-
PDF
- Split View
-
Views
-
Cite
Cite
Grzegorz Suwalski, Piotr Suwalski, Successful surgical ablation of atrial fibrillation does not disturb long-term sinus rhythm variability, Interactive CardioVascular and Thoracic Surgery, Volume 27, Issue 4, October 2018, Pages 520–524, https://doi.org/10.1093/icvts/ivy117
Close - Share Icon Share
Abstract
Surgical ablation of atrial fibrillation (AF) aims to restore normal sinus rhythm while protecting the sinus node from surgical damage. Surgical lesions may affect autonomic neural structures and influence physiological heart rate variability (HRV). The primary aim of this study was to describe long-term dynamics of HRV after successful surgical ablation of AF. The secondary aim was to compare sinus node function after successful ablation of AF with either left atrial modified Maze procedure or epicardial pulmonary vein isolation after long-term follow-up.
This retrospective study included 75 patients who underwent successful ablation of paroxysmal or persistent AF (53 patients, 71%) and long-standing persistent AF (22 patients, 29%). Standard variables were selected to describe HRV and the minimal and mean heart rates. In all patients, a 24-h Holter ECG study was performed preoperatively, at hospital discharge and after 3, 6, 12 and 24 months.
A significant reduction in the main HRV parameters and an increase in heart rate were observed at discharge when compared with the preoperative period. During follow-up, all HRV parameters returned to preoperative levels. No significant differences were observed in HRV parameters and HR between the modified Maze and pulmonary vein isolation procedure groups at any time.
Successful surgical ablation of paroxysmal, persistent and long-standing persistent AF using either pulmonary vein isolation or modified left atrial Maze procedure does not disrupt long-term HRV. A significant early postoperative reduction in HRV with a gradual increase in the following months is typical in patients after surgical restoration of sinus rhythm.
INTRODUCTION
Atrial fibrillation (AF) dramatically increases the risk of stroke and premature mortality [1]. Multiple surgical ablation techniques have proven their efficacy in terminating AF. In patients with long-standing persistent AF, excellent results are achieved using the modified Cox-Maze III procedure. However, this endocardial procedure requires an arrested heart technique. In patients with paroxysmal AF and those with persistent AF undergoing off-pump procedures, less invasive pulmonary vein isolation (PVI) may be executed. Performing concomitant surgical ablation is recommended in patients referred to cardiac surgery. Recently, surgical minimally invasive ablation techniques have also been recommended in patients with stand-alone AF [2].
The primary aim of surgical ablation is to restore normal sinus rhythm (SR). Restoration of normal SR reduces the risk of heart failure, arrhythmias and stroke [3]. Maintenance of physiological sinus node (SN) function after ablation is fundamental to gain the clinical benefits of the procedure. Both endocardial and epicardial surgical ablation techniques are designed to protect the SN from surgical damage. However, surgical lesions may affect both intracardiac and extracardiac autonomic neural structures [4]. Ablation procedures may, therefore, influence the physiological automaticity of the SN and heart rate variability (HRV). Only limited data are published in relation to the long-term SN function and HRV dynamics in patients following successful restoration of SR with surgical ablation.
The primary aim of this study was to describe the long-term dynamics of selected SN function parameters after successful surgical termination of AF. The secondary aim was to compare SN function after successful ablation with either left atrial Maze procedure or PVI during long-term follow-up.
METHODS
From the 250 patients who underwent surgical ablation of AF in our centre between 2004 and 2008, 75 patients met inclusion criteria and were retrospectively included in this study. Inclusion criteria were left atrial modified Maze III procedure performed in patients with long-standing AF, PVI performed in patients in SR with paroxysmal or persistent AF, SR confirmed by a 24-h Holter ECG at hospital discharge and after 3, 6, 12 and 24 months postoperatively, having no preoperative transcatheter ablation of AF, no postoperative pacemaker implantation and no postoperative oral antiarrhythmic therapy including amiodarone, sotalol and propaphenon.
Preoperatively, 53 (71%) patients had paroxysmal or persistent AF, and all these patients were in SR at hospital admission. In 22 (29%) patients, long-standing persistent AF was diagnosed. In patients with paroxysmal AF, the mean time since the onset of symptoms was 5 ± 6 years. Preoperatively, all patients were taking a beta-blocker. No patients took amiodarone or sotalol at the time of surgery. Seven (9%) patients were on propaphenon until surgery. Detailed population characteristics and data of the surgical procedures are presented in Tables 1 and 2.
| Parameters . | Mean value or number of patients (%) . |
|---|---|
| Age (years) | 64.2 ± 10 |
| Females | 28 (37) |
| Arterial hypertension | 43 (57) |
| Diabetes | 17 (23) |
| Ischaemic heart disease | 38 (51) |
| LVEF (%) | 57 ± 9 |
| NYHA class | 2 (1–3) |
| CCS class | 3 (1–3) |
| Long-standing persistent AF | 22 (29) |
| Paroxysmal or persistent AF | 53 (71) |
| AF duration (months) | 5 ± 6 |
| Preoperative LA diameter (mm) | 56 ± 13 |
| Logistic EuroSCORE I (%) | 3.9 ± 3 |
| Parameters . | Mean value or number of patients (%) . |
|---|---|
| Age (years) | 64.2 ± 10 |
| Females | 28 (37) |
| Arterial hypertension | 43 (57) |
| Diabetes | 17 (23) |
| Ischaemic heart disease | 38 (51) |
| LVEF (%) | 57 ± 9 |
| NYHA class | 2 (1–3) |
| CCS class | 3 (1–3) |
| Long-standing persistent AF | 22 (29) |
| Paroxysmal or persistent AF | 53 (71) |
| AF duration (months) | 5 ± 6 |
| Preoperative LA diameter (mm) | 56 ± 13 |
| Logistic EuroSCORE I (%) | 3.9 ± 3 |
AF: atrial fibrillation; CCS: canadian coronary score; LA: left atrium; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
| Parameters . | Mean value or number of patients (%) . |
|---|---|
| Age (years) | 64.2 ± 10 |
| Females | 28 (37) |
| Arterial hypertension | 43 (57) |
| Diabetes | 17 (23) |
| Ischaemic heart disease | 38 (51) |
| LVEF (%) | 57 ± 9 |
| NYHA class | 2 (1–3) |
| CCS class | 3 (1–3) |
| Long-standing persistent AF | 22 (29) |
| Paroxysmal or persistent AF | 53 (71) |
| AF duration (months) | 5 ± 6 |
| Preoperative LA diameter (mm) | 56 ± 13 |
| Logistic EuroSCORE I (%) | 3.9 ± 3 |
| Parameters . | Mean value or number of patients (%) . |
|---|---|
| Age (years) | 64.2 ± 10 |
| Females | 28 (37) |
| Arterial hypertension | 43 (57) |
| Diabetes | 17 (23) |
| Ischaemic heart disease | 38 (51) |
| LVEF (%) | 57 ± 9 |
| NYHA class | 2 (1–3) |
| CCS class | 3 (1–3) |
| Long-standing persistent AF | 22 (29) |
| Paroxysmal or persistent AF | 53 (71) |
| AF duration (months) | 5 ± 6 |
| Preoperative LA diameter (mm) | 56 ± 13 |
| Logistic EuroSCORE I (%) | 3.9 ± 3 |
AF: atrial fibrillation; CCS: canadian coronary score; LA: left atrium; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
| Parameters . | Modified Maze procedure group (n = 22) . | PVI procedure group (n = 53) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 63.8 ± 11 | 64.7 ± 9 | 0.6 |
| AF duration (years), mean ± SD | 5.4 ± 5 | 4.6 ± 5 | 0.5 |
| Preoperative LA diameter (mm), mean ± SD | 60 ± 7 | 56 ± 7 | 0.03 |
| LVEF (%), mean ± SD | 55 ± 10 | 58 ± 7 | 0.3 |
| NYHA class | 3 (2–4) | 2 (1–3) | 0.06 |
| CCS class | 3 (1–3) | 2 (1–3) | 0.1 |
| EuroSCORE logistic (%), mean ± SD | 4.1 ± 2.5 | 4 ± 3.2 | 0.8 |
| Parameters . | Modified Maze procedure group (n = 22) . | PVI procedure group (n = 53) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 63.8 ± 11 | 64.7 ± 9 | 0.6 |
| AF duration (years), mean ± SD | 5.4 ± 5 | 4.6 ± 5 | 0.5 |
| Preoperative LA diameter (mm), mean ± SD | 60 ± 7 | 56 ± 7 | 0.03 |
| LVEF (%), mean ± SD | 55 ± 10 | 58 ± 7 | 0.3 |
| NYHA class | 3 (2–4) | 2 (1–3) | 0.06 |
| CCS class | 3 (1–3) | 2 (1–3) | 0.1 |
| EuroSCORE logistic (%), mean ± SD | 4.1 ± 2.5 | 4 ± 3.2 | 0.8 |
AF: atrial fibrillation; CCS: canadian coronary score; LA: left atrium; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
| Parameters . | Modified Maze procedure group (n = 22) . | PVI procedure group (n = 53) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 63.8 ± 11 | 64.7 ± 9 | 0.6 |
| AF duration (years), mean ± SD | 5.4 ± 5 | 4.6 ± 5 | 0.5 |
| Preoperative LA diameter (mm), mean ± SD | 60 ± 7 | 56 ± 7 | 0.03 |
| LVEF (%), mean ± SD | 55 ± 10 | 58 ± 7 | 0.3 |
| NYHA class | 3 (2–4) | 2 (1–3) | 0.06 |
| CCS class | 3 (1–3) | 2 (1–3) | 0.1 |
| EuroSCORE logistic (%), mean ± SD | 4.1 ± 2.5 | 4 ± 3.2 | 0.8 |
| Parameters . | Modified Maze procedure group (n = 22) . | PVI procedure group (n = 53) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 63.8 ± 11 | 64.7 ± 9 | 0.6 |
| AF duration (years), mean ± SD | 5.4 ± 5 | 4.6 ± 5 | 0.5 |
| Preoperative LA diameter (mm), mean ± SD | 60 ± 7 | 56 ± 7 | 0.03 |
| LVEF (%), mean ± SD | 55 ± 10 | 58 ± 7 | 0.3 |
| NYHA class | 3 (2–4) | 2 (1–3) | 0.06 |
| CCS class | 3 (1–3) | 2 (1–3) | 0.1 |
| EuroSCORE logistic (%), mean ± SD | 4.1 ± 2.5 | 4 ± 3.2 | 0.8 |
AF: atrial fibrillation; CCS: canadian coronary score; LA: left atrium; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
Concomitant ablation was performed in 62 (83%) patients. Minimally invasive ablation of stand-alone AF was performed in 13 (7%) patients. Concomitant ablation was performed in patients operated through a median sternotomy. Minimally invasive procedures were performed through bilateral minithoracotomy supported with videoscopy. All patients with long-standing persistent AF underwent a modified left atrial Maze procedure. The ablation lesions that were created included PVI, connecting line in the roof of the left atrium, the line from the left inferior pulmonary vein to the mitral annulus. In those patients, ablation was performed endocardially using unipolar radiofrequency (Cardioblate, Medtronic, MN, USA) or cryothermy (Kriomedpol, Poland). All patients with paroxysmal and persistent AF were in the SR before surgery. The primary surgical procedure in that cohort did not require atriotomy. In all patients in that group, epicardial PVI with a bipolar radiofrequency device (Cardioblate, Medtronic) was performed. At each site, epicardial bipolar energy was delivered 4 times. After 2 initial applications, the probe was repositioned, and another 2 energy applications were done. An ablation protocol was standard at our centre during the study period. Assessment of bidirectional conduction block through the lesions created was not available during that time. In all patients included to the study, the left atrial appendage was left open.
During hospital stay, amiodarone, sotalol or propaphenon were not administered in patients. Beta-blockers were prescribed during the postoperative period to maintain a minimal heart rate (HR) >50 beats per minute (bpm) and maximal HR of <100 bpm.
In all patients, 24-h ECG studies were performed preoperatively (the paroxysmal and persistent AF group), at discharge and 3, 6, 12 and 24 months after surgery. At each time point, 24-h Holter electrocardiography was assessed using a 3-channel digital scanner (Aspect 702, Aspel, Poland). Electrocardiogram was digitally sampled at a 512- or 1025-Hz Inter-beat (RR) interval time series and were analysed from 1—the best quality—channel if the percentage of artefacts was less than 5. The following variables were selected to describe HRV: NN interval (HR), standard deviation of NN intervals (SDNN), standard deviation of averaged NN intervals (SDANN), root mean square of successive differences (rMSSD) and percentage of differences between adjacent intervals greater than 50% (p50NN). To achieve the aim of the study, HR and HRV parameters were compared between the groups.
The study population was divided into 2 groups. The 1st group consisted of patients with long-standing AF who remained constantly in AF for at least in the last 12 months before admission and were in AF on the day of surgery; in this group, the left atrial modified Maze procedure was performed. The 2nd group included patients with paroxysmal or persistent AF who were in SR on admission; in this group, PVI was performed. Comparison of the study groups is presented in Table 3.
| Primary surgical procedures . | Modified Maze procedure group (n = 22), n (%) . | PVI procedure group (n = 53), n (%) . |
|---|---|---|
| OPCAB | 0 (0) | 20 (38) |
| AVR | 0 (0) | 11 (21) |
| Minimally invasive ablation | 1 (5) | 12 (23) |
| MVR | 8 (36) | 0 (0) |
| AVR and CABG | 3 (14) | 6 (11) |
| On-pump CABG | 4 (18) | 4 (6) |
| MVR and TVR | 3 (14) | 0 (0) |
| MVR, AVR and TVR | 2 (9) | 0 (0) |
| AVR, MVR and CABG | 1 (5) | 0 (0) |
| Primary surgical procedures . | Modified Maze procedure group (n = 22), n (%) . | PVI procedure group (n = 53), n (%) . |
|---|---|---|
| OPCAB | 0 (0) | 20 (38) |
| AVR | 0 (0) | 11 (21) |
| Minimally invasive ablation | 1 (5) | 12 (23) |
| MVR | 8 (36) | 0 (0) |
| AVR and CABG | 3 (14) | 6 (11) |
| On-pump CABG | 4 (18) | 4 (6) |
| MVR and TVR | 3 (14) | 0 (0) |
| MVR, AVR and TVR | 2 (9) | 0 (0) |
| AVR, MVR and CABG | 1 (5) | 0 (0) |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; MVR: mitral valve replacement or repair; OPCAB: off-pump coronary artery bypass grafting; TVR: tricuspid valve repair.
| Primary surgical procedures . | Modified Maze procedure group (n = 22), n (%) . | PVI procedure group (n = 53), n (%) . |
|---|---|---|
| OPCAB | 0 (0) | 20 (38) |
| AVR | 0 (0) | 11 (21) |
| Minimally invasive ablation | 1 (5) | 12 (23) |
| MVR | 8 (36) | 0 (0) |
| AVR and CABG | 3 (14) | 6 (11) |
| On-pump CABG | 4 (18) | 4 (6) |
| MVR and TVR | 3 (14) | 0 (0) |
| MVR, AVR and TVR | 2 (9) | 0 (0) |
| AVR, MVR and CABG | 1 (5) | 0 (0) |
| Primary surgical procedures . | Modified Maze procedure group (n = 22), n (%) . | PVI procedure group (n = 53), n (%) . |
|---|---|---|
| OPCAB | 0 (0) | 20 (38) |
| AVR | 0 (0) | 11 (21) |
| Minimally invasive ablation | 1 (5) | 12 (23) |
| MVR | 8 (36) | 0 (0) |
| AVR and CABG | 3 (14) | 6 (11) |
| On-pump CABG | 4 (18) | 4 (6) |
| MVR and TVR | 3 (14) | 0 (0) |
| MVR, AVR and TVR | 2 (9) | 0 (0) |
| AVR, MVR and CABG | 1 (5) | 0 (0) |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; MVR: mitral valve replacement or repair; OPCAB: off-pump coronary artery bypass grafting; TVR: tricuspid valve repair.
This study was approved by the local ethics committee, and all patients received and signed informed consent.
Statistical analysis
Statistical analysis was performed using Statistica™ (StatSoft™, Inc. 2014, version 12). The Shapiro–Wilk W-test was used for testing normality. If the W statistic was significant, the hypothesis that the respective distribution was normal was rejected. Normally distributed continuous variables are expressed as mean ± standard deviation. Non-normally distributed variables are expressed as median and range. Longitudinal analyses of dependent sample data were analysed using the paired t-test. Comparison between groups was performed using the Student’s t-test for normally distributed continuous variables and the Mann–Whitney test for non-normally distributed continuous variables. Differences were considered statistically significant if P-value <0.05.
RESULTS
Longitudinal analysis of heart rate variability parameters
The analysis of changes in HRV parameters between preoperative and discharge studies was performed in patients in SR before surgery (53 patients). Following discharge, after 3, 6, 12 and 24 months, all patients (75) were in SR. Further longitudinal analysis on HRV since discharge was performed in the entire group of 75 patients.
In patients in SR before surgery, a significant increase in mean HR was observed between preoperative (66 ± 8.7 bpm) and discharge studies [80 ± 11 bpm; P < 0.001; 95% confidence interval (CI) 10.8–18.6]. In the whole study population, after 3, 6, 12 and 24 months, mean HR was 71 ± 9, 62 ± 9, 69 ± 9 and 66 ± 5 bpm, respectively. A significant decrease in mean HR was observed between discharge and after 3 months (P < 0.001; 95% CI −14 to −8). No significant changes in mean HR were observed between 3 and 6 months (P = 0.2; 95% CI −4 to 0.8) and between 6 and 12 months (P = 0.9; 95% CI −2.4 to 2.2). A significant decline in mean HR was observed between 12 and 24 months (P = 0.001; 95% CI −4.9 to −1.3).
In patients in SR before surgery, a significant increase in minimal HR was observed between preoperative (mean HR 49 ± 5 bpm) and discharge studies (mean HR 63 ± 18 bpm, P < 0.001; 95% CI 9.2–19.4). In the whole study population, after 3, 6, 12 and 24 months, the minimal HR was 55 ± 8, 52 ± 7, 52 ± 7 and 53 ± 7 bpm, respectively. A significant decrease in minimal HR was observed between discharge and after 3 months (P < 0.001; 95% CI −14 to −8) and between 3 and 6 months (P < 0.001; 95% CI −12 to −5). No significant changes were observed between 6 and 12 months (P = 0.9; 95% CI −1.4 to 1.3) and between 12 and 24 months (P = 0.3; 95% CI −1.1 to 2.8).
In patients in SR before surgery, a significant decrease in SDNN was observed between preoperative (117 ± 33 ms) and discharge studies (72 ± 35 ms; P < 0.001; 95% CI −57 to −32). In the whole study population, after 3, 6, 12 and 24 months, mean SDNN was 105 ± 31, 112 ± 30, 121 ± 47 and 118 ± 23 ms, respectively. A significant increase in SDDN was observed between discharge and after 3 months (P < 0.001; 95% CI 25–42). No significant changes in SDNN were observed between 3 and 6 months (P = 0.08; 95% CI −1.1 to 16), 6 and 12 months (P = 0.08; 95% CI −1.2 to 19) and between 12 and 24 months (P = 0.6; 95% CI −14 to 8).
In patients in SR before surgery, a significant decrease in SDANN was observed between preoperative (91 ± 20 ms) and discharge studies (57 ± 25 ms; P < 0.001; 95% CI −42 to −26). In the whole study population, after 3, 6, 12 and 24 months, mean SDANN was 82 ± 21, 89 ± 24, 95 ± 29 and 96 ± 17 ms, respectively. A significant increase in SDANN was observed between discharge and 3 months (P < 0.001; 95% CI 21–33) and between 3 and 6 months (P = 0.04; 95% CI 0.1–14). No significant changes in SDNN were observed between 6 and 12 months (P = 0.08; 95% CI −0.8 to 12) and between 12 and 24 months (P = 0.7; 95% CI −5 to 8).
In patients in SR before surgery, mean rMSSD was 61 ± 32 ms, and mean rMSSD at discharge was 53 ± 50 ms (P = 0.3; 95% CI −24 to 9). In the whole study population, after 3, 6, 12 and 24 months, mean rMSSD was 64 ± 42, 70 ± 52, 77 ± 62 and 63 ± 26 ms, respectively. No statistically significant change in rMSSD was observed between these time points.
In patients in SR before surgery, a significant decrease in p50NN was observed between preoperative (8.1 ± 6.1) and discharge studies (5 ± 5; P = 0.003; 95% CI −5.3 to −1.1). In the whole study population, after 3, 6, 12 and 24 months, mean p50NN was 9 ± 9, 6 ± 8, 9 ± 8 and 8 ± 7 ms, respectively. A significant increase in p50NN was observed between discharge and 3 months (P < 0.001; 95% CI 2–6). No significant changes in p50NN were observed between 3 and 6 months (P = 0.2; 95% CI −3 to 0.6), 6 and 12 months (P = 0.2; 95% CI −0.8 to 3) and 12 and 24 months (P = 0.5; 95% CI −3 to 1.6) (Fig. 1).
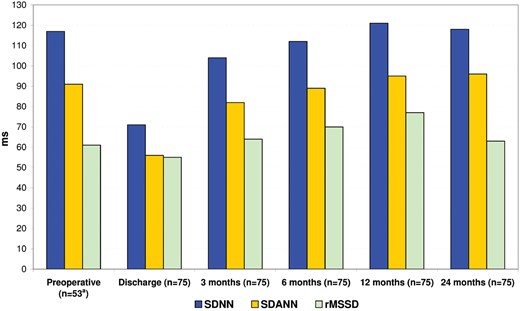
Dynamic changes of heart rate variability parameters in the whole study population. aData recorded on 53 patients in sinus rhythm before the surgery. rMSSD: root mean square of successive differences; SDANN: standard deviation of averaged NN-intervals; SDNN: standard deviation of NN-intervals.
Comparison of heart rate variability between the groups
HRV parameters were compared between the modified Maze group and PVI group. No significant differences in mean HR (bpm) were observed during the following time frames between the modified Maze group and PVI group, respectively: 84 ± 8 vs 80 ± 11 (P = 0.2) at discharge, 71 ± 4 vs 70.4 ± 10 (P = 0.8) after 3 months, 68 ± 8 vs 70 ± 10 (P = 0.5) after 6 months, 69 ± 9 vs 69 ± 8 (P = 0.8) after 12 months and 66 ± 5 vs 66 ± 5 (P = 0.9) after 24 months. No significant differences in minimal HR (bpm) were observed during the following time frames between the modified Maze group and PVI group, respectively: 64.2 ± 4 vs 63.4 ± 18 (P = 0.8) at discharge, 56.3 ± 6 vs 55 ± 9 (P = 0.5) after 3 months, 53.2 ± 6 vs 52 ± 7 (P = 0.4) after 6 months and 54 ± 6 vs 52 ± 7 (P = 0.1) after 12 months. After 24 months, minimal HR was significantly higher in patients following the modified Maze procedure (56 ± 11 bpm), when compared with patients following PVI (52 ± 4 bpm). During follow-up, no significant differences were observed between the groups for SDNN, SDANN, rMSSD and p50NN parameters. Only the mean p50NN parameter was significantly higher in the modified Maze group in comparison with the PVI group after 24 months (Table 4).
| HRV parameters . | Modified Maze group (mean ± SD) . | PVI group (mean ± SD) . | P-value . |
|---|---|---|---|
| SDNN at discharge (ms) | 67.7 ± 11 | 72.4 ± 35 | 0.5 |
| SDNN after 3 months (ms) | 103.9 ± 31 | 104.7 ± 32 | 0.9 |
| SDNN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDNN after 12 months (ms) | 124.4 ± 45 | 120 ± 47 | 0.7 |
| SDNN after 24 months (ms) | 115.1 ± 27 | 119.3 ± 21 | 0.5 |
| SDANN at discharge (ms) | 51 ± 10 | 57 ± 25 | 0.3 |
| SDANN after 3 months (ms) | 77.1 ± 11 | 85 ± 24 | 0.1 |
| SDANN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDANN after 12 months (ms) | 99 ± 33 | 93.4 ± 27 | 0.5 |
| SDANN after 24 months (ms) | 92 ± 16 | 98 ± 17 | 0.2 |
| rMSSD at discharge (ms) | 56 ± 17 | 55.3 ± 52 | 0.9 |
| rMSSD after 3 months (ms) | 70.4 ± 52 | 62 ± 37 | 0.4 |
| rMSSD after 6 months (ms) | 79 ± 46 | 66.3 ± 40 | 0.3 |
| rMSSD after 12 months (ms) | 80 ± 51 | 76 ± 66 | 0.8 |
| rMSSD after 24 months (ms) | 70 ± 39 | 60 ± 17 | 0.1 |
| p50NN at discharge (ms) | 5.2 ± 3.4 | 4.9 ± 5 | 0.8 |
| p50NN after 3 months (ms) | 9.5 ± 11 | 9 ± 7.7 | 0.7 |
| p50NN after 6 months (ms) | 8.8 ± 9.3 | 8 ± 8 | 0.5 |
| p50NN after 12 months (ms) | 10 ± 9 | 9 ± 8 | 0.6 |
| p50NN after 24 months (ms) | 11.3 ± 12 | 7 ± 2 | 0.01 |
| HRV parameters . | Modified Maze group (mean ± SD) . | PVI group (mean ± SD) . | P-value . |
|---|---|---|---|
| SDNN at discharge (ms) | 67.7 ± 11 | 72.4 ± 35 | 0.5 |
| SDNN after 3 months (ms) | 103.9 ± 31 | 104.7 ± 32 | 0.9 |
| SDNN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDNN after 12 months (ms) | 124.4 ± 45 | 120 ± 47 | 0.7 |
| SDNN after 24 months (ms) | 115.1 ± 27 | 119.3 ± 21 | 0.5 |
| SDANN at discharge (ms) | 51 ± 10 | 57 ± 25 | 0.3 |
| SDANN after 3 months (ms) | 77.1 ± 11 | 85 ± 24 | 0.1 |
| SDANN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDANN after 12 months (ms) | 99 ± 33 | 93.4 ± 27 | 0.5 |
| SDANN after 24 months (ms) | 92 ± 16 | 98 ± 17 | 0.2 |
| rMSSD at discharge (ms) | 56 ± 17 | 55.3 ± 52 | 0.9 |
| rMSSD after 3 months (ms) | 70.4 ± 52 | 62 ± 37 | 0.4 |
| rMSSD after 6 months (ms) | 79 ± 46 | 66.3 ± 40 | 0.3 |
| rMSSD after 12 months (ms) | 80 ± 51 | 76 ± 66 | 0.8 |
| rMSSD after 24 months (ms) | 70 ± 39 | 60 ± 17 | 0.1 |
| p50NN at discharge (ms) | 5.2 ± 3.4 | 4.9 ± 5 | 0.8 |
| p50NN after 3 months (ms) | 9.5 ± 11 | 9 ± 7.7 | 0.7 |
| p50NN after 6 months (ms) | 8.8 ± 9.3 | 8 ± 8 | 0.5 |
| p50NN after 12 months (ms) | 10 ± 9 | 9 ± 8 | 0.6 |
| p50NN after 24 months (ms) | 11.3 ± 12 | 7 ± 2 | 0.01 |
HRV: heart rate variability; p50NN: percentage of differences between adjacent intervals greater than 50%; rMSSD: root mean square of successive differences; SD: standard deviation; SDANN: standard deviation of averaged NN-intervals; SDNN: standard deviation of NN-intervals.
| HRV parameters . | Modified Maze group (mean ± SD) . | PVI group (mean ± SD) . | P-value . |
|---|---|---|---|
| SDNN at discharge (ms) | 67.7 ± 11 | 72.4 ± 35 | 0.5 |
| SDNN after 3 months (ms) | 103.9 ± 31 | 104.7 ± 32 | 0.9 |
| SDNN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDNN after 12 months (ms) | 124.4 ± 45 | 120 ± 47 | 0.7 |
| SDNN after 24 months (ms) | 115.1 ± 27 | 119.3 ± 21 | 0.5 |
| SDANN at discharge (ms) | 51 ± 10 | 57 ± 25 | 0.3 |
| SDANN after 3 months (ms) | 77.1 ± 11 | 85 ± 24 | 0.1 |
| SDANN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDANN after 12 months (ms) | 99 ± 33 | 93.4 ± 27 | 0.5 |
| SDANN after 24 months (ms) | 92 ± 16 | 98 ± 17 | 0.2 |
| rMSSD at discharge (ms) | 56 ± 17 | 55.3 ± 52 | 0.9 |
| rMSSD after 3 months (ms) | 70.4 ± 52 | 62 ± 37 | 0.4 |
| rMSSD after 6 months (ms) | 79 ± 46 | 66.3 ± 40 | 0.3 |
| rMSSD after 12 months (ms) | 80 ± 51 | 76 ± 66 | 0.8 |
| rMSSD after 24 months (ms) | 70 ± 39 | 60 ± 17 | 0.1 |
| p50NN at discharge (ms) | 5.2 ± 3.4 | 4.9 ± 5 | 0.8 |
| p50NN after 3 months (ms) | 9.5 ± 11 | 9 ± 7.7 | 0.7 |
| p50NN after 6 months (ms) | 8.8 ± 9.3 | 8 ± 8 | 0.5 |
| p50NN after 12 months (ms) | 10 ± 9 | 9 ± 8 | 0.6 |
| p50NN after 24 months (ms) | 11.3 ± 12 | 7 ± 2 | 0.01 |
| HRV parameters . | Modified Maze group (mean ± SD) . | PVI group (mean ± SD) . | P-value . |
|---|---|---|---|
| SDNN at discharge (ms) | 67.7 ± 11 | 72.4 ± 35 | 0.5 |
| SDNN after 3 months (ms) | 103.9 ± 31 | 104.7 ± 32 | 0.9 |
| SDNN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDNN after 12 months (ms) | 124.4 ± 45 | 120 ± 47 | 0.7 |
| SDNN after 24 months (ms) | 115.1 ± 27 | 119.3 ± 21 | 0.5 |
| SDANN at discharge (ms) | 51 ± 10 | 57 ± 25 | 0.3 |
| SDANN after 3 months (ms) | 77.1 ± 11 | 85 ± 24 | 0.1 |
| SDANN after 6 months (ms) | 87.5 ± 30 | 90 ± 22 | 0.7 |
| SDANN after 12 months (ms) | 99 ± 33 | 93.4 ± 27 | 0.5 |
| SDANN after 24 months (ms) | 92 ± 16 | 98 ± 17 | 0.2 |
| rMSSD at discharge (ms) | 56 ± 17 | 55.3 ± 52 | 0.9 |
| rMSSD after 3 months (ms) | 70.4 ± 52 | 62 ± 37 | 0.4 |
| rMSSD after 6 months (ms) | 79 ± 46 | 66.3 ± 40 | 0.3 |
| rMSSD after 12 months (ms) | 80 ± 51 | 76 ± 66 | 0.8 |
| rMSSD after 24 months (ms) | 70 ± 39 | 60 ± 17 | 0.1 |
| p50NN at discharge (ms) | 5.2 ± 3.4 | 4.9 ± 5 | 0.8 |
| p50NN after 3 months (ms) | 9.5 ± 11 | 9 ± 7.7 | 0.7 |
| p50NN after 6 months (ms) | 8.8 ± 9.3 | 8 ± 8 | 0.5 |
| p50NN after 12 months (ms) | 10 ± 9 | 9 ± 8 | 0.6 |
| p50NN after 24 months (ms) | 11.3 ± 12 | 7 ± 2 | 0.01 |
HRV: heart rate variability; p50NN: percentage of differences between adjacent intervals greater than 50%; rMSSD: root mean square of successive differences; SD: standard deviation; SDANN: standard deviation of averaged NN-intervals; SDNN: standard deviation of NN-intervals.
DISCUSSION
Surgical ablation of AF is a highly effective method for restoring SR, both as a concomitant procedure and stand-alone surgery [5, 6]. The pattern of surgical ablation lesions affects the primary mechanisms of AF: induction (triggering) and perpetuation (re-entry, rotors). Surgical PVI isolates triggers that are mainly located in the pulmonary vein. The more complex endocardial Maze procedure blocks triggering from the pulmonary vein, and by creating intra-atrial lesions, it blocks re-entry formation in other parts of atrial tissue. Surgical ablation may affect SN function via several mechanisms: direct surgical trauma of SN tissue, ischaemia due to injury to the arteries supplying the SN and necrosis induced by the ablation line. Moreover, intra-atrial ablation lesions may affect intrinsic cardiac pathways. PVI results in the partial autonomic denervation of the heart as the posterior wall of the left atrium is an entrance for many intrinsic nerves [7]. Surgical injury of the SN usually manifests immediately after the ablation procedure with bradycardia, sinus pauses or arrest, junctional rhythm, sinoatrial exit block or tachyarrhythmia. However, surgical modification of SN autonomic innervation may result in an HRV disorder. Few existing studies are published, which investigate how surgical ablation of AF affects long-term HRV after restoring SR [4].
This study showed that surgical ablation does not have a negative impact on HRV following long-term observations. When comparing HRV results with those observed in healthy adults and in the Framingham study population, we found no significant differences between these results in terms of preoperative and long-term parameters [8]. Several large studies revealed that reduced HRV is a strong predictor of cardiac death in the general population and in patients with heart disease [9, 10]. It has been known for decades that structural pathology of the SN often results in an increased HRV, which is mostly driven by differences in sinoatrial conduction time [11]. However, this was not observed in this study. This study confirmed that successful surgical termination of AF does not result in a significant reduction in SR variability, thus the benefit of restoring SR is not limited by a reduced HRV in these patients.
Another relevant finding of this study was that no differences in HRV dynamics were found in patients with preoperative paroxysmal and persistent AF. This shows that the Maze procedure restores physiological function of the SN, even in patients in whom AF has lasted for many years. Moreover, the study showed both PVI and the left atrial Maze procedure have similar effects on HRV. In the modified Maze procedure, 2 additional ablation lines were performed in comparison with PVI, and this had no impact on the long-term SN function.
Early results of this study revealed a significant postoperative decrease in HRV and an increase in HR. HRV parameters at discharge significantly decreased when compared with those in the healthy population. In the months following surgery, HRV parameters increased towards preoperative levels. These typical dynamics in HRV parameters were present in all groups with respect to the type of preoperative AF and ablation procedure. Several possible mechanisms contribute towards this. For instance, previous reports suggested that a decreased parasympathetic activity or an increased adrenergic activity early after cardiac surgery may result in a decreased HRV [12]. It is also known that cardiac ischaemia and heart failure lead to a reduced HRV [13]. Both these factors are present intraoperatively and in the early postoperative period. Left ventricular ejection function may temporarily decrease after surgery due to myocardial stunning. Similarly, a decrease in the HRV during the early postoperative period was observed in patients after transcatheter PVI [14], and this observation may be related to partial autonomic denervation of the heart. The epicardial surface of the left atrial wall has a high density of autonomic ganglionated plexi, and it has also been shown that surgical PVI results in a significant autonomic denervation of the heart [15].
Limitations
The results of this study have several clinical implications. A decrease in the HRV and an increase in the HR in the early postoperative period are typical after successful ablation of AF. This is not related to an unfavourable prognosis and does not require medical intervention. The dynamics of HRV is a characteristic for patients with a satisfactory long-term outcome. These findings may have implications towards a long-term out-patient monitoring. The major limitation of the study is the small cohort of patients included. A larger population would allow more extensive analysis of the long-standing persistent AF group.
CONCLUSIONS
Successful surgical ablation of persistent and paroxysmal AF using either the modified Maze procedure or PVI does not disrupt long-term HRV. A significant early postoperative reduction in HRV with a gradual increase in the following months is typical in patients after surgical restoration of SR.
Conflict of interest: none declared.




