postoperative management, which included intraoperative 2-dimensional echocardiographic monitoring for deairing of the left chambers, use of induced ventricular fibrillation, assessment of complete atrial septation by colour Doppler interrogation and bubble-test techniques, early extubation (in the operating room within 3 h from surgical repair) and early discharge from the intensive care unit (ICU) (<24 h) were described previously [5, 11].
The aim of this study was to evaluate our 20-year experience with minimally invasive approaches for closing ASD II, the evolution of our techniques, focusing on clinical results (incidence of postoperative morbidities, length of stay in the ICU and postoperative hospitalization), patient satisfaction and cost-effectiveness. Any complication requiring medical or surgical treatment was considered a postoperative morbidity.
At the time of discharge from the hospital, a 2-dimensional echocardiogram with Doppler examination of the femoral vessels was routinely performed in all patients who underwent remote CPB. As part of our programme, all patients were followed with a physical examination 1 month, 1 year and 3 years after surgery to assess their clinical status and the quality of the surgical result. Girls who had an RA minithoracotomy at a prepubescent age and who reached adulthood (with full breast development) were checked clinically with the aim of evaluating breast development [6].
Satisfaction with the cosmetic result of the minimally invasive approach was evaluated in all patients by means of direct contact with the patient (clinical examination) or via a phone interview. A satisfaction score was obtained using a Likert scale (1–5). A score of 5 indicated that the result of the minimally invasive approach was thought to be excellent; 4, very good; 3, good; 2, sufficient; and 1, unsatisfactory. During the phone interview, the patient was also asked to report his/her ongoing clinical status (as New York Heart Association functional class) and to provide the result of the most recent clinical/instrumental examination.
Actual hospital costs were calculated for each patient. To estimate the total cost, we multiplied the unit cost of the various components of care by the documented use and then summed the products [12] (Table 1).
List of items included in the total cost calculation for surgical ostium secundum atrial septal defect closure
| 1. Operating room (includes salaries of all personnel, costs of all medications and materials used during anaesthesia and costs of cardiopulmonary bypass equipment) |
| 2. Length of stay in the intensive care unit (days) |
| 3. Hospitalization (days) |
| 4. Blood products (units) |
| 5. Diagnostic testsa |
| 1. Operating room (includes salaries of all personnel, costs of all medications and materials used during anaesthesia and costs of cardiopulmonary bypass equipment) |
| 2. Length of stay in the intensive care unit (days) |
| 3. Hospitalization (days) |
| 4. Blood products (units) |
| 5. Diagnostic testsa |
Two-dimensional echocardiogram, electrocardiogram, chest radiographs, laboratory analyses and vascular sonography of the lower extremities.
List of items included in the total cost calculation for surgical ostium secundum atrial septal defect closure
| 1. Operating room (includes salaries of all personnel, costs of all medications and materials used during anaesthesia and costs of cardiopulmonary bypass equipment) |
| 2. Length of stay in the intensive care unit (days) |
| 3. Hospitalization (days) |
| 4. Blood products (units) |
| 5. Diagnostic testsa |
| 1. Operating room (includes salaries of all personnel, costs of all medications and materials used during anaesthesia and costs of cardiopulmonary bypass equipment) |
| 2. Length of stay in the intensive care unit (days) |
| 3. Hospitalization (days) |
| 4. Blood products (units) |
| 5. Diagnostic testsa |
Two-dimensional echocardiogram, electrocardiogram, chest radiographs, laboratory analyses and vascular sonography of the lower extremities.
A convenience sample of patients with ASD II (n = 30) who underwent surgical closure using a classic longitudinal full sternotomy during the same period (January 1996–December 1998) was considered a control group (Fig. 2).
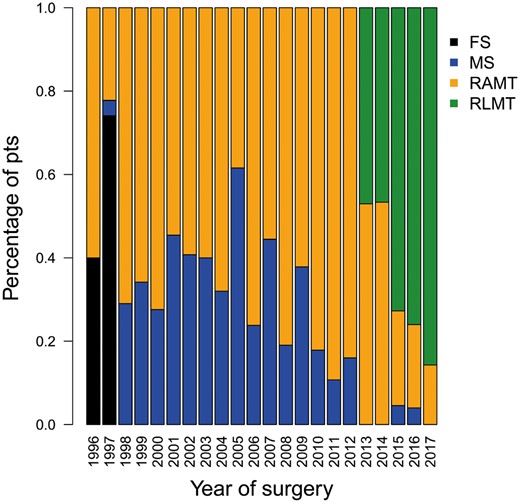
Bar graph showing the types of minimally invasive accesses for treating ostium secundum atrial septal defect, by year of surgery. FS: full sternotomy; MS: ministernotomy; pts: patients; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
Statistical analysis
Categorical variables were presented as percentage, and continuous variables were expressed as median, with interquartile range (IQR) as a measure of variability. To show the trend of patients who underwent the different types of accesses, we used a bar plot. Statistical differences among groups were computed for nominal variables using the Fisher’s exact test or Pearson’s χ2 test where appropriate, with the Mann–Whitney test (if groups = 2) and the Kruskal–Wallis test (if groups >2) for continuous variables. Linear or logistic regression was used to evaluate the association between the possible risk factors and the continuous or the dichotomic dependent variable, respectively. The results were shown as a scatter plot to which was added the curve estimated by the linear model. The cut-off point of the body weight in relation to the need for blood transfusions was calculated using logistic regression and the receiver operating characteristic curve. The level of significance was set at P-value <0.05. We analysed data with the R-System statistical package (https://www.r-project.org/) [13].
RESULTS
We included 538 patients who underwent ASD II closure using minimally invasive approaches. The majority of patients were girls/women (n = 353, 66%). Median age at surgery was 6.3 years (IQR 4–14 years); 52 patients were younger than 2 years (9.6%) and 108 patients (20%) were adults (≥18 years of age). Minimally invasive surgical accesses included RA minithoracotomy (n = 335 patients, 62%), ministernotomy (n = 135 patients, 25%) and RL minithoracotomy (n = 68 patients, 13%). We noticed a trend towards the lateral (RA minithoracotomy and RL minithoracotomy) over the central surgical approach (minithoracotomy) over time (Fig. 2). In none of the patients was it necessary to change our minimally invasive approach to a more extensive thoracotomy or to a full midline sternotomy.
A central cannulation for CPB was used in the majority of patients (n = 374, 69%). More recently, a peripheral cannulation for remote CPB was used in 164 selected patients (31%) [including 66/337 patients below a body weight of 30 kg (20%)]. The median CPB time was 34 min (IQR 27–45 min) (Table 2). In 531 (99%) patients, we induced ventricular fibrillation to achieve intracardiac repair; in the remaining 7 patients (1.3%) (all before 2006), the aorta was clamped and cardioplegic arrest was induced. The median induced ventricular fibrillation time was 16 min (IQR 10–24 min), and it was slightly longer in patients who had an RA minithoracotomy and RL minithoracotomy than in those who had a ministernotomy (Table 2). The ASD II was closed using an autologous pericardial patch in 476 patients (88%) and by direct suture in the remaining 49 (12%).
Patient characteristics, operative data and outcomes according to the type of surgical access
| Variables . | MS (n = 135, 25%) . | RAMT (n = 335, 63%) . | RLMT (n = 68, 12%) . |
|---|---|---|---|
| Girls/women, n (%) | 34 (25) | 287 (86) | 32 (47) |
| Age at surgery (years), median (IQR) | 4.2 (2–6.8) | 8.5 (4.3–21) | 7.4 (5.6–16.6) |
| Weight at surgery (kg), median (IQR) | 17 (11–23) | 28 (17–55) | 27 (19–59) |
| Peripheral cannulation, n (%) | 4 (3) | 96 (29) | 64 (94) |
| CPB time (min), median (IQR) | 33 (27–43) | 35 (27–50) | 32 (24–38) |
| IVF, n (%) | 130 (96) | 333 (99) | 68 (100) |
| IVF time (min), median (IQR) | 14 (9–21) | 16 (11–25) | 17 (14–25) |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Hospitalization (postoperative days), median (IQR) | 6 (5–7) | 6 (5–6) | 5 (4–5) |
| Postoperative complications, n (%) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome, n (%) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Blood transfusions, n (%) | 27 (20) | 35 (10) | 2 (2.9) |
| Cost of treatment (euros), median (IQR) | 7275 (6975–7575) | 7317 (7065–7515) | 6927 (6774–7227) |
| Variables . | MS (n = 135, 25%) . | RAMT (n = 335, 63%) . | RLMT (n = 68, 12%) . |
|---|---|---|---|
| Girls/women, n (%) | 34 (25) | 287 (86) | 32 (47) |
| Age at surgery (years), median (IQR) | 4.2 (2–6.8) | 8.5 (4.3–21) | 7.4 (5.6–16.6) |
| Weight at surgery (kg), median (IQR) | 17 (11–23) | 28 (17–55) | 27 (19–59) |
| Peripheral cannulation, n (%) | 4 (3) | 96 (29) | 64 (94) |
| CPB time (min), median (IQR) | 33 (27–43) | 35 (27–50) | 32 (24–38) |
| IVF, n (%) | 130 (96) | 333 (99) | 68 (100) |
| IVF time (min), median (IQR) | 14 (9–21) | 16 (11–25) | 17 (14–25) |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Hospitalization (postoperative days), median (IQR) | 6 (5–7) | 6 (5–6) | 5 (4–5) |
| Postoperative complications, n (%) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome, n (%) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Blood transfusions, n (%) | 27 (20) | 35 (10) | 2 (2.9) |
| Cost of treatment (euros), median (IQR) | 7275 (6975–7575) | 7317 (7065–7515) | 6927 (6774–7227) |
CBP: cardiopulmonary bypass; ICU: intensive care unit; IQR: interquartile range; IVF: induced ventricular fibrillation; MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
Patient characteristics, operative data and outcomes according to the type of surgical access
| Variables . | MS (n = 135, 25%) . | RAMT (n = 335, 63%) . | RLMT (n = 68, 12%) . |
|---|---|---|---|
| Girls/women, n (%) | 34 (25) | 287 (86) | 32 (47) |
| Age at surgery (years), median (IQR) | 4.2 (2–6.8) | 8.5 (4.3–21) | 7.4 (5.6–16.6) |
| Weight at surgery (kg), median (IQR) | 17 (11–23) | 28 (17–55) | 27 (19–59) |
| Peripheral cannulation, n (%) | 4 (3) | 96 (29) | 64 (94) |
| CPB time (min), median (IQR) | 33 (27–43) | 35 (27–50) | 32 (24–38) |
| IVF, n (%) | 130 (96) | 333 (99) | 68 (100) |
| IVF time (min), median (IQR) | 14 (9–21) | 16 (11–25) | 17 (14–25) |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Hospitalization (postoperative days), median (IQR) | 6 (5–7) | 6 (5–6) | 5 (4–5) |
| Postoperative complications, n (%) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome, n (%) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Blood transfusions, n (%) | 27 (20) | 35 (10) | 2 (2.9) |
| Cost of treatment (euros), median (IQR) | 7275 (6975–7575) | 7317 (7065–7515) | 6927 (6774–7227) |
| Variables . | MS (n = 135, 25%) . | RAMT (n = 335, 63%) . | RLMT (n = 68, 12%) . |
|---|---|---|---|
| Girls/women, n (%) | 34 (25) | 287 (86) | 32 (47) |
| Age at surgery (years), median (IQR) | 4.2 (2–6.8) | 8.5 (4.3–21) | 7.4 (5.6–16.6) |
| Weight at surgery (kg), median (IQR) | 17 (11–23) | 28 (17–55) | 27 (19–59) |
| Peripheral cannulation, n (%) | 4 (3) | 96 (29) | 64 (94) |
| CPB time (min), median (IQR) | 33 (27–43) | 35 (27–50) | 32 (24–38) |
| IVF, n (%) | 130 (96) | 333 (99) | 68 (100) |
| IVF time (min), median (IQR) | 14 (9–21) | 16 (11–25) | 17 (14–25) |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Hospitalization (postoperative days), median (IQR) | 6 (5–7) | 6 (5–6) | 5 (4–5) |
| Postoperative complications, n (%) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome, n (%) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Blood transfusions, n (%) | 27 (20) | 35 (10) | 2 (2.9) |
| Cost of treatment (euros), median (IQR) | 7275 (6975–7575) | 7317 (7065–7515) | 6927 (6774–7227) |
CBP: cardiopulmonary bypass; ICU: intensive care unit; IQR: interquartile range; IVF: induced ventricular fibrillation; MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
None of the patients died in the hospital. The median postoperative stay in the ICU was 1 day (IQR 1–1 day) and was similar between the different minimally invasive approaches (Table 2). On multivariate analyses, a longer CPB time (P = 0.0004) was associated with a longer stay in the ICU. None of the patients showed decreased cardiac function requiring major postoperative inotropic support.
There were 32 postoperative complications in 31 patients (5.8%) (Table 3); postcardiotomy syndrome (n = 20/538, 3.7%) was the most frequent postoperative complication. A difference in the incidence of postcardiotomy syndrome was detected between the different minimally invasive approaches, it being less frequent in the lateral minimally invasive approaches [8/335 (2.4%) with RA minithoracotomy and 2/68 (2.9%) with RL minithoracotomy vs 10/135 (7.4%) with the ministernotomy approach] (P = 0.02). Five patients (0.9%) required further surgical intervention including mediastinal revision for postoperative bleeding (n = 3) and femoral arterial revision (n = 2) (Tables 2, 3). Sixty-four patients (12%) required blood transfusions; the cut-off point of body weight for transfusion was 13 kg (with a sensitivity of 0.86 and a specificity of 0.91 on receiver operating characteristic analysis), independently of the type of minimally invasive approach.
Postoperative in-hospital complications in patients who underwent minimally invasive cardiac surgery (n = 32 in 31 patients)
| Type of complication . | Overall (n = 538) . | MS (n = 135) . | RAMT (n = 335) . | RLMT (n = 68) . |
|---|---|---|---|---|
| Overall number of complications | 32 (5.5) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome | 20 (3.7) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Postoperative temporary arrhythmias | 4 (0.3) | 2 (1.6) | 2 (0.5) | |
| Transient AV block | 2 | 1 | 1 | |
| AF treated with amiodarone | 2 | 1 | 1 | |
| Postoperative bleeding requiring reoperation | 3 (0.8) | 1 (0.7) | 2 (0.6) | |
| Vascular complications | 2 (0.4) | 1 (0.3) | 1 (1.5) | |
| Femoral artery dissection requiring revision | 1 | 1 | ||
| Femoral artery cannulation site patch augmentation | 1 | 1 | ||
| Temporary neurological complication (seizures) | 1 (0.2) | 1 (0.3) | ||
| Other less common complications (pneumothorax drainage, pleural effusion requiring drainage) | 2 (0.4) | 1 (0.7) | 1 (0.3) |
| Type of complication . | Overall (n = 538) . | MS (n = 135) . | RAMT (n = 335) . | RLMT (n = 68) . |
|---|---|---|---|---|
| Overall number of complications | 32 (5.5) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome | 20 (3.7) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Postoperative temporary arrhythmias | 4 (0.3) | 2 (1.6) | 2 (0.5) | |
| Transient AV block | 2 | 1 | 1 | |
| AF treated with amiodarone | 2 | 1 | 1 | |
| Postoperative bleeding requiring reoperation | 3 (0.8) | 1 (0.7) | 2 (0.6) | |
| Vascular complications | 2 (0.4) | 1 (0.3) | 1 (1.5) | |
| Femoral artery dissection requiring revision | 1 | 1 | ||
| Femoral artery cannulation site patch augmentation | 1 | 1 | ||
| Temporary neurological complication (seizures) | 1 (0.2) | 1 (0.3) | ||
| Other less common complications (pneumothorax drainage, pleural effusion requiring drainage) | 2 (0.4) | 1 (0.7) | 1 (0.3) |
Data are presented as number of complications and percentage (%).
AF: atrioventricular fibrillation; AV: atrioventricular; MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
Postoperative in-hospital complications in patients who underwent minimally invasive cardiac surgery (n = 32 in 31 patients)
| Type of complication . | Overall (n = 538) . | MS (n = 135) . | RAMT (n = 335) . | RLMT (n = 68) . |
|---|---|---|---|---|
| Overall number of complications | 32 (5.5) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome | 20 (3.7) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Postoperative temporary arrhythmias | 4 (0.3) | 2 (1.6) | 2 (0.5) | |
| Transient AV block | 2 | 1 | 1 | |
| AF treated with amiodarone | 2 | 1 | 1 | |
| Postoperative bleeding requiring reoperation | 3 (0.8) | 1 (0.7) | 2 (0.6) | |
| Vascular complications | 2 (0.4) | 1 (0.3) | 1 (1.5) | |
| Femoral artery dissection requiring revision | 1 | 1 | ||
| Femoral artery cannulation site patch augmentation | 1 | 1 | ||
| Temporary neurological complication (seizures) | 1 (0.2) | 1 (0.3) | ||
| Other less common complications (pneumothorax drainage, pleural effusion requiring drainage) | 2 (0.4) | 1 (0.7) | 1 (0.3) |
| Type of complication . | Overall (n = 538) . | MS (n = 135) . | RAMT (n = 335) . | RLMT (n = 68) . |
|---|---|---|---|---|
| Overall number of complications | 32 (5.5) | 14 (11) | 15 (4.1) | 3 (4.4) |
| Postcardiotomy syndrome | 20 (3.7) | 10 (7.4) | 8 (2.4) | 2 (2.9) |
| Postoperative temporary arrhythmias | 4 (0.3) | 2 (1.6) | 2 (0.5) | |
| Transient AV block | 2 | 1 | 1 | |
| AF treated with amiodarone | 2 | 1 | 1 | |
| Postoperative bleeding requiring reoperation | 3 (0.8) | 1 (0.7) | 2 (0.6) | |
| Vascular complications | 2 (0.4) | 1 (0.3) | 1 (1.5) | |
| Femoral artery dissection requiring revision | 1 | 1 | ||
| Femoral artery cannulation site patch augmentation | 1 | 1 | ||
| Temporary neurological complication (seizures) | 1 (0.2) | 1 (0.3) | ||
| Other less common complications (pneumothorax drainage, pleural effusion requiring drainage) | 2 (0.4) | 1 (0.7) | 1 (0.3) |
Data are presented as number of complications and percentage (%).
AF: atrioventricular fibrillation; AV: atrioventricular; MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
All patients were discharged in good clinical condition, without any significant residual intracardiac defect and without extracardiac organ impairment. Median postoperative hospitalization (from surgical intervention to discharge) was 5 days (IQR 5–6 days) (Table 2). According to scatterplot analysis, we demonstrated a progressive decrease of the length of postoperative hospitalization over time (P < 0.001) (Fig. 3). On multivariate analyses, the use of central cannulation (P = 0.002) and a longer CPB time (P = 0.0004) were both associated with a longer hospitalization time. Patient characteristics, operative data and outcomes according to the type of surgical access (classical full sternotomy versus minimally invasive approaches) are listed in Table 4.
Patient characteristics, operative data and outcomes according to the type of surgical access (classic full sternotomy versus minimally invasive surgical access)
| Variables . | FS (n = 30) . | Minimally invasive approaches (n = 538) . | P-value . |
|---|---|---|---|
| Girls/women, n (%) | 16 (47) | 353 (66) | 0.05 |
| Age at surgery (years), median (IQR) | 5.5 (2–19) | 6.3 (4–14) | 0.7 |
| Weight at surgery (kg), median (IQR) | 20 (12–50) | 23 (15–50) | 0.5 |
| Peripheral cannulation, n (%) | 164 (31) | <0.0001 | |
| CPB time (min), median (IQR) | 32 (23–43) | 34 (27–45) | 0.4 |
| IVF, n (%) | 7 (23) | 531 (99) | <0.0001 |
| IVF time (min), median (IQR) | 7 (6–9) | 16 (11–24) | <0.0001 |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 0.0004 |
| Hospitalization (postoperative days), median (IQR) | 8 (8–9) | 5 (5–6) | <0.0001 |
| Postoperative complications, n (%) | 4 (13) | 31 (5.8) | 0.1 |
| Postcardiotomy syndrome, n (%) | 4 (13) | 20 (3.7) | 0.03 |
| Blood transfusions, n (%) | 4 (13) | 64 (12) | 0.2 |
| Cost for treatment (euros), median (IQR) | 7875 (7705–8347) | 7275 (6975–7515) | <0.0001 |
| Variables . | FS (n = 30) . | Minimally invasive approaches (n = 538) . | P-value . |
|---|---|---|---|
| Girls/women, n (%) | 16 (47) | 353 (66) | 0.05 |
| Age at surgery (years), median (IQR) | 5.5 (2–19) | 6.3 (4–14) | 0.7 |
| Weight at surgery (kg), median (IQR) | 20 (12–50) | 23 (15–50) | 0.5 |
| Peripheral cannulation, n (%) | 164 (31) | <0.0001 | |
| CPB time (min), median (IQR) | 32 (23–43) | 34 (27–45) | 0.4 |
| IVF, n (%) | 7 (23) | 531 (99) | <0.0001 |
| IVF time (min), median (IQR) | 7 (6–9) | 16 (11–24) | <0.0001 |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 0.0004 |
| Hospitalization (postoperative days), median (IQR) | 8 (8–9) | 5 (5–6) | <0.0001 |
| Postoperative complications, n (%) | 4 (13) | 31 (5.8) | 0.1 |
| Postcardiotomy syndrome, n (%) | 4 (13) | 20 (3.7) | 0.03 |
| Blood transfusions, n (%) | 4 (13) | 64 (12) | 0.2 |
| Cost for treatment (euros), median (IQR) | 7875 (7705–8347) | 7275 (6975–7515) | <0.0001 |
CBP: cardiopulmonary bypass; FS: full sternotomy; ICU: intensive care unit; IQR: interquartile range; IVF: induced ventricular fibrillation.
Patient characteristics, operative data and outcomes according to the type of surgical access (classic full sternotomy versus minimally invasive surgical access)
| Variables . | FS (n = 30) . | Minimally invasive approaches (n = 538) . | P-value . |
|---|---|---|---|
| Girls/women, n (%) | 16 (47) | 353 (66) | 0.05 |
| Age at surgery (years), median (IQR) | 5.5 (2–19) | 6.3 (4–14) | 0.7 |
| Weight at surgery (kg), median (IQR) | 20 (12–50) | 23 (15–50) | 0.5 |
| Peripheral cannulation, n (%) | 164 (31) | <0.0001 | |
| CPB time (min), median (IQR) | 32 (23–43) | 34 (27–45) | 0.4 |
| IVF, n (%) | 7 (23) | 531 (99) | <0.0001 |
| IVF time (min), median (IQR) | 7 (6–9) | 16 (11–24) | <0.0001 |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 0.0004 |
| Hospitalization (postoperative days), median (IQR) | 8 (8–9) | 5 (5–6) | <0.0001 |
| Postoperative complications, n (%) | 4 (13) | 31 (5.8) | 0.1 |
| Postcardiotomy syndrome, n (%) | 4 (13) | 20 (3.7) | 0.03 |
| Blood transfusions, n (%) | 4 (13) | 64 (12) | 0.2 |
| Cost for treatment (euros), median (IQR) | 7875 (7705–8347) | 7275 (6975–7515) | <0.0001 |
| Variables . | FS (n = 30) . | Minimally invasive approaches (n = 538) . | P-value . |
|---|---|---|---|
| Girls/women, n (%) | 16 (47) | 353 (66) | 0.05 |
| Age at surgery (years), median (IQR) | 5.5 (2–19) | 6.3 (4–14) | 0.7 |
| Weight at surgery (kg), median (IQR) | 20 (12–50) | 23 (15–50) | 0.5 |
| Peripheral cannulation, n (%) | 164 (31) | <0.0001 | |
| CPB time (min), median (IQR) | 32 (23–43) | 34 (27–45) | 0.4 |
| IVF, n (%) | 7 (23) | 531 (99) | <0.0001 |
| IVF time (min), median (IQR) | 7 (6–9) | 16 (11–24) | <0.0001 |
| ICU (days), median (IQR) | 1 (1–1) | 1 (1–1) | 0.0004 |
| Hospitalization (postoperative days), median (IQR) | 8 (8–9) | 5 (5–6) | <0.0001 |
| Postoperative complications, n (%) | 4 (13) | 31 (5.8) | 0.1 |
| Postcardiotomy syndrome, n (%) | 4 (13) | 20 (3.7) | 0.03 |
| Blood transfusions, n (%) | 4 (13) | 64 (12) | 0.2 |
| Cost for treatment (euros), median (IQR) | 7875 (7705–8347) | 7275 (6975–7515) | <0.0001 |
CBP: cardiopulmonary bypass; FS: full sternotomy; ICU: intensive care unit; IQR: interquartile range; IVF: induced ventricular fibrillation.
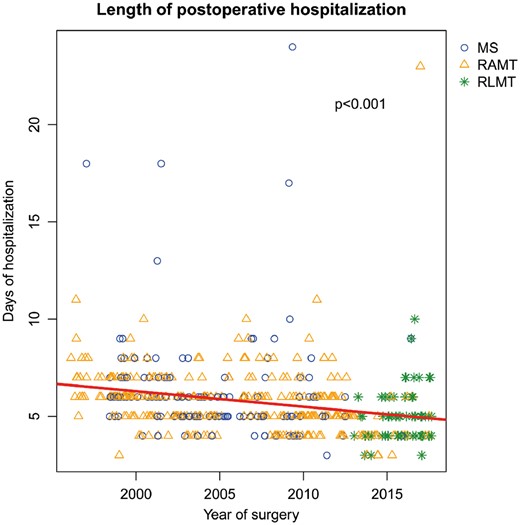
Scatter plot showing the variation of the length of postoperative hospitalizations of patients who underwent surgical ostium secundum atrial septal defect closure, by year of surgery. MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
At a median time of 12.1 years (IQR 0.6–14 years) after surgery, all patients are in good clinical condition with no limitation to physical activity; none of them required further intervention. None of our patients showed skeletal problems such as scoliosis or chest deformities at follow-up [5, 6]. The overall median satisfaction score for the cosmetic result of surgery was 5 (range 3–5) and was higher in the last 5 years of our experience (median score of 5, range 4–5) (P = 0.001). The result of surgery was judged as excellent/very good (scores 4–5) in 97% (524/538) of the patients and in 99% in the last 5 years (99/100 patients). Reasons for non-satisfaction were mainly related to the formation of a keloid at the level of the incision (n = 9) or to the presence of a ‘too long/visible scar’ (n = 9 with an RA minithoracotomy that extended medially, all before 2007); 3 patients who reached puberty had asymmetrical development of the right breast (all before 2007).
The median cost for treatment was 7275 euros (IQR 6975–7515 euros) (Table 2). According to scatterplot analysis, we showed a progressive reduction of hospital costs over time (P = 0.009) (Fig. 4).
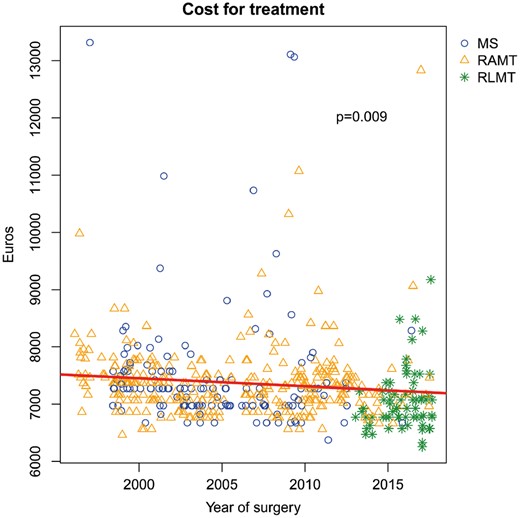
Scatter plot showing the variation of the overall cost of treatment for surgical ostium secundum atrial septal defect closure, by year of surgery. MS: ministernotomy; RAMT: right anterior minithoracotomy; RLMT: right lateral minithoracotomy.
DISCUSSION
Median sternotomy is the conventional approach for correction of congenital cardiac defects, but it often yields poor cosmetic results. In fact, especially in young female patients, unsightly midline scars cause displeasure and psychological distress [1–3]. Starting in the 1990s, with the aid of new technological advances in surgical instrumentation and in the perfusion technique, as well as competition from catheterization-based percutaneous procedures, many authors proposed the repair of simple congenital heart defects (mainly atrial septal defects) via a ministernotomy [14]. The inferior division of the lower half of the sternum ensures good access to the heart structures and to the great vessels for cannulation and guarantees better postoperative chest stability [5, 14–17]; in addition, it has the theoretical advantages of faster recovery [18]. These improvements in recovery may reflect the fact that the upper sternum (manubrium) is not divided during minimally invasive surgery, thus allowing the entire upper thoracic clavicular-sternal joint area to remain undisturbed, as opposed to being stretched during a full sternotomy [14]. Other surgeons criticized the ministernotomy access [19, 20] because they demonstrated that a ministernotomy only enhances an already improved aesthetic result.
The RA minithoracotomy or right submammary incision has been advocated for many years. It has been used as an alternative to a ministernotomy, particularly in the female gender for cosmetic reasons, and has steadily gained popularity for treatment of CHD due to the combined advantages of good cosmetic and functional results [5, 8, 21, 22]. Because of the risk of damage to the mammary gland in prepubescent girls, this approach was typically reserved for post-pubescent females in whom the location of the mammary gland is known and a submammary incision can be made [23].
More recently, a minimal incision on a more lateral aspect of the chest, in the subaxillary area, has been utilized with success for correcting simple CHDs [24, 25], with similar results.
At the beginning of our experience with minimally invasive approaches, we demonstrated that both a ministernotomy and an RA minithoracotomy were safe and effective surgical strategies for correcting simple CHD, offering similar outcomes in comparison to the more classic midline sternotomy, with minimal morbidity and good functional recovery, together with the additional value of a better cosmetic result, witnessed by the high level of patient satisfaction [5]. With time, we were able to progressively miniaturize the length of our chest incisions [6, 8], facilitated by the use of new retraction systems, especially in younger patients. In fact, the rib cages of young patients are more cartilaginous than those of older children and therefore easier to retract. Furthermore, since 2007, the use of the ministernotomy has been progressively abandoned and reserved for more complex CHD (e.g. ventricular septal defects, atrioventricular septal defects), favouring lateral accesses (away from the midline of the chest).
The utilization of peripheral cannulation for remote CPB avoided the insertion of arterial and venous cannulas into the chest incision, which sometimes may result in problems, especially in older patients due to the distance between the incision and the mediastinal structure. Some surgeons have resorted to femoral cannulation, especially in young children, owing to the high likelihood of femoral vessel injury [26]. Data from our experience showed that we were able to safely use a peripheral cannulation technique with a very low incidence of intra/postoperative complications. We found that femoral venous cannulation can be used safely in patients with a body weight above 7 kg; this observation was found particularly useful in patients presenting with an ASD located in the lower part of the atrial septum close to the orifice of the inferior vena cava (IVC); in these patients, the avoidance of direct IVC cannulation and of IVC snaring before a right atriotomy (since the tip of the femoral venous cannula is usually positioned a few centimetres below the IVC–right atrial junction) leaves the inferior border of the ASD II free and unstretched, and therefore, well visible for proper positioning of the patch.
The overall incidence of postoperative complications and of the need for further surgical interventions was low. The most frequent postoperative complication was the postcardiotomy syndrome, which occurred more frequently in patients who had a ministernotomy in comparison to those who had lateral approaches; the reason for this lower incidence in the latter could be linked to a more lateral incision in the pericardium, therefore allowing the reabsorption of the pericardial fluid in the intrapleural space. None of our patients, in particular the ones who underwent a lateral surgical access, had skeletal problems at follow-up.
We demonstrated that, with experience, we were able, with time, to progressively decrease the length of postoperative hospitalization. We believe that the main reason for a faster discharge could be related to a more prompt recovery due to our less invasive, minimally invasive approach, characterized by reduced skin incisions and by the close attention that we pay to the dissection of muscle planes (sparing almost all muscles), with particular attention to staying away from the mammary gland and from the major pectoralis muscle (RA minithoracotomy and RL minithoracotomy). Eventually, we were able to show a progressive and significant reduction of hospital costs, which could have been related to the reduced hospitalization time.
We had a very high overall satisfaction rate concerning the cosmetic results of the surgery. Reasons for dis-satisfaction were related to abnormal wound healing or excessive length of the incision (all reported before 2007). The only late complication was the asymmetric development of the right mammary gland in 3 patients. Since 2007, the process of lateralization of the RA minithoracotomy incisions (away from the midline) and their reduction in length [8], together with the introduction of RL minithoracotomy incisions (totally away from the breast tissue) were, according to us, important points for avoiding any improper future functional development of breast tissues. Lateral approaches are also definitively the approaches most frequently chosen for ASD II closure by our patients because the incision is completely hidden in the mammary sulcus in girls and women who have an RA minithoracotomy and within the subaxillary area in those who have an RL minithoracotomy [8].
Limitations
This study has some limitations: (i) it is a retrospective data analysis; (ii) the age groups within our minimally invasive approach cohorts are not comparable, which may bias our results; (iii) the follow-up period for patients having an RL minithoracotomy (more recently introduced minimally invasive approaches) is shorter than that of patients having other minimally invasive approaches, and the long-term functional results are not completely evaluable (i.e. growth of the mammary gland); and (iv) we did not include in this study the evaluation of the incidence of late arrhythmias. However, previously published data from our experience on this topic reveal no significant arrhythmias at midterm [27]. The many changes that occurred during this period (i.e. site of the incision and cannulation strategies) may bias the outcome parameters; nonetheless, they also represent the evolution of our approach over time, leading to our current results.
CONCLUSION
In conclusion, a minimally invasive approach has been our gold standard treatment for ASD II for more than 20 years, with a constant quest to achieve less invasiveness. Over time, we were able to progressively miniaturize the length of our incisions, thus further improving the cosmetic result and decreasing the patient’s surgical trauma; this latter achievement could also be associated with the progressively shorter length of hospitalization. Our current policy is to treat ASD II via a right thoracotomy (giving the patients the possibility to choose between RA minithoracotomy and RL minithoracotomy); this approach enhances the visualization of cardiac and vascular structures, is associated with a lower incidence of postoperative morbidities and can possibly facilitate future resolution of other acquired heart diseases requiring a midline full sternotomy (avoidance of resternotomy).
We believe that these data can be a stimulus for other congenital cardiac institutions to use these simple, easily reproducible techniques, which are extremely important for improving the quality of our surgical treatment.
ACKNOWLEDGEMENTS
The authors thank the cardiac anaesthesia and extracorporeal perfusion teams of our unit.
Conflict of interest: none declared.
REFERENCES
R Core Team.





