-
PDF
- Split View
-
Views
-
Cite
Cite
Milan Milojevic, Daniel J F M Thuijs, Stuart J Head, Carina T Domingues, Margreet W A Bekker, Felix Zijlstra, Joost Daemen, Peter P T de Jaegere, A Pieter Kappetein, Ron T van Domburg, Ad J J C Bogers, Life-long clinical outcome after the first myocardial revascularization procedures: 40-year follow-up after coronary artery bypass grafting and percutaneous coronary intervention in Rotterdam, Interactive CardioVascular and Thoracic Surgery, Volume 28, Issue 6, June 2019, Pages 852–859, https://doi.org/10.1093/icvts/ivz006
Close - Share Icon Share
Abstract
Our goal was to evaluate the outcomes of the first patients treated by venous coronary artery bypass grafting (CABG) or percutaneous coronary interventions (PCIs) with balloon angioplasty at a single centre who have reached up to 40 years of life-long follow-up.
We analysed the outcomes of the first consecutive patients who underwent (venous) CABG (n = 1041) from 1971 to 1980 and PCI (n = 856) with balloon angioplasty between 1980 and 1985. Follow-up was successfully achieved in 98% of patients (median 39 years, range 36–46) who underwent CABG and in 97% (median 33 years, range 32–36) of patients who had PCI.
The median age was 53 years in the CABG cohort and 57 years in the PCI cohort. A total of 82% of patients in the CABG group and 37% of those in the PCI group had multivessel coronary artery disease. The cumulative survival rates at 10, 20, 30 and 40 years were 77%, 39%, 14% and 4% after CABG, respectively, and at 10, 20, 30 and 35 years after PCI were 78%, 47%, 21% and 12%, respectively. The estimated life expectancy after CABG was 18 and 17 years after the PCI procedures. Repeat revascularization was performed in 36% and 57% of the patients in the CABG and PCI cohorts, respectively.
This unique life-long follow-up analysis demonstrates that both CABG and PCI were excellent treatment options immediately after their introduction as the standard of care. These procedures were lifesaving, thereby indirectly enabling patients to be treated with newly developed methods and medical therapies during the follow-up years.
INTRODUCTION
Since the introduction of the modern coronary artery bypass grafting (CABG) with venous and internal mammary artery (IMA) grafts in 1964 and percutaneous coronary intervention (PCI) with balloon angioplasty in 1977, these procedures have been performed extensively to treat coronary artery disease (CAD) worldwide [1, 2]. According to results from the Eurostat Database, coronary revascularization is one of the most common major hospital interventions performed in the European Union with an average rate of 258 per 100 000 inhabitants [3].
Advancements in both techniques and guideline-directed medical therapies have improved life expectancy (LE) and quality of life [4]. However, although long-term follow-up is available [5, 6], data on the life-long outcomes after CABG and PCI are still not published. Despite revascularization treatment has significantly changed and improved since its introduction, it is essential to establish the outcome of the first routinely treated patients, because life-long results (i) provide an opportunity to establish risk factors that show late sequelae, (ii) lend credibility for future studies and (iii) provide more insight into the real prognosis of patients. Therefore, we determined the outcome from life-long follow-up after the first CABG and PCI procedures.
METHODS
Study population
The study population and methods were described previously in detail [5, 6]. Briefly, the CABG population of this study comprised 1041 consecutive patients who underwent a first elective isolated coronary surgery with venous grafts between 1971 and 1980 at the Erasmus Medical Center. During that period, IMA grafts were not yet used at our institution. Indications for surgical revascularization were stable or unstable angina despite intensive pharmacological therapy. The intent was to achieve complete revascularization of significantly obstructed proximal coronary segments of the major arteries. Patients with any previous or concomitant cardiac surgical procedures were excluded from the current study.
The PCI cohort comprised 856 consecutive patients who underwent a first balloon angioplasty procedure between 1980 and 1985 at the Erasmus Medical Centre. Seventy-six patients were treated for acute myocardial infarction (MI), and other patients had either stable or unstable angina. At that time, all patients had intensive pharmacological therapy with beta-blockers, calcium channel blockers and nitrates, with intravenous heparin for unstable patients. Before the PCI procedure, 250 mg of acetylsalicylic acid and 100 mg of unfractionated heparin were administered intravenously, following additional boluses of 50 mg per hour. At hospital discharge, conventional treatment included acetylsalicylic acid of 500 mg daily for at least 6 months as well as a high dose of nifedipine.
The primary end point of this study was all-cause death. Secondary end points were repeat revascularization and a composite of death and repeat revascularization. Patients with multivessel disease were defined as having 2- or 3-vessel disease. Left ventricular (LV) dysfunction was defined as an ejection fraction <50%. For this observational study, patients were not subject of additional treatment or diagnostic procedures; neither was any mode of behaviour imposed other than as part of their regular treatment. Therefore, according to Dutch law at that time, written informed consent for a patient to be enrolled in this study was not required. This study was conducted according to the privacy policy of the Erasmus Medical Centre and to the Erasmus Medical Centre regulations for the appropriate use of data in patient-oriented research, which are based on international regulations, including the Declaration of Helsinki.
Follow-up
Patients who had CABG or PCI were followed from the date of the index procedure until the time of death or the time of the last available follow-up. Data regarding death or repeat revascularization were updated at 12 months after the index procedure and every 5–7 years after that, by reviewing hospital and general practitioner records and the civil registry or by telephone interviews with patients/family members. Follow-up was complete in 98% of patients who had CABG and 97% of patients who had PCI who were recruited in the cohorts. The survival status of 22 patients who had CABG and 25 patients who had PCI could not be retrieved because they had emigrated, and these patients were censored at the date of the last follow-up. Since the early 1980s, all data were prospectively entered into a dedicated database.
Statistical analysis
No statistical comparisons were performed between PCI and CABG because the entry criteria differed as to inclusion period, clinical presentation and the complexity of the CAD.
Data are presented using descriptive statistics, as a percentage, count of sample size or median ± interquartile range (IQR). Cumulative time-to-event Kaplan–Meier estimates were used to assess the clinical outcomes after PCI at 35 years and CABG at 40 years among overall cohorts and according to the number of diseased vessels. LE after CABG and PCI was calculated from the area under the Kaplan–Meier curves [5]. The expected survival in a reference population was calculated using age- and gender-specific mortality data from the Netherlands during the study period (www.cbs.nl) and was compared with survival rates of patients after CABG and PCI. Multivariable Cox proportional hazards models were constructed to identify independent prognostic factors for very long-term mortality rates using baseline characteristics: age, gender, history of smoking, diabetes, hypertension, dyslipidaemia, 3-vessel disease and LV dysfunction. A 2-sided P-value of <0.05 was considered to be statistically significant. Analyses were performed using SPSS Statistics version 21.0 (IBM Corporation, Armonk, NY, USA).
RESULTS
Coronary artery bypass grafting cohort
The median age was 53 years (IQR 48–58 years), and 88% were male (Table 1). A total of 9% had diabetes, 31% had a diagnosis of LV dysfunction and the majority 73% had multivessel disease.
| Characteristics . | CABG (n = 1041) . | PCI (n = 856) . |
|---|---|---|
| Age (years) | 53.0 (47.7–58.4) | 56.9 (50.4–62.7) |
| Range | 28–70 | 22–80 |
| Male | 915 (87.9) | 684 (79.9) |
| Smoking (history) | 589 (57.8) | 497 (58.0) |
| Diabetes | 82 (8.6) | 99 (11.6) |
| Hypertension | 224 (21.6) | 338 (40.5) |
| Dyslipidaemia | 232 (22.4) | 241 (27.4) |
| LV dysfunction | 274 (31.4) | 104 (16.6) |
| Normal (≥50%) | 601 (68.6) | 523 (83.4) |
| Moderate (30–49%) | 247 (28.3) | 97 (15.5) |
| Low (<30%) | 27 (3.1) | 7 (1.1) |
| Vessel disease | ||
| 1-Vessel disease | 192 (18.4) | 543 (63.4) |
| 2-Vessel disease | 320 (30.7) | 198 (23.6) |
| 3-Vessel disease | 445 (42.7) | 97 (11.8) |
| Left main | 84 (8.1) | 11 (1.3) |
| Characteristics . | CABG (n = 1041) . | PCI (n = 856) . |
|---|---|---|
| Age (years) | 53.0 (47.7–58.4) | 56.9 (50.4–62.7) |
| Range | 28–70 | 22–80 |
| Male | 915 (87.9) | 684 (79.9) |
| Smoking (history) | 589 (57.8) | 497 (58.0) |
| Diabetes | 82 (8.6) | 99 (11.6) |
| Hypertension | 224 (21.6) | 338 (40.5) |
| Dyslipidaemia | 232 (22.4) | 241 (27.4) |
| LV dysfunction | 274 (31.4) | 104 (16.6) |
| Normal (≥50%) | 601 (68.6) | 523 (83.4) |
| Moderate (30–49%) | 247 (28.3) | 97 (15.5) |
| Low (<30%) | 27 (3.1) | 7 (1.1) |
| Vessel disease | ||
| 1-Vessel disease | 192 (18.4) | 543 (63.4) |
| 2-Vessel disease | 320 (30.7) | 198 (23.6) |
| 3-Vessel disease | 445 (42.7) | 97 (11.8) |
| Left main | 84 (8.1) | 11 (1.3) |
Numbers are presented as n (%) or as median with IQR.
CABG: coronary artery bypass grafting; IQR: interquartile range; LV: left ventricular; PCI: percutaneous coronary intervention.
| Characteristics . | CABG (n = 1041) . | PCI (n = 856) . |
|---|---|---|
| Age (years) | 53.0 (47.7–58.4) | 56.9 (50.4–62.7) |
| Range | 28–70 | 22–80 |
| Male | 915 (87.9) | 684 (79.9) |
| Smoking (history) | 589 (57.8) | 497 (58.0) |
| Diabetes | 82 (8.6) | 99 (11.6) |
| Hypertension | 224 (21.6) | 338 (40.5) |
| Dyslipidaemia | 232 (22.4) | 241 (27.4) |
| LV dysfunction | 274 (31.4) | 104 (16.6) |
| Normal (≥50%) | 601 (68.6) | 523 (83.4) |
| Moderate (30–49%) | 247 (28.3) | 97 (15.5) |
| Low (<30%) | 27 (3.1) | 7 (1.1) |
| Vessel disease | ||
| 1-Vessel disease | 192 (18.4) | 543 (63.4) |
| 2-Vessel disease | 320 (30.7) | 198 (23.6) |
| 3-Vessel disease | 445 (42.7) | 97 (11.8) |
| Left main | 84 (8.1) | 11 (1.3) |
| Characteristics . | CABG (n = 1041) . | PCI (n = 856) . |
|---|---|---|
| Age (years) | 53.0 (47.7–58.4) | 56.9 (50.4–62.7) |
| Range | 28–70 | 22–80 |
| Male | 915 (87.9) | 684 (79.9) |
| Smoking (history) | 589 (57.8) | 497 (58.0) |
| Diabetes | 82 (8.6) | 99 (11.6) |
| Hypertension | 224 (21.6) | 338 (40.5) |
| Dyslipidaemia | 232 (22.4) | 241 (27.4) |
| LV dysfunction | 274 (31.4) | 104 (16.6) |
| Normal (≥50%) | 601 (68.6) | 523 (83.4) |
| Moderate (30–49%) | 247 (28.3) | 97 (15.5) |
| Low (<30%) | 27 (3.1) | 7 (1.1) |
| Vessel disease | ||
| 1-Vessel disease | 192 (18.4) | 543 (63.4) |
| 2-Vessel disease | 320 (30.7) | 198 (23.6) |
| 3-Vessel disease | 445 (42.7) | 97 (11.8) |
| Left main | 84 (8.1) | 11 (1.3) |
Numbers are presented as n (%) or as median with IQR.
CABG: coronary artery bypass grafting; IQR: interquartile range; LV: left ventricular; PCI: percutaneous coronary intervention.
Median follow-up was 39 ± 2 years (range 36–46), during which 979 deaths occurred. Cumulative survival rates were 77% at 10 years, 39% at 20 years, 14% at 30 years and 4% at 40 years (Fig. 1). Estimated LE was 18 years.
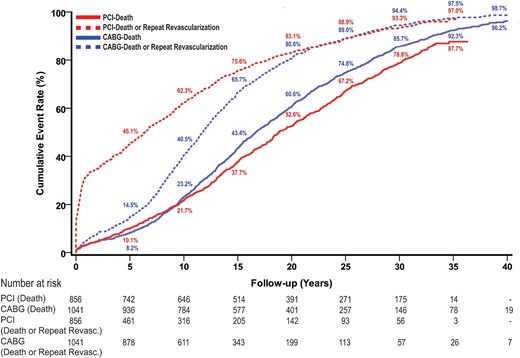
Survival and event-free survival estimates after CABG and PCI. Values are Kaplan–Meier event rates. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; Revasc.: revascularization.
The number of diseased vessels was strongly correlated with higher mortality rates (Fig. 2A). The survival rates in patients with 2-vessel disease were significantly higher compared to those in patients with 3-vessel disease at 10 years (82% vs 71%), 20 years (46% vs 28%), 30 years (16% vs 9%) and 40 years (4% vs 2%). Independent predictors of the 40-year mortality rate were age, diabetes, hypertension, LV dysfunction and 3-vessel disease (Table 2).
| . | HR (95% CI) . | P-value . |
|---|---|---|
| CABG cohort (n = 1041) | ||
| Age (per 5-year increments) | 1.30 (1.23–1.37) | <0.001 |
| Male gender | 1.22 (0.97–1.54) | 0.092 |
| Smoking (history) | 0.91 (0.78–1.05) | 0.21 |
| Diabetes | 1.31 (1.01–1.70) | 0.042 |
| Hypertension | 1.24 (1.04–1.48) | 0.018 |
| Dyslipidaemia | 0.95 (0.80–1.14) | 0.60 |
| 3-Vessel disease | 1.17 (1.08–1.27) | <0.001 |
| LV dysfunction | 1.79 (1.53–2.10) | <0.001 |
| PCI cohort (n = 856) | ||
| Age (per 5-year increase) | 1.40 (1.31–1.48) | <0.001 |
| Male gender | 1.01 (0.81–1.27) | 0.93 |
| Smoking (history) | 1.29 (1.08–1.55) | 0.005 |
| Diabetes | 1.19 (0.88–1.61) | 0.25 |
| Hypertension | 1.20 (1.01–1.44) | 0.039 |
| Dyslipidaemia | 0.86 (0.69–1.07) | 0.17 |
| 3-Vessel disease | 1.53 (1.19–1.96) | 0.001 |
| LV dysfunction | 1.38 (1.09–1.75) | 0.007 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| CABG cohort (n = 1041) | ||
| Age (per 5-year increments) | 1.30 (1.23–1.37) | <0.001 |
| Male gender | 1.22 (0.97–1.54) | 0.092 |
| Smoking (history) | 0.91 (0.78–1.05) | 0.21 |
| Diabetes | 1.31 (1.01–1.70) | 0.042 |
| Hypertension | 1.24 (1.04–1.48) | 0.018 |
| Dyslipidaemia | 0.95 (0.80–1.14) | 0.60 |
| 3-Vessel disease | 1.17 (1.08–1.27) | <0.001 |
| LV dysfunction | 1.79 (1.53–2.10) | <0.001 |
| PCI cohort (n = 856) | ||
| Age (per 5-year increase) | 1.40 (1.31–1.48) | <0.001 |
| Male gender | 1.01 (0.81–1.27) | 0.93 |
| Smoking (history) | 1.29 (1.08–1.55) | 0.005 |
| Diabetes | 1.19 (0.88–1.61) | 0.25 |
| Hypertension | 1.20 (1.01–1.44) | 0.039 |
| Dyslipidaemia | 0.86 (0.69–1.07) | 0.17 |
| 3-Vessel disease | 1.53 (1.19–1.96) | 0.001 |
| LV dysfunction | 1.38 (1.09–1.75) | 0.007 |
CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; LV: left ventricular; PCI: percutaneous coronary intervention.
| . | HR (95% CI) . | P-value . |
|---|---|---|
| CABG cohort (n = 1041) | ||
| Age (per 5-year increments) | 1.30 (1.23–1.37) | <0.001 |
| Male gender | 1.22 (0.97–1.54) | 0.092 |
| Smoking (history) | 0.91 (0.78–1.05) | 0.21 |
| Diabetes | 1.31 (1.01–1.70) | 0.042 |
| Hypertension | 1.24 (1.04–1.48) | 0.018 |
| Dyslipidaemia | 0.95 (0.80–1.14) | 0.60 |
| 3-Vessel disease | 1.17 (1.08–1.27) | <0.001 |
| LV dysfunction | 1.79 (1.53–2.10) | <0.001 |
| PCI cohort (n = 856) | ||
| Age (per 5-year increase) | 1.40 (1.31–1.48) | <0.001 |
| Male gender | 1.01 (0.81–1.27) | 0.93 |
| Smoking (history) | 1.29 (1.08–1.55) | 0.005 |
| Diabetes | 1.19 (0.88–1.61) | 0.25 |
| Hypertension | 1.20 (1.01–1.44) | 0.039 |
| Dyslipidaemia | 0.86 (0.69–1.07) | 0.17 |
| 3-Vessel disease | 1.53 (1.19–1.96) | 0.001 |
| LV dysfunction | 1.38 (1.09–1.75) | 0.007 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| CABG cohort (n = 1041) | ||
| Age (per 5-year increments) | 1.30 (1.23–1.37) | <0.001 |
| Male gender | 1.22 (0.97–1.54) | 0.092 |
| Smoking (history) | 0.91 (0.78–1.05) | 0.21 |
| Diabetes | 1.31 (1.01–1.70) | 0.042 |
| Hypertension | 1.24 (1.04–1.48) | 0.018 |
| Dyslipidaemia | 0.95 (0.80–1.14) | 0.60 |
| 3-Vessel disease | 1.17 (1.08–1.27) | <0.001 |
| LV dysfunction | 1.79 (1.53–2.10) | <0.001 |
| PCI cohort (n = 856) | ||
| Age (per 5-year increase) | 1.40 (1.31–1.48) | <0.001 |
| Male gender | 1.01 (0.81–1.27) | 0.93 |
| Smoking (history) | 1.29 (1.08–1.55) | 0.005 |
| Diabetes | 1.19 (0.88–1.61) | 0.25 |
| Hypertension | 1.20 (1.01–1.44) | 0.039 |
| Dyslipidaemia | 0.86 (0.69–1.07) | 0.17 |
| 3-Vessel disease | 1.53 (1.19–1.96) | 0.001 |
| LV dysfunction | 1.38 (1.09–1.75) | 0.007 |
CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; LV: left ventricular; PCI: percutaneous coronary intervention.
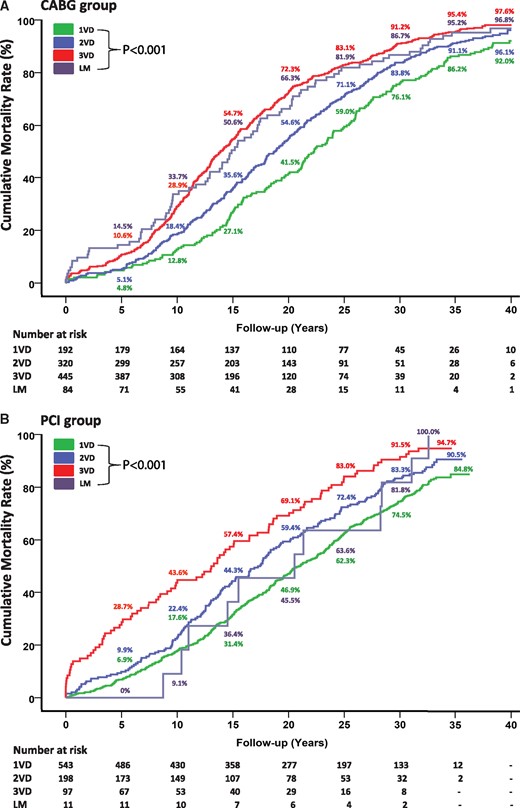
Death after CABG (A) and PCI (B) according to the number of diseased vessels. Values are Kaplan–Meier event rates. CABG: coronary artery bypass grafting; LM: left main; PCI: percutaneous coronary intervention; VD: vessel disease.
A total of 668 repeat revascularizations were performed in 375 patients (36%). Of those patients who required repeat procedures, repeat CABG procedures were needed in 315 (84%) patients, and 164 patients underwent at least 2 repeat revascularizations (Fig. 3). The hazard of repeat revascularization was highest 7–13 years after the initial procedure (Fig. 4A). Freedom from death or repeat revascularization was 60% at 10 years, 19% at 20 years, 6% at 30 years and 1% at 40 years (Fig. 1).
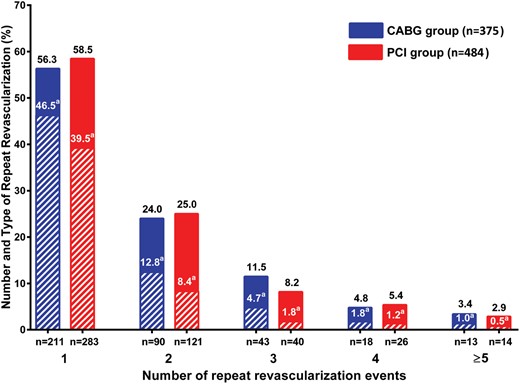
The proportion of patients undergoing the different numbers and types of repeat revascularization after CABG (blue) and PCI (red). Striped rectangles represent repeat CABG revascularization. aPercentage of CABG procedures. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
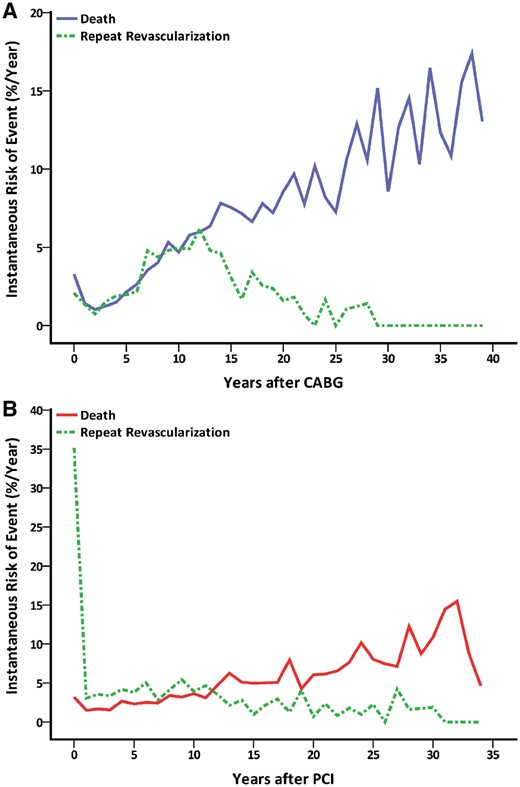
Instantaneous risk of death and repeat revascularization after CABG (A) and PCI (B). CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
Percutaneous coronary intervention cohort
The median age at the time of PCI was 57 years (IQR 50–63 years), and 80% were men (Table 1). Diabetes was present in 12%, LV dysfunction in 17%; the majority (63%) had 1-vessel disease.
Mean follow-up was 33 ± 1 years (range 32–36), during which 707 deaths occurred. Cumulative survival rates were 78% at 10 years, 47% at 20 years, 21% at 30 years and 12% at 35 years (Fig. 1). Estimated LE was 17 years.
The number of diseased vessels at baseline was an essential determinant of an increase in mortality rates (Fig. 2B). Cumulative survival rates were markedly higher among patients with 2-vessel disease versus those with 3-vessel disease at 10 years (78% vs 56%), 20 years (40% vs 31%), 30 years (17% vs 9%) and 35 years (10% vs 5%). Independent predictors of the 35-year mortality rate were age, history of smoking, hypertension, LV dysfunction and 3-vessel disease (Table 2).
A total of 831 repeat revascularization procedures were performed in 484 patients (57%). A CABG procedure was performed in 325 of these patients (67%), and at least 2 repeat revascularizations were required in 201 patients during the follow-up period (Fig. 3). The hazard of repeat revascularization reached its peak during the first year after the initial procedure (Fig. 4B). Freedom from death or repeat revascularization was 38% at 10 years, 17% at 20 years, 7% at 30 years and 3% at 35 years (Fig. 1).
DISCUSSION
To our knowledge, the presented data on life-long results after the first isolated venous CABG and PCI with balloon angioplasty procedures provide the longest, most unique and most complete follow-up information published to date. The main findings of this analysis can be summarized as follows: (i) overall LE after venous CABG and PCI with balloon angioplasty was 18 and 17 years, respectively; (ii) the degree of complexity of the coronary disease had a significant impact on long-term survival, especially after PCI; (iii) in addition to the degree of coronary complexity, independent predictors of the 40-year survival rate were age, hypertension, diabetes, smoking and LV dysfunction; and (iv) rates of repeat revascularization were highest during the first year after PCI and during the 7–13 years follow-up period after CABG.
The coronary artery bypass grafting cohort
Advancements in the whole spectrum of patient care have led to significantly improved outcomes after CABG, which is still the standard of care in patients with advanced complex CAD [7, 8]. Whereas other studies have reported 20–35 years of follow-up [9–11], this is the first study that provides life-long follow-up results after CABG surgery, with only 2% of the patients being lost to follow-up. Impressively, the 10-year survival rate is comparable to the findings from the more recent trials [12, 13]. We found that venous CABG was associated with acceptable survival rates, probably for the following reasons: first, the majority (75%) of patients were under 58 years of age with preserved LV function and without significant comorbidities such as diabetes, thereby prolonging the lifespan of venous grafts. Secondly, considering that all patients were treated in the pre-PCI era, in situ stents did not complicate the surgical technique [14]. Lastly, secondary prevention medications, modification of risk factors and repeat treatment interventions have changed notably during the follow-up period, which may have improved the LE.
The rate of repeat revascularization increased significantly 7 years after the initial CABG, most likely due to the loss of graft patency. However, these findings were derived before the widespread use of statins as secondary prevention, control of risk factors and modified surgical techniques that may have resulted in improved venous graft patency [15, 16]. Furthermore, although the benefits of arterial grafts tend to increase with the duration of the follow-up [17], current European and American practice guidelines for arterial grafts are recommended for younger patients whose LE is beyond the observed benefit of the vein graft [8, 18]. This observation has motivated the design of the ongoing Arterial Revascularization Trial (ART) study that compares the 10-year survival rates of patients with bilateral versus single IMA grafting. The recent results of an interim analysis performed at 5 years follow-up show no significant differences between the 2 groups in the rates of major adverse events [19]. However, considering our results, an increase in cardiac adverse events can be expected in the 5- to 10-year follow-up period, which may result in the benefit of multiple arterial grafts at the 10-year end point of the ART study hypothesis. Nevertheless, the use of even the single IMA as the gold-standard conduit for CABG produced surprisingly suboptimal results in recent studies [20], making the current results still clinically meaningful.
The percutaneous coronary intervention cohort
Treatment with PCI has evolved significantly over the last 40 years and has become a life-saving procedure in patients with acute indications [21] but also a treatment of choice for many patients with stable disease [8]. Only a few studies have reported survival results longer than 10 years after PCI [22], whereas no very long-term follow-up data are available. In our study, only 3% of patients who had PCI were lost to follow-up, thereby providing an accurate estimate of long-term survival.
In 2012, Yamaji et al. [22] reported a mortality rate of 59% at 20 years compared to 53% in our study. However, the present study enrolled younger patients, only a few of whom had diabetes and a history of MI. In the Bypass Angioplasty Revascularization Investigation (BARI) clinical trial, the 10-year mortality rate was 55% in patients with diabetes compared to 23% in patients without diabetes [13]. Patients with diabetes presenting for PCI are more likely to have more extensive CAD with accelerated atherosclerosis, thereby increasing the risk of MI, repeat revascularization and death [23]. A recent pooled analysis of individual patient data from clinical trials shows that diabetes is one of the most critical determinants of the 5-year survival rate after PCI [24]. We did not find diabetes to be a predictor of long-term death, probably because of the low number of patients who had diabetes. Additionally, the number of diseased vessels had a crucial impact on long-term survival after PCI. Similarly to results from previous trials using balloon angioplasty or bare-metal stents [25], the presence of 3-vessel disease was associated with a 3-fold increase in the mortality risk at 5 years compared to that of single- or 2-vessel disease. These data reflect the historical trends in PCI and may serve as a baseline for comparisons with the results of future PCI studies.
We found that the first PCI procedures with balloon angioplasty, in general, were associated with a high risk of repeat revascularization during the first year of follow-up. This risk was similar to rates reported from large randomized trials [26]. However, it should be noted that the introduction of bare-metal stents and drug-eluting stents and the change from anticoagulation to antiplatelet therapies have markedly improved the efficacy of PCI by reducing acute coronary occlusion or the process of restenosis and hence the need for repeat revascularization [27]. Furthermore, the results from the Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial showed that the use of drug-eluting stents significantly decreased the necessity for repeat CABG procedures among patients randomized to the PCI group, although this was only assessed up to the 5-year follow-up [28]. Nevertheless, the results from recent clinical trials comparing newer-generation drug-eluting stents with CABG have shown that the significant advantage of CABG over PCI concerning the incidence of repeat revascularization has remained, especially in patients with multivessel disease [29].
Limitations
Our study has several significant limitations. Firstly, it is a cohort study of (s)elective patients from a tertiary referral centre that was designed 40 years ago. At that time, knowledge about the existence of risk factors was almost non-existent. Therefore, only a limited number of baseline variables were collected. Secondly, surgical and percutaneous technology and techniques, periprocedural therapy and long-term guideline-directed secondary prevention medication have changed substantially since the time that the procedures in the current study were performed. Our findings therefore are not directly translatable to the current practice of CABG or PCI. In addition, no data were available on MIs and strokes occurring during the follow-up period.
CONCLUSIONS
Our findings demonstrate that CABG and PCI turned out to be excellent, durable treatments immediately after becoming a routine treatment, with a LE of 18 and 17 years, respectively. Life-long follow-up studies such as these provide essential information for patients, clinicians and health care systems and may serve as landmark results for future life-long CABG and PCI reports.
Conflict of interest: A.P. Kappetein is a full-time employee of Medtronic. All other authors declare that they have no conflict of interest.
REFERENCES
Organisation for Economic Cooperation and Development.
BARI Investigators.





