-
PDF
- Split View
-
Views
-
Cite
Cite
Fleur M M Meijer, Philippine Kies, Monique R M Jongbloed, Mark G Hazekamp, David R Koolbergen, Nico A Blom, Albert de Roos, Martin J Schalij, Hubert W Vliegen, Excellent durability of homografts in pulmonary position analysed in a predefined adult group with tetralogy of Fallot, Interactive CardioVascular and Thoracic Surgery, Volume 28, Issue 2, February 2019, Pages 279–283, https://doi.org/10.1093/icvts/ivy242
Close - Share Icon Share
Abstract
In repaired tetralogy of Fallot, surgical pulmonary valve replacement (PVR) is in certain cases required. Our institution reported earlier about 26 patients who received a pulmonary homograft via PVR. To date, we have data from more than 17 years of follow-up. The aim of this retrospective study was to evaluate the late haemodynamic and clinical outcomes in this predefined patient group.
Between 1993 and 2001, 26 patients underwent PVR for pulmonary regurgitation (58% men; 30.4 ± 8.9 years). The rates of mortality and of complications (re-PVR, ablation and cardioverter defibrillator implants) were analysed. Other main study outcomes were haemodynamic parameters determined from cardiovascular magnetic resonance imaging: pulmonary regurgitation; right ventricular (RV) end-diastolic volume; RV ejection fraction; left ventricular (LV) end-diastolic volume; LV ejection fraction; New York Heart Association functional class at the latest follow-up visit; and echocardiographic parameters of the right ventricle.
The median follow-up time was 17 ± 1.1 years. Overall freedom from complications was 61.5% (95% confidence interval 47.5–78.6%). One patient died 18 months after surgery of unknown causes. Two patients needed replacement of the homograft at 24 and 39 months after PVR. The indication in both patients was recurrence of severe homograft regurgitation with important RV dilatation. Six patients received an implantable cardioverter defibrillator at a median age of 41 years (interquartile range 36–47); 12 patients experienced supra- and/or ventricular arrhythmias and 6 of these needed ablation. There was no significant deterioration of haemodynamic function or functional class.
The patients who underwent PVR exhibited long-term follow-up stabilization of RV function and impressive functional durability of the graft. After a follow-up of 17 years, 23 out of 26 patients (89%) were alive without redo PVR. Event-free survival was good (61.5%).
INTRODUCTION
Tetralogy of Fallot (TOF) is the most common type of cyanotic congenital heart disease [1, 2]. Advanced surgical techniques have drastically increased the survival rate of this patient group and have led to an increasing prevalence of adult survivors of TOF repair. These adult patients are prone to develop severe pulmonary regurgitation (PR), which may lead to volume overload, right ventricular (RV) dilatation, heart failure, arrhythmias and/or death [3–6]. Eventually, most of these patients require pulmonary valve replacement (PVR). Initially there was great concern about the durability of a pulmonary homograft. Today, there is a large group of patients with a repaired TOF who underwent PVR, and more clinical data are available. In 2002, Vliegen et al. [7] reported 26 patients who received a pulmonary homograft via a PVR: to date, we have data from more than 17 years of follow-up. The aim of this retrospective study was to evaluate clinical outcomes and late haemodynamics in this predefined patient group.
PATIENTS AND METHODS
Between 1993 and 2001, 26 patients underwent PVR with a cryopreserved pulmonary homograft for PR (58% men; 30.4 ± 8.9 years). Homografts were inserted in the orthotopic pulmonary position with 1 proximal and 1 distal end-to-end running suture after longitudinally opening the proximal pulmonary artery and slightly extending this incision if necessary across the former pulmonary annulus. Calcified outflow tract patch material was resected as much as possible. The medical records of all patients were reviewed. Cardiovascular magnetic resonance imaging was executed as previously described [7] and was performed in 19 patients at least 9.5 years after PVR with a median of 130 months [interquartile range (IQR) 86–196].
Primary study outcomes were mortality rate, redo PVR, ablation and use of an implantable cardioverter defibrillator (ICD). Moreover, haemodynamic parameters determined with cardiovascular magnetic resonance imaging at the latest follow-up visit were analysed: PR fraction; RV end-diastolic volume; RV ejection fraction; left ventricular (LV) end-diastolic volume; and LV ejection fraction. The volume parameters were indexed for body surface area. Echocardiographic parameters were also evaluated: maximum gradient over the pulmonary valve, presence of PR and presence of tricuspid regurgitation. PR was defined as mild when the PR fraction was below 20%, moderate when the PR fraction was between 20% and 40% and severe when the fraction was >40%.
Statistical analyses
Results are expressed as mean ± standard deviation, number with frequencies and percentages or median with IQR. Comparisons between postoperative and late follow-up data were performed with the paired Student’s t-tests. P-values of <0.05 were considered to be statistically significant. Event-free survival was analysed using the Kaplan–Meier method with 95% confidence intervals.
RESULTS
Table 1 lists the personal and surgical characteristics of those patients who had PVR. The median follow-up time was 17 ± 1.1 years. In Table 2, the preoperative, early postoperative and late follow-up parameters are shown. The latest cardiac magnetic resonance image (MRI) was performed at a median of 130 (IQR 86–196) months after PVR. Not every patient operated on received a follow-up MRI due to ICD implantation. Haemodynamic RV- and LV function remained stable over time; the same applies to RV volume. Only the PR fraction had mildly but significantly increased (P = 0.028) from 3.7% to 8% (both in the range of mild PR). Echocardiograms confirmed the excellent durability of the graft; 89% had none to mild PR at the latest follow-up. Moreover, there was no deterioration of New York Heart Association functional class. There were no symptoms of endocarditis documented. Overall event-free survival (no death, ablation, ICD insertion or redo surgery) was 61.5% (95% confidence interval 47.5–78.6%) after 18 years (Fig. 1). One patient died unexpectedly 18 months after surgery; no autopsy was performed. This patient had a good validity of 98% on an exercise test 9 months prior to death; no other details are known. Two patients needed redo surgery of the homograft at 24 and 39 months after PVR. The indication for redo surgery in the first patient was the recurrence of severe pulmonary homograft regurgitation in combination with serious RV dilatation. This patient received a surgical Contegra conduit and reconstruction of the RV outflow tract. The other patient received a percutaneous Edward Sapiens prosthesis due to recurrence of severe pulmonary homograft regurgitation. Twelve patients experienced arrhythmias (supraventricular or ventricular), and 6 of them needed 1 or more ablations. Six patients received an ICD at a median age of 41 (IQR 36–47) (Table 3); freedom from ICD implantation was 77% at 12 years (Fig. 1). In all cases, the indication for an ICD was secondary prevention of ventricular tachycardias.
| Number of patients | 26 |
| Male | 15 (58) |
| Age at initial correction (years) | 5 ± 4.2 |
| Initial surgical correction/type of RVOT reconstruction at initial correction | |
| Total correction | 12 (46.2) |
| Myectomy/valvulotomy | 5 (19) |
| Right ventricle patch | 1 (3.8) |
| Transannular patch | 10 (38.5) |
| Unknown | 10 (38.5) |
| Previous shunt procedure | |
| Waterston | 3 (11.5) |
| Blalock–Taussig | 8 (30.8) |
| Potts anastomosis | 1 (3.8) |
| Hancock conduit | 1 (3.8) |
| Unknown | 1 (3.8) |
| Surgical PVR | |
| Age at PVR (years) | 30.4 ± 8.9 |
| Concomitant procedures | |
| Resection infundibulum | 1 (3.8) |
| Tricuspid valve repair | 4 (11.5) |
| Closure VSD | 4 (15.4) |
| Closure of atrial septal defect and tricuspid valve repair | 1 (3.8) |
| Xenopericardial reconstruction | 3 (11.5) |
| No additional procedure | 14 (53.8) |
| Number of patients | 26 |
| Male | 15 (58) |
| Age at initial correction (years) | 5 ± 4.2 |
| Initial surgical correction/type of RVOT reconstruction at initial correction | |
| Total correction | 12 (46.2) |
| Myectomy/valvulotomy | 5 (19) |
| Right ventricle patch | 1 (3.8) |
| Transannular patch | 10 (38.5) |
| Unknown | 10 (38.5) |
| Previous shunt procedure | |
| Waterston | 3 (11.5) |
| Blalock–Taussig | 8 (30.8) |
| Potts anastomosis | 1 (3.8) |
| Hancock conduit | 1 (3.8) |
| Unknown | 1 (3.8) |
| Surgical PVR | |
| Age at PVR (years) | 30.4 ± 8.9 |
| Concomitant procedures | |
| Resection infundibulum | 1 (3.8) |
| Tricuspid valve repair | 4 (11.5) |
| Closure VSD | 4 (15.4) |
| Closure of atrial septal defect and tricuspid valve repair | 1 (3.8) |
| Xenopericardial reconstruction | 3 (11.5) |
| No additional procedure | 14 (53.8) |
Data are described as numbers with frequency or percentage and mean with SD.
PVR: pulmonary valve replacement; RVOT: right ventricular outflow tract; SD: standard deviation; VSD: ventricular septal defect.
| Number of patients | 26 |
| Male | 15 (58) |
| Age at initial correction (years) | 5 ± 4.2 |
| Initial surgical correction/type of RVOT reconstruction at initial correction | |
| Total correction | 12 (46.2) |
| Myectomy/valvulotomy | 5 (19) |
| Right ventricle patch | 1 (3.8) |
| Transannular patch | 10 (38.5) |
| Unknown | 10 (38.5) |
| Previous shunt procedure | |
| Waterston | 3 (11.5) |
| Blalock–Taussig | 8 (30.8) |
| Potts anastomosis | 1 (3.8) |
| Hancock conduit | 1 (3.8) |
| Unknown | 1 (3.8) |
| Surgical PVR | |
| Age at PVR (years) | 30.4 ± 8.9 |
| Concomitant procedures | |
| Resection infundibulum | 1 (3.8) |
| Tricuspid valve repair | 4 (11.5) |
| Closure VSD | 4 (15.4) |
| Closure of atrial septal defect and tricuspid valve repair | 1 (3.8) |
| Xenopericardial reconstruction | 3 (11.5) |
| No additional procedure | 14 (53.8) |
| Number of patients | 26 |
| Male | 15 (58) |
| Age at initial correction (years) | 5 ± 4.2 |
| Initial surgical correction/type of RVOT reconstruction at initial correction | |
| Total correction | 12 (46.2) |
| Myectomy/valvulotomy | 5 (19) |
| Right ventricle patch | 1 (3.8) |
| Transannular patch | 10 (38.5) |
| Unknown | 10 (38.5) |
| Previous shunt procedure | |
| Waterston | 3 (11.5) |
| Blalock–Taussig | 8 (30.8) |
| Potts anastomosis | 1 (3.8) |
| Hancock conduit | 1 (3.8) |
| Unknown | 1 (3.8) |
| Surgical PVR | |
| Age at PVR (years) | 30.4 ± 8.9 |
| Concomitant procedures | |
| Resection infundibulum | 1 (3.8) |
| Tricuspid valve repair | 4 (11.5) |
| Closure VSD | 4 (15.4) |
| Closure of atrial septal defect and tricuspid valve repair | 1 (3.8) |
| Xenopericardial reconstruction | 3 (11.5) |
| No additional procedure | 14 (53.8) |
Data are described as numbers with frequency or percentage and mean with SD.
PVR: pulmonary valve replacement; RVOT: right ventricular outflow tract; SD: standard deviation; VSD: ventricular septal defect.
| . | Before PVR . | Early after PVR . | Late follow-up . | Δ Post-late follow-up . | P-value, post-late follow-up . |
|---|---|---|---|---|---|
| CMR | n = 25 | n = 25 | n = 19 | ||
| Time, CMR from PVR (months) | −4.5 (−7 to −3) | 10 (8–16) | 130 (86–196) | ||
| PR fraction (%)a | 46 ± 11 | 3.7 ± 6.8 | 8 ± 9 | 4 ± 10 | 0.028 |
| RV | |||||
| EDV (ml)a | 302 ± 60 | 207 ± 69 | 212 ± 67 | −6 ± 30 | 0.46 |
| EDV-I (ml/m²) | 163 ± 33 | 115 ± 40 | 111 ± 35 | −9 ± 17 | 0.09 |
| ESV (ml)a | 177 ± 53 | 120 ± 65 | 115 ± 59 | −14 ± 34 | 0.3 |
| ESV-I (ml/m²) | 97 ± 36 | 67 ± 41 | 60 ± 32 | −11 ± 20 | 0.06 |
| EF (%)a | 43 ± 11 | 44 ± 11 | 46 ± 10 | 2 ± 7 | 0.47 |
| LV | |||||
| EDV (ml)a | 145 ± 40 | 152 ± 34 | 156 ± 44 | 5 ± 34 | 0.53 |
| EDV-I (ml/m²) | 80 ± 23 | 83 ± 15 | 85 ± 17 | −1 ± 19 | 0.8 |
| ESV (ml)a | 64 ± 32 | 68 ± 19 | 76 ± 26 | 6 ± 19 | 0.41 |
| ESV-I (ml/m²) | 36 ± 18 | 37 ± 9 | 41 ± 12 | 1 ± 10 | 0.9 |
| EF (%)a | 55 ± 14 | 56 ± 8 | 55 ± 7 | −1 ± 8 | 0.94 |
| NYHA class | 3.0 ± 0.2 | 1.3 ± 0.5 | 1.1 ± 0.3 | ||
| Echo | n = 25 | n = 25 | |||
| Time, echo from PVR (months) | −10 (−11.5 to −3.5) | 187 (183–207) | |||
| PV maximum gradient (mmHg) | 27 ± 26 | 19 ± 13 | |||
| PR present (%) | |||||
| Severe (Grade 4) | 66 | 0 | |||
| Moderate–severe (Grade 3) | 31 | 0 | |||
| Moderate (Grade 2) | 0 | 8 | |||
| Mild (Grade 1) | 0 | 89 | |||
| TR present (%) | |||||
| Moderate/severe | 85 | 20 | |||
| None/mild | 15 | 80 | |||
| Residual lesions | 0 | 0 |
| . | Before PVR . | Early after PVR . | Late follow-up . | Δ Post-late follow-up . | P-value, post-late follow-up . |
|---|---|---|---|---|---|
| CMR | n = 25 | n = 25 | n = 19 | ||
| Time, CMR from PVR (months) | −4.5 (−7 to −3) | 10 (8–16) | 130 (86–196) | ||
| PR fraction (%)a | 46 ± 11 | 3.7 ± 6.8 | 8 ± 9 | 4 ± 10 | 0.028 |
| RV | |||||
| EDV (ml)a | 302 ± 60 | 207 ± 69 | 212 ± 67 | −6 ± 30 | 0.46 |
| EDV-I (ml/m²) | 163 ± 33 | 115 ± 40 | 111 ± 35 | −9 ± 17 | 0.09 |
| ESV (ml)a | 177 ± 53 | 120 ± 65 | 115 ± 59 | −14 ± 34 | 0.3 |
| ESV-I (ml/m²) | 97 ± 36 | 67 ± 41 | 60 ± 32 | −11 ± 20 | 0.06 |
| EF (%)a | 43 ± 11 | 44 ± 11 | 46 ± 10 | 2 ± 7 | 0.47 |
| LV | |||||
| EDV (ml)a | 145 ± 40 | 152 ± 34 | 156 ± 44 | 5 ± 34 | 0.53 |
| EDV-I (ml/m²) | 80 ± 23 | 83 ± 15 | 85 ± 17 | −1 ± 19 | 0.8 |
| ESV (ml)a | 64 ± 32 | 68 ± 19 | 76 ± 26 | 6 ± 19 | 0.41 |
| ESV-I (ml/m²) | 36 ± 18 | 37 ± 9 | 41 ± 12 | 1 ± 10 | 0.9 |
| EF (%)a | 55 ± 14 | 56 ± 8 | 55 ± 7 | −1 ± 8 | 0.94 |
| NYHA class | 3.0 ± 0.2 | 1.3 ± 0.5 | 1.1 ± 0.3 | ||
| Echo | n = 25 | n = 25 | |||
| Time, echo from PVR (months) | −10 (−11.5 to −3.5) | 187 (183–207) | |||
| PV maximum gradient (mmHg) | 27 ± 26 | 19 ± 13 | |||
| PR present (%) | |||||
| Severe (Grade 4) | 66 | 0 | |||
| Moderate–severe (Grade 3) | 31 | 0 | |||
| Moderate (Grade 2) | 0 | 8 | |||
| Mild (Grade 1) | 0 | 89 | |||
| TR present (%) | |||||
| Moderate/severe | 85 | 20 | |||
| None/mild | 15 | 80 | |||
| Residual lesions | 0 | 0 |
Data were described as median with interquartile range and mean with standard deviation. Δ Post-late follow-up, change between early after PVR and late follow-up; P-value, significance level between early and late follow-up. The bold significance of P-value is <0.05.
Was not measured in all patients.
CMR: cardiovascular magnetic resonance; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; I: indexed for body surface area; LV: left ventricle; NYHA: New York Heart Association; PR: pulmonary regurgitation; PR: pulmonary regurgitation; PVR: pulmonary valve replacement; RV: right ventricle; TR: tricuspid regurgitation.
| . | Before PVR . | Early after PVR . | Late follow-up . | Δ Post-late follow-up . | P-value, post-late follow-up . |
|---|---|---|---|---|---|
| CMR | n = 25 | n = 25 | n = 19 | ||
| Time, CMR from PVR (months) | −4.5 (−7 to −3) | 10 (8–16) | 130 (86–196) | ||
| PR fraction (%)a | 46 ± 11 | 3.7 ± 6.8 | 8 ± 9 | 4 ± 10 | 0.028 |
| RV | |||||
| EDV (ml)a | 302 ± 60 | 207 ± 69 | 212 ± 67 | −6 ± 30 | 0.46 |
| EDV-I (ml/m²) | 163 ± 33 | 115 ± 40 | 111 ± 35 | −9 ± 17 | 0.09 |
| ESV (ml)a | 177 ± 53 | 120 ± 65 | 115 ± 59 | −14 ± 34 | 0.3 |
| ESV-I (ml/m²) | 97 ± 36 | 67 ± 41 | 60 ± 32 | −11 ± 20 | 0.06 |
| EF (%)a | 43 ± 11 | 44 ± 11 | 46 ± 10 | 2 ± 7 | 0.47 |
| LV | |||||
| EDV (ml)a | 145 ± 40 | 152 ± 34 | 156 ± 44 | 5 ± 34 | 0.53 |
| EDV-I (ml/m²) | 80 ± 23 | 83 ± 15 | 85 ± 17 | −1 ± 19 | 0.8 |
| ESV (ml)a | 64 ± 32 | 68 ± 19 | 76 ± 26 | 6 ± 19 | 0.41 |
| ESV-I (ml/m²) | 36 ± 18 | 37 ± 9 | 41 ± 12 | 1 ± 10 | 0.9 |
| EF (%)a | 55 ± 14 | 56 ± 8 | 55 ± 7 | −1 ± 8 | 0.94 |
| NYHA class | 3.0 ± 0.2 | 1.3 ± 0.5 | 1.1 ± 0.3 | ||
| Echo | n = 25 | n = 25 | |||
| Time, echo from PVR (months) | −10 (−11.5 to −3.5) | 187 (183–207) | |||
| PV maximum gradient (mmHg) | 27 ± 26 | 19 ± 13 | |||
| PR present (%) | |||||
| Severe (Grade 4) | 66 | 0 | |||
| Moderate–severe (Grade 3) | 31 | 0 | |||
| Moderate (Grade 2) | 0 | 8 | |||
| Mild (Grade 1) | 0 | 89 | |||
| TR present (%) | |||||
| Moderate/severe | 85 | 20 | |||
| None/mild | 15 | 80 | |||
| Residual lesions | 0 | 0 |
| . | Before PVR . | Early after PVR . | Late follow-up . | Δ Post-late follow-up . | P-value, post-late follow-up . |
|---|---|---|---|---|---|
| CMR | n = 25 | n = 25 | n = 19 | ||
| Time, CMR from PVR (months) | −4.5 (−7 to −3) | 10 (8–16) | 130 (86–196) | ||
| PR fraction (%)a | 46 ± 11 | 3.7 ± 6.8 | 8 ± 9 | 4 ± 10 | 0.028 |
| RV | |||||
| EDV (ml)a | 302 ± 60 | 207 ± 69 | 212 ± 67 | −6 ± 30 | 0.46 |
| EDV-I (ml/m²) | 163 ± 33 | 115 ± 40 | 111 ± 35 | −9 ± 17 | 0.09 |
| ESV (ml)a | 177 ± 53 | 120 ± 65 | 115 ± 59 | −14 ± 34 | 0.3 |
| ESV-I (ml/m²) | 97 ± 36 | 67 ± 41 | 60 ± 32 | −11 ± 20 | 0.06 |
| EF (%)a | 43 ± 11 | 44 ± 11 | 46 ± 10 | 2 ± 7 | 0.47 |
| LV | |||||
| EDV (ml)a | 145 ± 40 | 152 ± 34 | 156 ± 44 | 5 ± 34 | 0.53 |
| EDV-I (ml/m²) | 80 ± 23 | 83 ± 15 | 85 ± 17 | −1 ± 19 | 0.8 |
| ESV (ml)a | 64 ± 32 | 68 ± 19 | 76 ± 26 | 6 ± 19 | 0.41 |
| ESV-I (ml/m²) | 36 ± 18 | 37 ± 9 | 41 ± 12 | 1 ± 10 | 0.9 |
| EF (%)a | 55 ± 14 | 56 ± 8 | 55 ± 7 | −1 ± 8 | 0.94 |
| NYHA class | 3.0 ± 0.2 | 1.3 ± 0.5 | 1.1 ± 0.3 | ||
| Echo | n = 25 | n = 25 | |||
| Time, echo from PVR (months) | −10 (−11.5 to −3.5) | 187 (183–207) | |||
| PV maximum gradient (mmHg) | 27 ± 26 | 19 ± 13 | |||
| PR present (%) | |||||
| Severe (Grade 4) | 66 | 0 | |||
| Moderate–severe (Grade 3) | 31 | 0 | |||
| Moderate (Grade 2) | 0 | 8 | |||
| Mild (Grade 1) | 0 | 89 | |||
| TR present (%) | |||||
| Moderate/severe | 85 | 20 | |||
| None/mild | 15 | 80 | |||
| Residual lesions | 0 | 0 |
Data were described as median with interquartile range and mean with standard deviation. Δ Post-late follow-up, change between early after PVR and late follow-up; P-value, significance level between early and late follow-up. The bold significance of P-value is <0.05.
Was not measured in all patients.
CMR: cardiovascular magnetic resonance; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; I: indexed for body surface area; LV: left ventricle; NYHA: New York Heart Association; PR: pulmonary regurgitation; PR: pulmonary regurgitation; PVR: pulmonary valve replacement; RV: right ventricle; TR: tricuspid regurgitation.
| Total follow-up (years) | 17 (1.1) |
| Deaths | 1 (3.8) |
| Arrhythmias | 12 (46.2) |
| Ablation performed | 6 (23.1) |
| ICDs | 6 (23.1) |
| Age at ICD insertion (years) | 41 (36–47) |
| Time from PVR to ICD insertion (years) | 7.1 (4–11) |
| ICD shocks | 2 (7.7) |
| Redo PVR (%) | 2 (8) |
| Time from PVR to redo PVR (months) (minimum–maximum) | 32 (24–39) |
| Total follow-up (years) | 17 (1.1) |
| Deaths | 1 (3.8) |
| Arrhythmias | 12 (46.2) |
| Ablation performed | 6 (23.1) |
| ICDs | 6 (23.1) |
| Age at ICD insertion (years) | 41 (36–47) |
| Time from PVR to ICD insertion (years) | 7.1 (4–11) |
| ICD shocks | 2 (7.7) |
| Redo PVR (%) | 2 (8) |
| Time from PVR to redo PVR (months) (minimum–maximum) | 32 (24–39) |
Data are expressed as number of patients (%), mean with SD, median with IQR and mean with minimum and maximum.
ICD: implantable cardioverter defibrillator; IQR: interquartile range; PVR: pulmonary valve replacement; SD: standard deviation.
| Total follow-up (years) | 17 (1.1) |
| Deaths | 1 (3.8) |
| Arrhythmias | 12 (46.2) |
| Ablation performed | 6 (23.1) |
| ICDs | 6 (23.1) |
| Age at ICD insertion (years) | 41 (36–47) |
| Time from PVR to ICD insertion (years) | 7.1 (4–11) |
| ICD shocks | 2 (7.7) |
| Redo PVR (%) | 2 (8) |
| Time from PVR to redo PVR (months) (minimum–maximum) | 32 (24–39) |
| Total follow-up (years) | 17 (1.1) |
| Deaths | 1 (3.8) |
| Arrhythmias | 12 (46.2) |
| Ablation performed | 6 (23.1) |
| ICDs | 6 (23.1) |
| Age at ICD insertion (years) | 41 (36–47) |
| Time from PVR to ICD insertion (years) | 7.1 (4–11) |
| ICD shocks | 2 (7.7) |
| Redo PVR (%) | 2 (8) |
| Time from PVR to redo PVR (months) (minimum–maximum) | 32 (24–39) |
Data are expressed as number of patients (%), mean with SD, median with IQR and mean with minimum and maximum.
ICD: implantable cardioverter defibrillator; IQR: interquartile range; PVR: pulmonary valve replacement; SD: standard deviation.
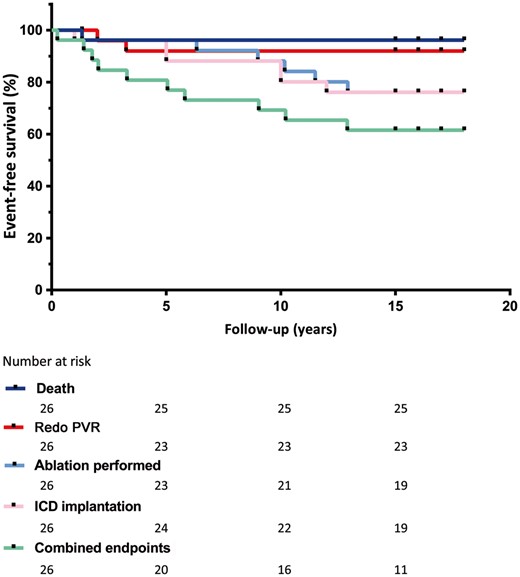
Event-free survival after PVR. Patient who died was excluded from the analysis. ICD: implantable cardioverter defibrillator; PVR: pulmonary valve replacement.
DISCUSSION
In this update of a previous study [7], we evaluated the long-term haemodynamic and clinical outcomes of previously corrected patients with TOF. The present study demonstrates a stabilization of RV function and impressive durability of the pulmonary homograft. Furthermore, there was good event-free survival.
PVR has been advocated to reduce RV volume overload, thereby improving RV performance and decreasing the risk of sudden death and ventricular failure. One patient who needed redo PVR had relatively early severe homograft regurgitation in combination with serious RV dilatation. There is a vicious circle between PR, RV dilatation and tricuspid regurgitation that results in deterioration of RV function and the rapid development of symptoms [8, 9]. One of the mechanisms underlying PR of the homograft is this dilatation of the RV. It has been suggested that including resection of a RV outflow tract aneurysm during pulmonary valve surgery will result in improved RV mechanics [10]. In this patient, no additional RV resection was done during the initial PVR operation. In retrospect, it might have been better to include RV outflow tract reconstruction at the time of the PVR. In later patients, this procedure became the routine in our centre. In contrast, Geva et al. [11] performed a randomized controlled trial and concluded that there was no benefit from RV remodelling. However, the postoperative evaluation was done 6 months after surgery, which could be too early to detect changes.
Timing of the pulmonary valve replacement
We recognize that the indications for PVR are a matter of debate, especially in patients who are asymptomatic. RV deterioration can be irreversible if PVR is performed too late [4, 12]. A recent study from our group stated that preoperative RV end-systolic volume is superior to RV end-diastolic volume and RV ejection fraction in predicting mid-to-late RV normalization. In the same study, half of the patients operated on with preoperative RV end-systolic volume >95 ml/m2 had a suboptimal mid-to-late outcome, whereas in patients operated on when preoperative RV end-systolic volume was 80–95 ml/m2, a suboptimal mid-to-late outcome was unlikely. The present study demonstrates reverse remodelling to some extent, especially in the early stages after the PVR. In the longer term, there is stabilization of the remodelling. This result underlines the fact that intensive monitoring of this patient group is necessary to detect sudden deterioration and prevent the ‘point of no return’.
Durability of the homograft
In our group, there was excellent durability of the homograft with an 88% freedom from redo PVR or death after a mean of 17 years (Fig. 1). In addition, none of the patients met the criteria of echocardiographically measured moderate to severe PR at the latest follow-up visit. In the literature, long-term (>10 years) analysis is lacking [13]. The 10-year freedom from redo PVR in other reports ranges from 69% to 85% [13–16]. An explanation for the varying numbers in previous studies could be that the other groups comprise a mix of adults and children. Children are susceptible to earlier reoperation due to increased somatic growth and accelerated valve degeneration.
Event-free survival
Event-free survival (no deaths, ablation, ICDs or redo PVR in the overall group) was 61.5% after 18 years. Although long-term results for PVR are good, this finding is not surprising because a substantial number of patients still have adverse events during the follow-up period. Almost half of the patients (46.2%) developed arrhythmias. Gengsakul et al. [17] and Harrild et al. [18] found no significant improvement in the frequency of arrhythmias after PVR whereas Lee et al. [19] and Therrien et al. [20] reported a reduction, in combination with antiarrhythmic surgery during PVR. The latter is done more frequently nowadays.
Limitations
The current study is retrospective; the results are influenced by this study design, in terms of missing data, and there was no structured follow-up protocol. Therefore, the MRIs, echocardiograms and clinical parameters are not measured on exactly the same time points. In terms of selection bias, the patients who had available MRI data may have had a better overall outcome because they did not receive an ICD. The small sample size is another limitation of our study. However, the study comprises a group that was defined 17 years ago and has been followed for 17 years.
CONCLUSION
In 2002, Vliegen et al. [7] described a remarkable early postoperative haemodynamic improvement after PVR in patients with TOF who had a total correction. This follow-up study of the predefined group showed at 17.1 years of follow-up stabilization of RV function and impressive functional durability of the graft. Event-free survival was good (61.5%).
ACKNOWLEDGEMENTS
The authors thank all participating patients.
Funding
The Department of Cardiology receives unrestricted grants from Biotronik (Berlin, Germany), Boston Scientific (Natick, Massachusetts) and Medtronic (Minneapolis, Minnesota).
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 67th Annual Scientific Session & Expo of the American College of Cardiology, Orlando, 11 March 2018.





