-
PDF
- Split View
-
Views
-
Cite
Cite
Ryu Kanzaki, Takashi Kanou, Naoko Ose, Soichiro Funaki, Yasushi Shintani, Masato Minami, Hiroshi Kida, Kazuhiko Ogawa, Atsushi Kumanogoh, Meinoshin Okumura, Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience, Interactive CardioVascular and Thoracic Surgery, Volume 28, Issue 3, March 2019, Pages 360–367, https://doi.org/10.1093/icvts/ivy276
Close - Share Icon Share
Abstract
The results of preoperative chemotherapy or chemoradiotherapy followed by surgery for locally advanced thymoma were analysed.
Between 1997 and 2016, 29 patients with a thymoma underwent preoperative chemotherapy or chemoradiotherapy followed by surgery. These cases were retrospectively reviewed.
The study population included 9 men and 20 women, with a mean age of 48.8 years (range 31–68 years). The preoperative Masaoka stage was III in 12, IVa in 13 and IVb in 4 patients, whereas histological type was B3 in 11, B2 in 9 and others in 5 patients. The mean tumour size was 8.0 ± 2.5 cm (3.4–15.0 cm). The site of infiltration shown in preoperative radiological examinations was the aorta in 6 patients, the superior vena cava in 14 patients and the pulmonary artery trunk in 3 patients, with pleural dissemination detected in 14. Three patients underwent chemoradiotherapy. Chemotherapy regimens given were cisplatin + doxorubicin + vincristine + cyclophosphamide in 9 patients, carboplatin + paclitaxel in 6 patients, cisplatin + doxorubicin + methylprednisolone in 5 patients and others in 9 patients, with partial response obtained in 11 patients and stable disease noted in 18 patients. Complete resection was achieved in 24 (83%) cases. There were no perioperative mortalities, whereas 6 (21%) patients developed postoperative complications. The 5- and 10-year overall survival rates were 100% and 87%, respectively, and 5- and 10-year disease-free survival rates were 50% and 50%, respectively.
Preoperative chemotherapy or chemoradiotherapy followed by surgery for locally advanced thymoma can be performed with an acceptable degree of surgical risk. Such a strategy should be proactively considered, as it can lead to favourable long-term results.
INTRODUCTION
A thymoma is a common type of malignancy that occurs in the anterior mediastinum. Although lymphomas are common in children and germ cell tumours in young men, a thymoma is the most common primary malignancy found in the anterior mediastinum of adults. Complete surgical resection is an important factor for treatment of thymic epithelial tumours [1–4].
The standard treatment option for the Masaoka stage I and II disease is primarily surgery, with complete resection achieved in nearly all cases without preoperative treatment [3–5]. In contrast, it is often difficult to attain complete resection by surgery alone in cases of locally advanced thymoma with infiltration into an adjacent structure (stage III) or those with involvement of pleural dissemination (stage IVa).
Until the 1980s, our treatment strategy for these diseases was to perform surgery first, with postoperative radiotherapy and/or chemotherapy administered for selected patients. In 1999, we reported surgical results of 194 consecutive thymoma patients in that era (study period 1957–1996) [6], which demonstrated that in patients with stage III disease, resection completeness and great vessel invasion were associated with prognosis. Furthermore, we found that the rate of complete resection in cases of a thymoma with great vessel invasion was lower as compared to those with a thymoma with invasion to other structures (70% vs 100%). Thus, complete resection of a thymoma with great vessel invasion was considered to be a challenging task at that time. On the other hand, complete resection of a thymoma with pleural dissemination has also been shown to be difficult to achieve, and outcomes of patients with stage IVA disease following surgery are limited [5, 6].
On the basis of these observations and findings, we introduced preoperative chemotherapy or chemoradiotherapy in the early 1990s for cases of thymoma with great vessel invasion and/or pleural dissemination, with the aim of achieving a high complete resection rate. In the present study, we analysed short-term and long-term outcomes of chemotherapy followed by surgery for advanced thymoma in patients treated at our institution within the recent 2 decades.
METHODS
Patients and methods
Between January 1997 and April 2018, a total of 283 patients underwent surgical resection for a primary thymoma at Osaka University Hospital. Among them, 29 (10%) patients received preoperative chemotherapy or chemoradiotherapy followed by surgical resection, and their clinical records were retrospectively reviewed. The present cohort included patients for whom induction therapy followed by surgery was planned and those who underwent salvage surgery, whose disease was primarily considered to be unresectable and for whom surgery was indicated based on re-evaluation after chemotherapy. Surgical samples were examined after receiving approval from the Ethical Review Board for Clinical Studies at Osaka University (control number 10026-3, 14461, 15558). Disease stage was determined according to the Masaoka staging system for thymic epithelial tumours and TNM classification (8th edition) [7, 8]. All surgical specimens were reviewed by pathologists working at our hospital and diagnosed according to the World Health Organization classification [9]. Postoperative complications were graded according to the Clavien–Dindo classification [10].
Patient selection and preoperative chemotherapy
At our hospital, the following criteria are used for indicating preoperative chemotherapy: (i) pathological diagnosis of thymoma obtained from a biopsy sample; (ii) detection of great vessel and/or adjacent organ invasion, or encirclement based on radiological examination findings and/or detection of large amount of dissemination in preoperative radiological examinations; (iii) eastern cooperative oncology group (ECOG) performance status 0 or 1; and (iv) in patients with autoimmune disease, stable disease and chemotherapy considered to be tolerable. Concurrent chemoradiotherapy (40 Gy) was indicated for patients with preoperative Masaoka stage III, superior vena cava (SVC) invasion or a radiotherapy field deemed to be limited. The chemotherapy regimen was determined on an individual patient basis by our tumour board. Generally, 2 cycles of cisplatin- or carboplatin-based chemotherapy were administered, with the final number of chemotherapy cycles determined by the attending thoracic surgeon based on response and noted adverse effects. Changes in chemotherapy due to an insufficient response or adverse effect were determined by the tumour board for each case. Clinical response was judged according to response evaluation criteria in solid tumors (RECIST) [11]. For patients diagnosed with myasthenia gravis (MG), treatment was done according to the degree of symptom severity, as previously described [12].
Surgical approach and type of resection
The standard mode for resection in cases of advanced thymoma is an extended thymectomy via a median sternotomy. For cases that required a combined lobectomy procedure, a sternotomy combined with an anterior thoracotomy or hemiclamshell approach was selected [13]. When a pleuropneumonectomy was performed, a sternotomy combined with posterolateral thoracotomy was selected [14]. For cases in which the disseminated tumour was large and easily approachable by a unilateral thoracotomy, and in which the dissemination could be resected, a thoracotomy was selected. For pleural dissemination cases, resection of dissemination was the first choice with regard to the type of resection. When preservation of the lung was deemed impossible because of tumour invasion to the hilum, especially to the main pulmonary artery, and massive invasion of the lung, a pleuropneumonectomy was considered to be the best choice for treatment [14]. Combined resection of surrounding organs infiltrated by the tumour, such as the SVC, lung and phrenic nerve, was proactively performed [15]. However, according to our institutional policy, resection of the aorta was not indicated when the thymoma had infiltrated the aorta; rather, subtotal resection was selected, and adjuvant radiotherapy was performed in these cases. When the thymoma invaded the lung, a lung wedge resection or lobectomy was performed. In cases with massive involvement of the phrenic nerve, it was resected to achieve complete resection, and plication of the diaphragm was also performed [16]. Because we generally perform an extended thymectomy for these patients, anterior region lymph nodes (N1) defined by TNM classification were dissected in all cases [9]. For dissection of deep-region lymph nodes (N2), the following methods were employed. When (i) thymic cancer was suspected based on preoperative or intraoperative findings or (ii) the disease was diagnosed as a thymoma, and N2 node metastasis was suspected based on preoperative and/or intraoperative findings, an upper mediastinal lymph node dissection was performed. Cervical lymph node dissection was performed only when cervical lymph node metastasis was suspected. Complete surgical resection was defined as a macroscopically radical resection procedure and performed when postoperative histological examination results revealed disease-free margins. For pleural dissemination, resection of at least a 5-mm margin and subpleural fatty tissue of all macroscopic nodules was defined as complete surgical resection for the present study. Intercostal muscle tissues were also partially resected in some cases to secure an adequate vertical surgical margin. Postoperative chemotherapy or radiation was planned for patients with incomplete resection, as well as these were judged to be at high risk for recurrence (i.e. narrow margin).
Follow-up and statistical analysis
Although there were no strict rules for postoperative follow-up examinations, it was common to obtain computed tomography (CT) of the chest once each year [17, 18]. Recurrence was diagnosed based on imaging or pathological examinations found in a review of the case records. Disease-free survival (DFS) and overall survival (OS) were calculated based on the interval between thymoma resection and detection of recurrence using the Kaplan–Meier method. Values are expressed as mean ± standard deviation. Statistical analyses were performed using the JMP software programme (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
In the present study cohort, the interval between the surgery and latest follow-up examination ranged from 1 to 223 months (median 84 months). Patient characteristics are listed in Table 1. Twenty-four (83%) patients underwent preoperative therapy as intended induction therapy, whereas 5 (17%) patients underwent surgery after chemotherapy as salvage surgery. A pathological diagnosis of thymoma was obtained based on examinations of biopsy samples prior to chemotherapy in all patients, which were obtained by the CT-guided core-needle biopsy procedure in 27 patients, the thoracoscopic surgical biopsy in 1 patient and a thoracotomy in 1 patient. Five patients were diagnosed with MG, each of whom was positive for serum anti-Ach receptor antibodies. Treatments for these patients were cholinesterase inhibitor in 3 patients and steroid administration in 1 patient, whereas none was done in 1 patient. Another 9 patients were positive for serum anti-Ach receptor antibodies but did not show MG symptoms.
| Characteristics . | . |
|---|---|
| Age (years) | |
| Average ± SD | 48.8 ± 8.5 |
| Range | 31–68 |
| Sex, n (%) | |
| Male | 9 (31) |
| Female | 20 (69) |
| Preoperative Masaoka stage, n (%) | |
| III | 12 (41) |
| Iva | 13 (44) |
| IVb | 4 (15) |
| Preoperative TNM stage, n (%) | |
| IIIa | 7 (24) |
| IIIb | 5 (17) |
| Iva | 14 (49) |
| IVb | 3 (10) |
| Staging by FDG-PET or FDG-PET/CT, n (%) | |
| Before preoperative therapy | 4 (14) |
| After preoperative therapy | 13 (45) |
| Before and after preoperative therapy | 1 (3) |
| Not done | 11 (38) |
| Thymoma-related syndrome, n (%) | |
| MG | 5 (17) |
| PRCA | 1 (4) |
| Histological type, n (%) | |
| A | 1 (3) |
| AB | 0 (0) |
| B1 | 4 (14) |
| B2 | 11 (38) |
| B3 | 13 (45) |
| Tumour size before chemotherapy (cm) | |
| Mean ± SD | 8.0 ± 2.5 |
| Range | 3.4–15.0 |
| Site of infiltration based on preoperative radiological examinations, n (%) | |
| Aorta | 6 (21) |
| SVC | 14 (48) |
| Pulmonary artery trunk | 3 (10) |
| Left atrial appendage | 1 (3) |
| Lung | 19 (66) |
| Chest wall | 2 (7) |
| Pleural dissemination | 14 (48) |
| Method for detection of thymoma, n (%) | |
| Screening chest X-ray | 12 (41) |
| Symptom | 12 (41) |
| Other disease | 5 (18) |
| Postoperative treatment, n (%) | |
| Radiotherapy | 5 (17) |
| Chemotherapy | 1 (3) |
| Characteristics . | . |
|---|---|
| Age (years) | |
| Average ± SD | 48.8 ± 8.5 |
| Range | 31–68 |
| Sex, n (%) | |
| Male | 9 (31) |
| Female | 20 (69) |
| Preoperative Masaoka stage, n (%) | |
| III | 12 (41) |
| Iva | 13 (44) |
| IVb | 4 (15) |
| Preoperative TNM stage, n (%) | |
| IIIa | 7 (24) |
| IIIb | 5 (17) |
| Iva | 14 (49) |
| IVb | 3 (10) |
| Staging by FDG-PET or FDG-PET/CT, n (%) | |
| Before preoperative therapy | 4 (14) |
| After preoperative therapy | 13 (45) |
| Before and after preoperative therapy | 1 (3) |
| Not done | 11 (38) |
| Thymoma-related syndrome, n (%) | |
| MG | 5 (17) |
| PRCA | 1 (4) |
| Histological type, n (%) | |
| A | 1 (3) |
| AB | 0 (0) |
| B1 | 4 (14) |
| B2 | 11 (38) |
| B3 | 13 (45) |
| Tumour size before chemotherapy (cm) | |
| Mean ± SD | 8.0 ± 2.5 |
| Range | 3.4–15.0 |
| Site of infiltration based on preoperative radiological examinations, n (%) | |
| Aorta | 6 (21) |
| SVC | 14 (48) |
| Pulmonary artery trunk | 3 (10) |
| Left atrial appendage | 1 (3) |
| Lung | 19 (66) |
| Chest wall | 2 (7) |
| Pleural dissemination | 14 (48) |
| Method for detection of thymoma, n (%) | |
| Screening chest X-ray | 12 (41) |
| Symptom | 12 (41) |
| Other disease | 5 (18) |
| Postoperative treatment, n (%) | |
| Radiotherapy | 5 (17) |
| Chemotherapy | 1 (3) |
CT: computed tomography; MG: myasthenia gravis; FDG-PET: 18F fluoro-deoxyglucose positron emission tomography; PRCA: pure red cell aplasia; SD: standard deviation; SVC: superior vena cava.
| Characteristics . | . |
|---|---|
| Age (years) | |
| Average ± SD | 48.8 ± 8.5 |
| Range | 31–68 |
| Sex, n (%) | |
| Male | 9 (31) |
| Female | 20 (69) |
| Preoperative Masaoka stage, n (%) | |
| III | 12 (41) |
| Iva | 13 (44) |
| IVb | 4 (15) |
| Preoperative TNM stage, n (%) | |
| IIIa | 7 (24) |
| IIIb | 5 (17) |
| Iva | 14 (49) |
| IVb | 3 (10) |
| Staging by FDG-PET or FDG-PET/CT, n (%) | |
| Before preoperative therapy | 4 (14) |
| After preoperative therapy | 13 (45) |
| Before and after preoperative therapy | 1 (3) |
| Not done | 11 (38) |
| Thymoma-related syndrome, n (%) | |
| MG | 5 (17) |
| PRCA | 1 (4) |
| Histological type, n (%) | |
| A | 1 (3) |
| AB | 0 (0) |
| B1 | 4 (14) |
| B2 | 11 (38) |
| B3 | 13 (45) |
| Tumour size before chemotherapy (cm) | |
| Mean ± SD | 8.0 ± 2.5 |
| Range | 3.4–15.0 |
| Site of infiltration based on preoperative radiological examinations, n (%) | |
| Aorta | 6 (21) |
| SVC | 14 (48) |
| Pulmonary artery trunk | 3 (10) |
| Left atrial appendage | 1 (3) |
| Lung | 19 (66) |
| Chest wall | 2 (7) |
| Pleural dissemination | 14 (48) |
| Method for detection of thymoma, n (%) | |
| Screening chest X-ray | 12 (41) |
| Symptom | 12 (41) |
| Other disease | 5 (18) |
| Postoperative treatment, n (%) | |
| Radiotherapy | 5 (17) |
| Chemotherapy | 1 (3) |
| Characteristics . | . |
|---|---|
| Age (years) | |
| Average ± SD | 48.8 ± 8.5 |
| Range | 31–68 |
| Sex, n (%) | |
| Male | 9 (31) |
| Female | 20 (69) |
| Preoperative Masaoka stage, n (%) | |
| III | 12 (41) |
| Iva | 13 (44) |
| IVb | 4 (15) |
| Preoperative TNM stage, n (%) | |
| IIIa | 7 (24) |
| IIIb | 5 (17) |
| Iva | 14 (49) |
| IVb | 3 (10) |
| Staging by FDG-PET or FDG-PET/CT, n (%) | |
| Before preoperative therapy | 4 (14) |
| After preoperative therapy | 13 (45) |
| Before and after preoperative therapy | 1 (3) |
| Not done | 11 (38) |
| Thymoma-related syndrome, n (%) | |
| MG | 5 (17) |
| PRCA | 1 (4) |
| Histological type, n (%) | |
| A | 1 (3) |
| AB | 0 (0) |
| B1 | 4 (14) |
| B2 | 11 (38) |
| B3 | 13 (45) |
| Tumour size before chemotherapy (cm) | |
| Mean ± SD | 8.0 ± 2.5 |
| Range | 3.4–15.0 |
| Site of infiltration based on preoperative radiological examinations, n (%) | |
| Aorta | 6 (21) |
| SVC | 14 (48) |
| Pulmonary artery trunk | 3 (10) |
| Left atrial appendage | 1 (3) |
| Lung | 19 (66) |
| Chest wall | 2 (7) |
| Pleural dissemination | 14 (48) |
| Method for detection of thymoma, n (%) | |
| Screening chest X-ray | 12 (41) |
| Symptom | 12 (41) |
| Other disease | 5 (18) |
| Postoperative treatment, n (%) | |
| Radiotherapy | 5 (17) |
| Chemotherapy | 1 (3) |
CT: computed tomography; MG: myasthenia gravis; FDG-PET: 18F fluoro-deoxyglucose positron emission tomography; PRCA: pure red cell aplasia; SD: standard deviation; SVC: superior vena cava.
Preoperative chemotherapy
Preoperative chemotherapy details are summarized in Table 2. Three patients classified as Masaoka stage III with SVC invasion prior to surgery underwent concurrent chemoradiotherapy (40 Gy). The number of chemotherapy cycles for all patients ranged from 1 to 6. The chemotherapy regimen was changed in 6 (21%) cases, which was due to adverse effects in 1 patient and insufficient response in 5 patients. One patient experienced myasthenic crisis, which was caused by preoperative chemotherapy [19]. The overall response rate was 38%. Downstaging was achieved in 2 (7%) patients.
| . | n (%) . |
|---|---|
| Preoperative treatment mode | |
| Chemotherapy | 26 (90) |
| Chemoradiotherapy | 3 (10) |
| Chemotherapy regimen | |
| ADOC | 9 (31) |
| CBDCA + PTX | 6 (21) |
| CAMP | 5 (17) |
| CDDP + VP16 | 4 (14) |
| CODE | 3 (10) |
| CDDP + DTX | 2 (7) |
| Response to chemotherapy | |
| PR | 11 (38) |
| SD | 18 (62) |
| . | n (%) . |
|---|---|
| Preoperative treatment mode | |
| Chemotherapy | 26 (90) |
| Chemoradiotherapy | 3 (10) |
| Chemotherapy regimen | |
| ADOC | 9 (31) |
| CBDCA + PTX | 6 (21) |
| CAMP | 5 (17) |
| CDDP + VP16 | 4 (14) |
| CODE | 3 (10) |
| CDDP + DTX | 2 (7) |
| Response to chemotherapy | |
| PR | 11 (38) |
| SD | 18 (62) |
ADOC: cisplatin + doxorubicin + vincristine + cyclophosphamide; CAMP: cisplatin + doxorubicin + methylprednisolone; CBDCA: carboplatin; CDDP: cisplatin; CODE: cisplatin + vincristine + doxorubicin + etoposide; DTX: docetaxel; PR: partial response; PTX: paclitaxel; SD: stable disease; VP16: etoposide.
| . | n (%) . |
|---|---|
| Preoperative treatment mode | |
| Chemotherapy | 26 (90) |
| Chemoradiotherapy | 3 (10) |
| Chemotherapy regimen | |
| ADOC | 9 (31) |
| CBDCA + PTX | 6 (21) |
| CAMP | 5 (17) |
| CDDP + VP16 | 4 (14) |
| CODE | 3 (10) |
| CDDP + DTX | 2 (7) |
| Response to chemotherapy | |
| PR | 11 (38) |
| SD | 18 (62) |
| . | n (%) . |
|---|---|
| Preoperative treatment mode | |
| Chemotherapy | 26 (90) |
| Chemoradiotherapy | 3 (10) |
| Chemotherapy regimen | |
| ADOC | 9 (31) |
| CBDCA + PTX | 6 (21) |
| CAMP | 5 (17) |
| CDDP + VP16 | 4 (14) |
| CODE | 3 (10) |
| CDDP + DTX | 2 (7) |
| Response to chemotherapy | |
| PR | 11 (38) |
| SD | 18 (62) |
ADOC: cisplatin + doxorubicin + vincristine + cyclophosphamide; CAMP: cisplatin + doxorubicin + methylprednisolone; CBDCA: carboplatin; CDDP: cisplatin; CODE: cisplatin + vincristine + doxorubicin + etoposide; DTX: docetaxel; PR: partial response; PTX: paclitaxel; SD: stable disease; VP16: etoposide.
Surgery
Operation-related factors are presented in Table 3. Complete resection was achieved in 24 (83%) patients. In cases of incomplete resection, the residual tumour site was the pulmonary artery trunk in 1 patient, the aorta in 1 patient and pleural dissemination in 3 patients. The SVC was resected in 9 (31%) patients, whereas an intracardiac tumour was also resected with the SVC in 1 patient. SVC reconstruction could not be achieved in 1 patient because of insufficient collateral flow.
| Factors . | . |
|---|---|
| Surgical approach, n (%) | |
| Median sternotomy | 17 (59) |
| Hemiclamshell | 3 (10) |
| Median sternotomy and thoracotomy | 6 (21) |
| Thoracotomy | 3 (10) |
| Site of combined resection, n (%) | |
| SVC (mode of reconstruction) | 9 (31) |
| Patch closure | 2 (7) |
| Prosthetic replacement | 6 (21) |
| None | 1 (3) |
| Lung (type of resection) | 22 (76) |
| Pneumonectomy | 3 (10) |
| Lobectomy | 6 (21) |
| Partial resection | 13 (45) |
| Dissemination (type of resection) | 14 (48) |
| Resection of dissemination | 11 (38) |
| Pleuropneumonectomy | 3 (10) |
| Phrenic nerve | 13 (45) |
| Left atrial appendage | 1 (3) |
| Lymph node dissection, n (%) | |
| Anterior region lymph nodes | 22 (76) |
| Anterior region and upper mediastinal lymph nodes | 6 (21) |
| Anterior region, upper mediastinal and cervical lymph nodes | 1 (3) |
| Operative time (min) | |
| Mean ± SD | 410 ± 169 |
| Range | 120–811 |
| Blood loss (ml) | |
| Mean ± SD | 1268 ± 918 |
| Range | 100–3650 |
| Patients who received blood transfusion, n (%) | 16 (55) |
| Postoperative Masaoka stage, n (%) | |
| II | 2 (7) |
| III | 10 (34) |
| Iva | 13 (45) |
| IVb | 4 (14) |
| Postoperative TNM stage, n (%) | |
| I | 2 (7) |
| IIIa | 9 (31) |
| IIIb | 1 (3) |
| Iva | 14 (48) |
| IVb | 3 (10) |
| Factors . | . |
|---|---|
| Surgical approach, n (%) | |
| Median sternotomy | 17 (59) |
| Hemiclamshell | 3 (10) |
| Median sternotomy and thoracotomy | 6 (21) |
| Thoracotomy | 3 (10) |
| Site of combined resection, n (%) | |
| SVC (mode of reconstruction) | 9 (31) |
| Patch closure | 2 (7) |
| Prosthetic replacement | 6 (21) |
| None | 1 (3) |
| Lung (type of resection) | 22 (76) |
| Pneumonectomy | 3 (10) |
| Lobectomy | 6 (21) |
| Partial resection | 13 (45) |
| Dissemination (type of resection) | 14 (48) |
| Resection of dissemination | 11 (38) |
| Pleuropneumonectomy | 3 (10) |
| Phrenic nerve | 13 (45) |
| Left atrial appendage | 1 (3) |
| Lymph node dissection, n (%) | |
| Anterior region lymph nodes | 22 (76) |
| Anterior region and upper mediastinal lymph nodes | 6 (21) |
| Anterior region, upper mediastinal and cervical lymph nodes | 1 (3) |
| Operative time (min) | |
| Mean ± SD | 410 ± 169 |
| Range | 120–811 |
| Blood loss (ml) | |
| Mean ± SD | 1268 ± 918 |
| Range | 100–3650 |
| Patients who received blood transfusion, n (%) | 16 (55) |
| Postoperative Masaoka stage, n (%) | |
| II | 2 (7) |
| III | 10 (34) |
| Iva | 13 (45) |
| IVb | 4 (14) |
| Postoperative TNM stage, n (%) | |
| I | 2 (7) |
| IIIa | 9 (31) |
| IIIb | 1 (3) |
| Iva | 14 (48) |
| IVb | 3 (10) |
SD: standard deviation; SVC: superior vena cava.
| Factors . | . |
|---|---|
| Surgical approach, n (%) | |
| Median sternotomy | 17 (59) |
| Hemiclamshell | 3 (10) |
| Median sternotomy and thoracotomy | 6 (21) |
| Thoracotomy | 3 (10) |
| Site of combined resection, n (%) | |
| SVC (mode of reconstruction) | 9 (31) |
| Patch closure | 2 (7) |
| Prosthetic replacement | 6 (21) |
| None | 1 (3) |
| Lung (type of resection) | 22 (76) |
| Pneumonectomy | 3 (10) |
| Lobectomy | 6 (21) |
| Partial resection | 13 (45) |
| Dissemination (type of resection) | 14 (48) |
| Resection of dissemination | 11 (38) |
| Pleuropneumonectomy | 3 (10) |
| Phrenic nerve | 13 (45) |
| Left atrial appendage | 1 (3) |
| Lymph node dissection, n (%) | |
| Anterior region lymph nodes | 22 (76) |
| Anterior region and upper mediastinal lymph nodes | 6 (21) |
| Anterior region, upper mediastinal and cervical lymph nodes | 1 (3) |
| Operative time (min) | |
| Mean ± SD | 410 ± 169 |
| Range | 120–811 |
| Blood loss (ml) | |
| Mean ± SD | 1268 ± 918 |
| Range | 100–3650 |
| Patients who received blood transfusion, n (%) | 16 (55) |
| Postoperative Masaoka stage, n (%) | |
| II | 2 (7) |
| III | 10 (34) |
| Iva | 13 (45) |
| IVb | 4 (14) |
| Postoperative TNM stage, n (%) | |
| I | 2 (7) |
| IIIa | 9 (31) |
| IIIb | 1 (3) |
| Iva | 14 (48) |
| IVb | 3 (10) |
| Factors . | . |
|---|---|
| Surgical approach, n (%) | |
| Median sternotomy | 17 (59) |
| Hemiclamshell | 3 (10) |
| Median sternotomy and thoracotomy | 6 (21) |
| Thoracotomy | 3 (10) |
| Site of combined resection, n (%) | |
| SVC (mode of reconstruction) | 9 (31) |
| Patch closure | 2 (7) |
| Prosthetic replacement | 6 (21) |
| None | 1 (3) |
| Lung (type of resection) | 22 (76) |
| Pneumonectomy | 3 (10) |
| Lobectomy | 6 (21) |
| Partial resection | 13 (45) |
| Dissemination (type of resection) | 14 (48) |
| Resection of dissemination | 11 (38) |
| Pleuropneumonectomy | 3 (10) |
| Phrenic nerve | 13 (45) |
| Left atrial appendage | 1 (3) |
| Lymph node dissection, n (%) | |
| Anterior region lymph nodes | 22 (76) |
| Anterior region and upper mediastinal lymph nodes | 6 (21) |
| Anterior region, upper mediastinal and cervical lymph nodes | 1 (3) |
| Operative time (min) | |
| Mean ± SD | 410 ± 169 |
| Range | 120–811 |
| Blood loss (ml) | |
| Mean ± SD | 1268 ± 918 |
| Range | 100–3650 |
| Patients who received blood transfusion, n (%) | 16 (55) |
| Postoperative Masaoka stage, n (%) | |
| II | 2 (7) |
| III | 10 (34) |
| Iva | 13 (45) |
| IVb | 4 (14) |
| Postoperative TNM stage, n (%) | |
| I | 2 (7) |
| IIIa | 9 (31) |
| IIIb | 1 (3) |
| Iva | 14 (48) |
| IVb | 3 (10) |
SD: standard deviation; SVC: superior vena cava.
Postoperative complications
There were no perioperative mortalities. Six (21%) patients developed postoperative complications. Details regarding postoperative complications are summarized in Table 4. Three of these 6 patients underwent pleuropneumonectomy, whereas the other 3 patients underwent an SVC or left atrial appendage resection procedure. Patient 1 was positive for serum anti-Ach receptor antibodies (4 nmol/l) without MG symptoms in preoperative findings but later experienced new-onset MG. Five patients underwent postoperative radiation (40–50 Gy) and 1 postoperative chemotherapy.
| Patients . | Extent of lung resection . | SVC resection . | Postoperative complications (grade) . |
|---|---|---|---|
| 1 | Pleuropneumonectomy | No | Myasthenic crisis (3a) and pneumonia (3a) |
| 2 | Pleuropneumonectomy | No | Postoperative bleeding (2) and recurrent nerve palsy (2) |
| 3 | Pleuropneumonectomy | Yes | Cardiac hernia due to resection of the pericardium (3b) |
| 4 | Lobectomy | Yes | Chylothorax (2) |
| 5 | Partial resection | Yes | Wound infection (2) |
| 6 | Lobectomy | Noa | Respiratory failure (4) |
| Patients . | Extent of lung resection . | SVC resection . | Postoperative complications (grade) . |
|---|---|---|---|
| 1 | Pleuropneumonectomy | No | Myasthenic crisis (3a) and pneumonia (3a) |
| 2 | Pleuropneumonectomy | No | Postoperative bleeding (2) and recurrent nerve palsy (2) |
| 3 | Pleuropneumonectomy | Yes | Cardiac hernia due to resection of the pericardium (3b) |
| 4 | Lobectomy | Yes | Chylothorax (2) |
| 5 | Partial resection | Yes | Wound infection (2) |
| 6 | Lobectomy | Noa | Respiratory failure (4) |
Left atrial appendage resection performed in this case.
SVC: superior vena cava.
| Patients . | Extent of lung resection . | SVC resection . | Postoperative complications (grade) . |
|---|---|---|---|
| 1 | Pleuropneumonectomy | No | Myasthenic crisis (3a) and pneumonia (3a) |
| 2 | Pleuropneumonectomy | No | Postoperative bleeding (2) and recurrent nerve palsy (2) |
| 3 | Pleuropneumonectomy | Yes | Cardiac hernia due to resection of the pericardium (3b) |
| 4 | Lobectomy | Yes | Chylothorax (2) |
| 5 | Partial resection | Yes | Wound infection (2) |
| 6 | Lobectomy | Noa | Respiratory failure (4) |
| Patients . | Extent of lung resection . | SVC resection . | Postoperative complications (grade) . |
|---|---|---|---|
| 1 | Pleuropneumonectomy | No | Myasthenic crisis (3a) and pneumonia (3a) |
| 2 | Pleuropneumonectomy | No | Postoperative bleeding (2) and recurrent nerve palsy (2) |
| 3 | Pleuropneumonectomy | Yes | Cardiac hernia due to resection of the pericardium (3b) |
| 4 | Lobectomy | Yes | Chylothorax (2) |
| 5 | Partial resection | Yes | Wound infection (2) |
| 6 | Lobectomy | Noa | Respiratory failure (4) |
Left atrial appendage resection performed in this case.
SVC: superior vena cava.
Long-term outcomes
At the time of writing, 4 (14%) patients have died of disease and 12 (41%) experienced recurrence. The site of recurrence was local in 1, cervical lymph node in 1, mediastinal lymph node in 2 and pleural dissemination in 8 patients. Of the 12 patients who experienced recurrence, 10 received treatment for recurrent disease (surgical resection in 4, chemotherapy in 3, radiotherapy in 3). OS and DFS curves are shown in Fig. 1. The 5- and 10-year OS rates were 100% and 87%, respectively, and the 5- and 10-year DFS rates were 50% and 50%, respectively.
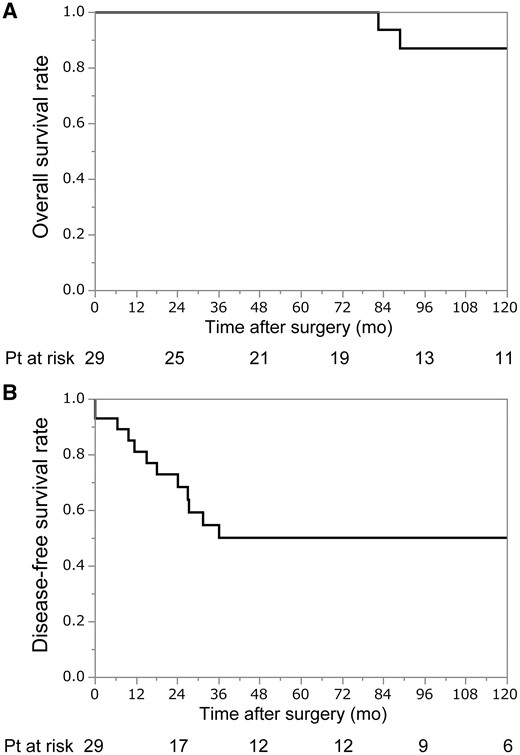
(A) Overall survival after surgery. The 5- and 10-year overall survival rates were 100% and 87%, respectively. (B) Disease-free survival after surgery. The 5- and 10-year disease-free survival rates were 50% and 50%, respectively.
Univariate analysis
To determine factors associated with long-term outcomes, univariate analysis was performed using age (<50, ≥50 years), gender (male, female), presence of MG (yes, no), clinical response (partial response, stable disease), histological type (B3, others), macroscopic invasion to great vessels (yes, no) and the postoperative Masaoka stage (II/III, IV) as factors (Table 5). The results showed that only the postoperative Masaoka stage was significantly associated with long-term outcomes. In terms of clinical response, the outcomes of patients with partial response showed a trend to be better as compared to those with stable disease, though the difference was not significant (Fig. 2). On the other hand, 2 patients who achieved downstaging had a favourable outcome (216 months and 23 months with no evidence of disease).
| Factors . | 10-Year OS (%) . | P-value . | 5-Year DFS (%) . | P-value . |
|---|---|---|---|---|
| Age (<50 vs ≥50 years) | 100 vs 81 | NS | 45 vs 55 | NS |
| Sex (male versus female) | 100 vs 83 | NS | 64 vs 45 | NS |
| Presence of MG (yes versus no) | 100 vs 100 | NS | 50 vs 50 | NS |
| Clinical response (PR versus SD) | 100 vs 76 | NS | 68 vs 39 | NS |
| Histological type (B3 versus others) | 78 vs 100 | NS | 38 vs 60 | NS |
| Macroscopic invasion to great vessels (yes versus no) | 100 vs 80 | NS | 63 vs 43 | NS |
| Postoperative Masaoka stage (II/III versus IV) | 100 vs 71 | 0.01 | 89 vs 23 | <0.01 |
| Factors . | 10-Year OS (%) . | P-value . | 5-Year DFS (%) . | P-value . |
|---|---|---|---|---|
| Age (<50 vs ≥50 years) | 100 vs 81 | NS | 45 vs 55 | NS |
| Sex (male versus female) | 100 vs 83 | NS | 64 vs 45 | NS |
| Presence of MG (yes versus no) | 100 vs 100 | NS | 50 vs 50 | NS |
| Clinical response (PR versus SD) | 100 vs 76 | NS | 68 vs 39 | NS |
| Histological type (B3 versus others) | 78 vs 100 | NS | 38 vs 60 | NS |
| Macroscopic invasion to great vessels (yes versus no) | 100 vs 80 | NS | 63 vs 43 | NS |
| Postoperative Masaoka stage (II/III versus IV) | 100 vs 71 | 0.01 | 89 vs 23 | <0.01 |
DFS: disease-free survival; MG: myasthenia gravis; OS: overall survival; PR: partial response; SD: stable disease.
| Factors . | 10-Year OS (%) . | P-value . | 5-Year DFS (%) . | P-value . |
|---|---|---|---|---|
| Age (<50 vs ≥50 years) | 100 vs 81 | NS | 45 vs 55 | NS |
| Sex (male versus female) | 100 vs 83 | NS | 64 vs 45 | NS |
| Presence of MG (yes versus no) | 100 vs 100 | NS | 50 vs 50 | NS |
| Clinical response (PR versus SD) | 100 vs 76 | NS | 68 vs 39 | NS |
| Histological type (B3 versus others) | 78 vs 100 | NS | 38 vs 60 | NS |
| Macroscopic invasion to great vessels (yes versus no) | 100 vs 80 | NS | 63 vs 43 | NS |
| Postoperative Masaoka stage (II/III versus IV) | 100 vs 71 | 0.01 | 89 vs 23 | <0.01 |
| Factors . | 10-Year OS (%) . | P-value . | 5-Year DFS (%) . | P-value . |
|---|---|---|---|---|
| Age (<50 vs ≥50 years) | 100 vs 81 | NS | 45 vs 55 | NS |
| Sex (male versus female) | 100 vs 83 | NS | 64 vs 45 | NS |
| Presence of MG (yes versus no) | 100 vs 100 | NS | 50 vs 50 | NS |
| Clinical response (PR versus SD) | 100 vs 76 | NS | 68 vs 39 | NS |
| Histological type (B3 versus others) | 78 vs 100 | NS | 38 vs 60 | NS |
| Macroscopic invasion to great vessels (yes versus no) | 100 vs 80 | NS | 63 vs 43 | NS |
| Postoperative Masaoka stage (II/III versus IV) | 100 vs 71 | 0.01 | 89 vs 23 | <0.01 |
DFS: disease-free survival; MG: myasthenia gravis; OS: overall survival; PR: partial response; SD: stable disease.
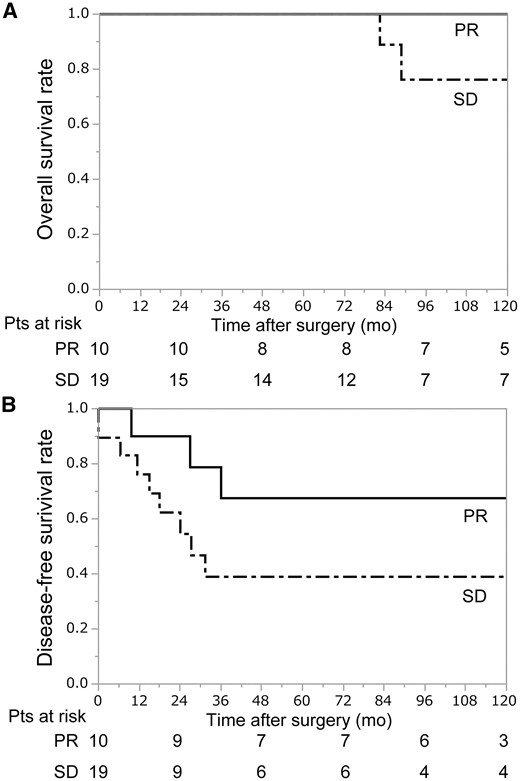
(A) Overall survival after surgery according to clinical response. The 5- and 10-year overall survival rates for the PR group were 100% and 100%, respectively, while those for the SD group were 100% and 76%, respectively. (B) Disease-free survival after surgery according to clinical response. The 5- and 10-year disease-free survival rates for the PR group were 68% and 68%, respectively, while those for the SD group were 39% and 39%, respectively. PR: partial response; SD: stable disease.
DISCUSSION
The results of the present study demonstrated that surgery could be safely performed following chemotherapy or chemoradiotherapy for patients with a thymoma with great vessel invasion and/or pleural dissemination. We found a complete resection rate of 83%, and long-term outcomes were favourable.
Preoperative chemotherapy or chemoradiotherapy for advanced thymoma is generally applied in leading institutions in Europe and North America. The indications for such preoperative therapy are Masaoka stage III [20] or IVa [21, 22] disease or both [20, 23–26]. English language reports of preoperative chemotherapy or chemoradiotherapy followed by surgery for invasive thymoma that include detailed information for more than 10 patients and published since 2006 are shown in Table 6. Some of these studies included patients with thymic cancer. Besides the report presented by Korst et al., [24] who described the performance of preoperative chemoradiotherapy, the other authors selected preoperative chemotherapy rather than chemoradiotherapy. The reported complete resection rates range from 67% to 95% and 10-year OS rates from 65% to 82%. A variety of regimens were used for these reported procedures, though the majority chosen were cisplatin-based. In comparison with the results reported by world-leading institutions, those in the present study (complete resection rate 83%, 10-year OS rate 87%) are quite compatible.
Reports regarding preoperative chemotherapy or chemoradiotherapy followed by surgery for invasive thymoma
| Author names . | Year . | Study period . | Total number of patients . | Number of patients with thymoma . | Mode of preoperative therapy (CT/CRT) . | Stage (III/IV) . | Rate of complete resection (%) . | 5- and 10-Year OS . | 5- and 10-Year DFS . |
|---|---|---|---|---|---|---|---|---|---|
| Lucchi et al. | 2006 | 1989–2004 | 30 | 30 | 30/0 | 20/10 | 83 | 82, 82 | NA |
| Huang et al. | 2007 | 1996–2006 | 18 | 18 | 18/0 | 0/18 | 67 | 78, 65 | 90 and 34 |
| Rea et al. | 2011 | 1980–2008 | 38 | 32 | 38/0 | 23/15 | NA | NA | NA |
| Korst et al. | 2014 | 2007–2012 | 22 | 15 | 0/22 | 12/3 | 95 | NA | NA |
| Cardillo et al. | 2016 | 1990–2010 | 108 | 88 | 103/5 | 108/0 | 85 | 79, NA | 69 and NA |
| This article | 2017 | 1997–2016 | 25 | 25 | 22/3 | 10/17 | 83 | 100, 87 | 50 and 50 |
| Author names . | Year . | Study period . | Total number of patients . | Number of patients with thymoma . | Mode of preoperative therapy (CT/CRT) . | Stage (III/IV) . | Rate of complete resection (%) . | 5- and 10-Year OS . | 5- and 10-Year DFS . |
|---|---|---|---|---|---|---|---|---|---|
| Lucchi et al. | 2006 | 1989–2004 | 30 | 30 | 30/0 | 20/10 | 83 | 82, 82 | NA |
| Huang et al. | 2007 | 1996–2006 | 18 | 18 | 18/0 | 0/18 | 67 | 78, 65 | 90 and 34 |
| Rea et al. | 2011 | 1980–2008 | 38 | 32 | 38/0 | 23/15 | NA | NA | NA |
| Korst et al. | 2014 | 2007–2012 | 22 | 15 | 0/22 | 12/3 | 95 | NA | NA |
| Cardillo et al. | 2016 | 1990–2010 | 108 | 88 | 103/5 | 108/0 | 85 | 79, NA | 69 and NA |
| This article | 2017 | 1997–2016 | 25 | 25 | 22/3 | 10/17 | 83 | 100, 87 | 50 and 50 |
CT: computed tomography; DFS: disease-free survival; OS: overall survival.
Reports regarding preoperative chemotherapy or chemoradiotherapy followed by surgery for invasive thymoma
| Author names . | Year . | Study period . | Total number of patients . | Number of patients with thymoma . | Mode of preoperative therapy (CT/CRT) . | Stage (III/IV) . | Rate of complete resection (%) . | 5- and 10-Year OS . | 5- and 10-Year DFS . |
|---|---|---|---|---|---|---|---|---|---|
| Lucchi et al. | 2006 | 1989–2004 | 30 | 30 | 30/0 | 20/10 | 83 | 82, 82 | NA |
| Huang et al. | 2007 | 1996–2006 | 18 | 18 | 18/0 | 0/18 | 67 | 78, 65 | 90 and 34 |
| Rea et al. | 2011 | 1980–2008 | 38 | 32 | 38/0 | 23/15 | NA | NA | NA |
| Korst et al. | 2014 | 2007–2012 | 22 | 15 | 0/22 | 12/3 | 95 | NA | NA |
| Cardillo et al. | 2016 | 1990–2010 | 108 | 88 | 103/5 | 108/0 | 85 | 79, NA | 69 and NA |
| This article | 2017 | 1997–2016 | 25 | 25 | 22/3 | 10/17 | 83 | 100, 87 | 50 and 50 |
| Author names . | Year . | Study period . | Total number of patients . | Number of patients with thymoma . | Mode of preoperative therapy (CT/CRT) . | Stage (III/IV) . | Rate of complete resection (%) . | 5- and 10-Year OS . | 5- and 10-Year DFS . |
|---|---|---|---|---|---|---|---|---|---|
| Lucchi et al. | 2006 | 1989–2004 | 30 | 30 | 30/0 | 20/10 | 83 | 82, 82 | NA |
| Huang et al. | 2007 | 1996–2006 | 18 | 18 | 18/0 | 0/18 | 67 | 78, 65 | 90 and 34 |
| Rea et al. | 2011 | 1980–2008 | 38 | 32 | 38/0 | 23/15 | NA | NA | NA |
| Korst et al. | 2014 | 2007–2012 | 22 | 15 | 0/22 | 12/3 | 95 | NA | NA |
| Cardillo et al. | 2016 | 1990–2010 | 108 | 88 | 103/5 | 108/0 | 85 | 79, NA | 69 and NA |
| This article | 2017 | 1997–2016 | 25 | 25 | 22/3 | 10/17 | 83 | 100, 87 | 50 and 50 |
CT: computed tomography; DFS: disease-free survival; OS: overall survival.
It is difficult to assess whether the outcome of preoperative therapy followed by surgery is superior to that of surgery performed as the initial treatment for advanced thymoma, as a randomized trial is not practical due to the rarity of the disease. Theoretically, preoperative therapy has both advantages and disadvantages in comparison with surgery as the initial treatment. Advantages include the following: First, systemic treatment can be performed initially, and micrometastasis that develops through blood flow or pleural dissemination can be treated in the early period. Second, tolerability towards preoperative chemotherapy may be better as compared to postoperative chemotherapy because the general condition of the patient may change after surgery. Third, response to chemotherapy can be assessed and may be useful for planning postoperative chemotherapy or chemotherapy given for recurrent disease. Fourth, it is possible that preoperative therapy is associated with a higher rate of complete resection in comparison with surgery as the initial treatment, especially in cases with tumour invasion of the great vessels. Finally, when downstaging is achieved, the long-term outcome is often favourable. A recent study performed in China supports this notion [27], with the same phenomenon also observed in the present cohort. On the other hand, there are some disadvantages with preoperative chemotherapy. First, if no response to chemotherapy can be obtained (i.e. tumour becomes enlarged during chemotherapy), the timing of surgery will be delayed and may be less than optimal. Second, common adverse effects of chemotherapy such as infection may occur, while rare complications such as myasthenic crisis can also occur, as previously reported [19]. Third, the rate of postoperative complications may be higher due to tissue damage caused by chemotherapy.
In our opinion, the most important advantage of induction treatment is higher possibility of achieving complete resection. As noted in the Introduction section above, we performed surgery as the initial treatment for advanced thymoma until the 1980s, and the rate for complete resection in cases of thymoma with great vessel invasion was low at 70% [6]. Although the present findings cannot be directly compared with those in that previous report, it is conceivable that preoperative treatment may contribute to an increased rate of complete resection rate for advanced thymoma. Our speculation is supported by results obtained in a previous large-scale study. Yamada et al. [28] used a Japan nationwide database to investigate surgical outcomes of patients with stage III thymoma treated between 1991 and 2010. They found that 126 (41%) of 310 patients had a thymoma with great vessel invasion, of whom 36 (12%) patients received preoperative chemotherapy or chemoradiotherapy, with a complete resection rate of 90% and great vessel invasion found to not influence survival. These results suggested that the rate of complete resection of a thymoma with great vessel invasion was increased by the use of preoperative therapy, and the outcome was favourable in these patients. The finding that great vessel invasion does not influence survival could be explained by possible improvement in outcome in patients with great vessel invasion. On the other hand, chest wall invasion was found to be the only independent predictor of worse DFS in that study [28]. Therefore, it is considered that patients with chest wall invasion may be good candidates for preoperative chemotherapy.
The standard mode of resection for an advanced thymoma is an extended thymectomy via a median sternotomy and has been widely accepted for patients with MG [29]. In patients without MG, the aim of this mode of resection is tumour resection with a sufficient margin and prevention of occult intrathymic micrometastasis. However, in those with a large tumour and dissemination, partial resection of the thymus and a lateral thoracotomy are generally selected. On the other hand, when the phrenic nerve is involved by the tumour, whether resection or preservation should be chosen remains controversial. The effects of preservation of the phrenic nerve in such cases was analysed by Yano et al. [30], who reported that phrenic nerve resection caused a decrease in vital capacity (66% of predicted value) as well as forced expiratory volume in the first second of expiration (69% of predicted value). On the basis of their findings, they speculated that preservation of the phrenic nerve may be an option, especially in patients with serious complications, because there was no difference in OS between the resected and preserved groups. In contrast, at our institution, when the phrenic nerve is massively involved, it is resected to achieve complete resection, and diaphragm plication is also performed, and we previously reported the efficacy of intraoperative unilateral diaphragm plication for patients undergoing a unilateral phrenic nerve resection procedure [16]. In that study cohort, postoperative lung function tests revealed that mean vital capacity and forced expiratory volume in the first second of expiration were 88.2% and 98.5%, respectively, of the predicted postoperative values. Regardless, the balance between radicality and preservation of lung function must be considered in affected patients.
Limitations
The present study has some limitations. First, marked advancements in radiological examination methods were achieved during the study period, which might have affected patient selection. Second, the preoperative chemotherapy regimens were not unified due to the retrospective nature of the study, and future investigations are needed to seek an optimal regimen. Third, the number of patients was limited, and a multicentre study with a larger study population should be performed to clarify the significance of preoperative therapy for advanced thymoma. In addition, because planning for the use of an appropriate multimodal treatment strategy for advanced thymoma patients requires experience with such cases, it is considered that such procedures should only be attempted by experienced doctors in high-volume centres.
CONCLUSION
In conclusion, preoperative chemotherapy or chemoradiotherapy followed by surgery for locally advanced thymoma can be performed with an acceptable degree of surgical risk. Nevertheless, such a strategy should be proactively considered, as it may provide favourable long-term results.
Conflict of interest: none declared.






Comments
We have read with great interest the article by Ryu Kanzaki et al. [1] reporting on the long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery [1]. Although the Masaoka staging system is a valuable prognostic factor, it is well known that the category of Masaoka stage III is heterogeneous and consists of 2 groups with distinct prognoses depending also on the involvement of the great vessels [2].
In the study of Okumura et al. [2], in fact the involvement of the great vessels was a single independent prognostic factor in patients with stage III disease with 10- and 20-year survivals in patients with stage III disease that were 97% and 75% in the absence of involvement of the great vessels, and 70% and 29% in the presence of it. We all also know that stage IVA thymoma patients with pleural dissemination sometimes achieve long-term survival after resection, especially in single localization [3]. According to the findings of [1-3] and the new model concerning the prognosis predictability after complete resection [4] we would suggest performing resection prior to the induction therapy in all cases in whom radicality could be reached, except in cases of vascular infiltration (any WHO). In this specific subset of patients we agree with the authors that neoadjuvant therapy could have an impact because one could select that subgroup of patients (responders) in whom the surgical resection could be really useful.
References
[1] Kanzaki R, Kanou T, Ose N, Funaki S, Shintani Y, Minami M et al. Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience. Interact CardioVasc Thorac Surg 2019;28:360–367.
[2] Okumura M, Miyoshi S, Takeuchi Y, Yoon HE, Minami M, Takeda SI et al. Results of surgical treatment of thymomas with special reference to the involved organs. J Thorac Cardiovasc Surg 1999;117:605-13.
[3] Okumura M, Utsumi T. Treatment strategy for thymoma and thymic carcinoma. Kyobu Geka 2011;64(8 Suppl):719-24.
[4] Lococo F, Cafarotti S, Cesario A, Dall'Armi V, Cusumano G, Lauriola L et al. Prognostic grading after complete resection for thymic malignancies. Eur Rev Med Pharmacol Sci 2015;19:2882-91.