-
PDF
- Split View
-
Views
-
Cite
Cite
Takeshi Konuma, Syunsuke Sakamoto, Syuhei Toba, Ayano Futsuki, Naoki Yamamoto, Shinji Kanemitsu, Hideto Shimpo, Novel aortic arch reconstruction using a modified Norwood procedure based on hypoplastic left heart syndrome-specific anatomical malformations, Interactive CardioVascular and Thoracic Surgery, Volume 27, Issue 2, August 2018, Pages 243–249, https://doi.org/10.1093/icvts/ivy047
Close - Share Icon Share
Abstract
Postoperative left pulmonary artery (PA) or bronchus compression occasionally follows narrowing of the retroaortic space after the Norwood procedure. We examined hypoplastic left heart syndrome (HLHS)-specific anatomical malformations and hypothesized that a long main pulmonary arterial trunk might contribute to a larger retroaortic space. In this study, we introduced a modified Norwood procedure [pulmonary artery trunk-saving method (PATS)] and evaluated the results.
HLHS-specific anatomical malformations were examined using computed tomographic data from 23 consecutive patients who underwent bilateral pulmonary banding. Fourteen patients had HLHS or associated conditions (Group H), and 9 patients had other complex cardiac defects and underwent biventricular repair (Group B). Five consecutive HLHS patients underwent PATS as a modified Norwood operation, and 6 controls underwent a conventional Norwood procedure. We used computed tomography to measure the lengths of the aorta and PA and the angle of both pulmonary branches to evaluate the effect of PATS.
Preoperative examination confirmed HLHS-specific right PA branching adjacent to the pulmonary valve and a trend towards a shorter main pulmonary arterial trunk with the conventional Norwood procedure. Also, both right and left pulmonary arterial branching from the dorsal aspect of the main pulmonary arterial trunk and the PA branch angle were minimal in HLHS patients. Postoperative data showed the retroaortic space behind the reconstructed neoaorta was significantly wider in PATS patients than in conventional Norwood patients. Longitudinal measurements (between the aortic arch and pulmonary valve) and sagittal measurements (ascending aorta to descending aorta) were larger in PATS patients, and no left PA or airway obstruction was observed following PATS.
PATS resulted in a wider aortopulmonary space without left pulmonary arterial stenosis or bronchus obstruction. HLHS-specific anatomical malformations suggested that PATS may avoid extrinsic compression of the left PA and bronchus by the neoaorta and can be an alternative for HLHS patients.
INTRODUCTION
In 1980, Norwood et al. [1] reported the first successful surgical palliation of hypoplastic left heart syndrome (HLHS). The first stage of this surgical palliation is now commonly called the Norwood procedure. Autologous tissue-only aortic arch reconstruction techniques have changed the original description; numerous modifications now use biological or synthetic materials designed to simplify the operative technique and improve outcomes. However, left pulmonary arterial (LPA) stenosis after the Norwood procedure has been reported to be as high as 27–77% [2–4] and is a significant cause of morbidity and mortality and a risk factor predicting poor Fontan outcome as well as aortic coarctation. Bichell et al. [4] reported that in Norwood procedures including autologous arch reconstruction with or without patch augmentation, LPA stenosis requiring plasty occurred in 10 of 13 (77%) patients. Poirier et al. [5] reported that tension-free autologous tissue-only aortic arch reconstruction is possible by longitudinally splitting the medial descending aorta and using as much main pulmonary arterial (MPA) length as possible. The authors also stated that a short MPA trunk can result in insufficient autologous aortic tissue and narrowing of the aortopulmonary space. Appropriate tailoring of the allograft material patch is essential to avoid excessive dilatation of the ascending aorta and compression of the left pulmonary artery (PA) [6, 7]. Our modification of the Norwood procedure [pulmonary artery trunk-saving method (PATS)] consists of transecting both pulmonary branches and leaving the MPA trunk longer to widen the aortopulmonary space to avoid extrinsic compression of the left PA and bronchus by the neoaorta. Divided pulmonary branches are anastomosed to each other end-to-end. In our technique, the aortic arch is reconstructed by anastomosing the MPA and aortic arch and descending aorta without patch augmentation. The aim of this study was to investigate aortic arch geometry after our modified Norwood procedure and to evaluate the HLHS-specific anatomical malformations.
PATIENTS AND METHODS
Hypoplastic left heart syndrome-specific anatomical malformations
HLHS-specific anatomical malformations were examined with computed tomographic (CT) data from 23 consecutive patients who underwent bilateral pulmonary banding at a single institution, Mie University Hospital, Tsu, Japan. Fourteen patients had HLHS or associated conditions (Group H), and 9 patients had other complex cardiac defects (interruption of aortic arch in 4 patients, coarctation of the aorta in 4 patients and pure pulmonic atresia in 1 patient) and underwent biventricular repair (Group B). The mean age and weight at the time of the bilateral pulmonary banding was 4.1 days (range 2–7 days) and 2.8 kg (range 2.6–3.0 kg), respectively.
Great vessel measurements and geometric analysis
This study was approved by the ethics committee of our institution. Data were collected from patient records, and all patients underwent CT before the Norwood procedure at 1–4 months of age. The distance between the pulmonary valve and the base of the right pulmonary branch (P-RPA) and the distance between the pulmonary valve and base of the left pulmonary branch (P-LPA) and distance between pulmonary valve and the base of the patent ductus arteriosus (P-PDA) were measured with data adjusted for patient body weight and evaluated as an index. The angle between the right pulmonary arterial (RPA) and LPA branches was considered the ‘PA angle’ (Fig. 1).
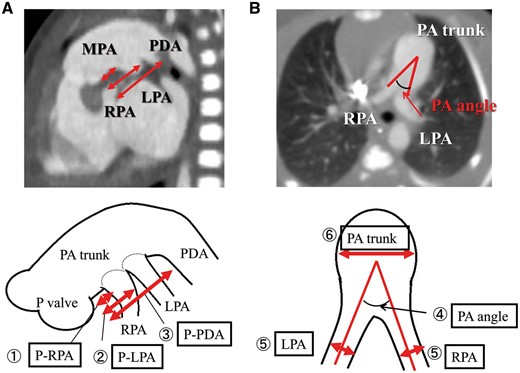
Preoperative geometric data for the hypoplastic left heart syndrome-specific anatomical malformations. We measured the following: (1) P-RPA, (2) P-LPA and (3) P-PDA. Data were adjusted for patient body weight and evaluated as an index. (4) The angle between the RPA and LPA branches was expressed as the ‘PA angle’. (5) The diameter of the RPA and LPA branches. (6) PA trunk. LPA: left pulmonary arterial; MPA: main pulmonary artery; PDA: patent ductus arteriosus; RPA: right pulmonary arterial; P-LPA: the distance between the pulmonary valve and the right pulmonary arterial branch and the base of the left pulmonary arterial branch; P-PDA: the distance between the pulmonary valve and the base of the PDA; P-RPA: the distance between the pulmonary valve and the base of the right pulmonary arterial branch; PA trunk: the diameter of the pulmonary arterial trunk.
Modified aortic reconstruction in the Norwood procedure
Between May 2014 and June 2015, 5 patients underwent PATS as a modified Norwood procedure at the Mie University Hospital. Six controls underwent a conventional Norwood procedure before 2014, and postoperative geometric data for the aortic arch were collected by measuring the following selected parameters in postoperative CT data: (i) ‘arch height’, the distance between the inferior margin of the aortic arch and the pulmonary valve; (ii) ‘arch width’, the distance between the posterior margin of the pulmonary trunk and the anterior margin of the descending aorta at the level of the left main bronchus and (iii) ‘arch angle’, the angle between the 2 centre line of ascending aorta and descending aorta, on sagittal oblique multiplanar reconstruction images. Data were adjusted for patient body weight and evaluated as an index (Fig. 2).
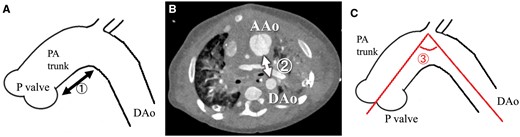
Postoperative geometric data for the aortopulmonary space: (1) ‘arch height’, the distance between the inferior margin of the aortic arch and the pulmonary valve; (2) ‘arch width’, the distance between the posterior margin of the pulmonary trunk and the anterior margin of the DAo at the level of the left main bronchus and (3) ‘arch angle’, the angle between the 2 centre line of AAo and DAo, on sagittal oblique multiplanar reconstruction images. AAo: ascending aorta; DAo: descending aorta; PA trunk: the diameter of the pulmonary arterial trunk.
Statistical analysis
Normally distributed data were expressed as mean ± standard deviation and non-normal data as median (range). Continuous variables, such as valve gradient, were compared using the unpaired Student’s t-test. Correlations were assessed using the Pearson’s correlation coefficient for normally distributed, continuous variables. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using the StatView 5.0 software package (SAS Institute Inc., Cary, NC, USA).
Operative technique
Norwood and bidirectional Glenn procedures were performed as a second-stage operation for all patients at 4–6 months, as a hybrid therapy after bilateral pulmonary banding with prostaglandin E1 infusion. The mean age and weight at the time of the Norwood procedure was 4.5 months (range 4.1–5.3 months) and 5.1 kg (range 4.7–5.1 kg), respectively. After median sternotomy, the great vessels, especially the pulmonary branches, were dissected. Hypothermic cardiopulmonary bypass was used with an alpha-stat strategy, and cerebral perfusion was performed with an expanded polytetrafluoroethylene graft (3.0–3.5 mm) to the brachiocephalic artery. Lower body perfusion was performed with a cannula to the descending aorta. After bilateral PA debanding, the right and left pulmonary branches were divided at the base of the pulmonary trunk. Defects in the pulmonary trunk were closed directly to reduce the diameter of the pulmonary trunk, and the pulmonary branches were anastomosed end-to-end. Myocardial protection was achieved with cold crystalloid cardioplegia. Arch reconstruction was performed with preserved pulmonary trunk without patch argumentation after excising all duct tissues and the coarctation ridge. After arch reconstruction, the superior vena cava was anastomosed to the right PA as the Glenn procedure (Fig. 3).
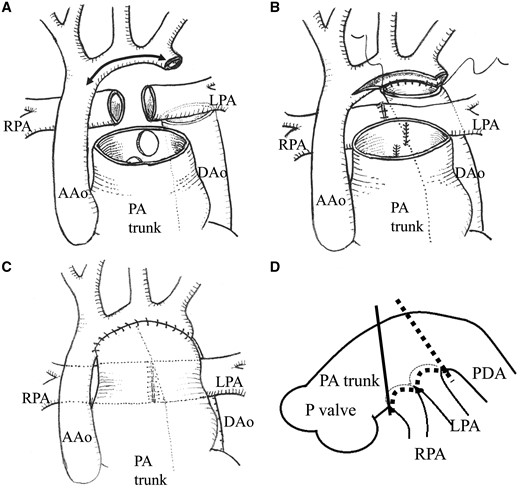
The modified Norwood procedure (pulmonary artery trunk-saving method): (A) The patent ductus arteriosus was divided, all ductal tissues were resected and the right and left pulmonary branches were divided at the base of the pulmonary trunk. (B) Defects in the pulmonary trunk were closed directly to reduce the diameter of the pulmonary trunk, and pulmonary branches were anastomosed end-to-end. (C) The arch was reconstructed by anastomosing the DAo to the aortic isthmus and distal arch. The preserved pulmonary trunk without patching was sutured to a combination of the ascending aorta. (D) Schema showing the point of pulmonary trunk dissection. Solid line indicates the point during the conventional Norwood procedure; the broken line is the point during pulmonary artery trunk-saving method. AAo: ascending aorta; DAo: descending aorta; LPA: left pulmonary arterial; P: pulmonary; RPA: right pulmonary arterial; PA trunk: the diameter of the pulmonary arterial trunk.
RESULTS
Postoperative outcomes
In the 5 PATS patients, cardiopulmonary bypass time was 294 min (range 272–383 min), and the intensive care unit stay was 7 days on average. One patient needed extracorporeal membrane oxygenation due to pulmonary bleeding and hypoxia and stayed 39 days in the intensive care unit. We encountered no operative deaths and 1 hospital death secondary to pneumonia in a patient with known chromosomal abnormality. Four patients were discharged, and 1 patient underwent the Fontan operation at the time of this report, and 4 patients underwent the pre-Fontan catheter study and showed the PA index of 125 (range 116–141) and superior vena cava pressure of 12.2 mmHg (range 10–14 mmHg) (Fig. 4). In 3 PATS patients, PTA was performed (RPA: 1 case and LPA: 2 cases) mainly for hypoplastic pulmonary branches, not for the central anastomosed site. Preoperative bronchial obstruction and respiratory failure was resolved in 1 patient after PATS.
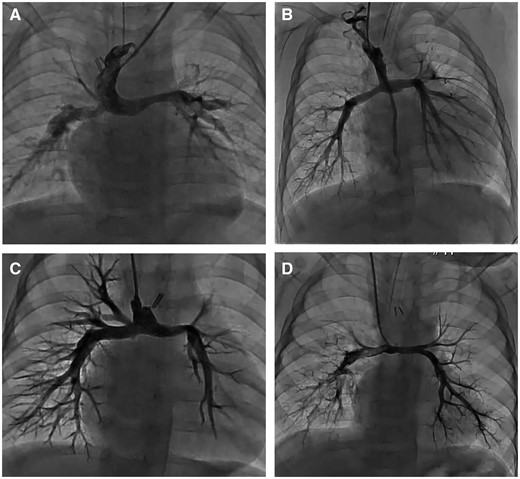
Angiogram of the pulmonary artery after the pulmonary artery trunk-saving procedure and Glenn operation. A, B, C and D are the angiogram of 4 patients who underwent the pre-Fontan catheter study and showed no stenosis of central anastomosed site.
Preoperative great vessel geometry as hypoplastic left heart syndrome-specific anatomical malformations
CT scan measurements revealed HLHS-specific anatomical malformations of patients. No significant differences were observed in the position and portion of the bifurcation of the pulmonary branches [the distance index between the pulmonary valve and the pulmonary artery (P-PAI), P-LPA and the distance index between the pulmonary valve and the base of the patent ductus arteriosus (P-PDAI)] and the diameter of the pulmonary trunk and branches [the diameter index of the RPA branch (RPAI), the diameter index of the LPA branch (LPAI) and the diameter index of the pulmonary arterial trunk (PATI)]. The angle of both pulmonary arteries was significantly more acute in HLHS patients than in control patients (Table 1, Fig. 5).
Preoperative great vessel geometry in HLHS-specific anatomical malformations
| . | HLHS (n = 14) . | Biventricular (n = 9) . | P-value . |
|---|---|---|---|
| Mean age (months) | 3.0 | 0.9 | |
| Mean weight (kg) | 4.2 | 3.1 | |
| P-RPAI (mm/kg), mean ± SD | 1.41 ± 0.46 | 1.88 ± 0.68 | 0.06 |
| P-LPAI (mm/kg), mean ± SD | 3.10 ± 0.60 | 3.06 ± 0.64 | 0.60 |
| P-PDAI (mm/kg), mean ± SD | 4.66 ± 1.00 | 5.03 ± 1.02 | 0.41 |
| PA angle (°),mean ± SD | 34.7 ± 11.2 | 69.3 ± 17.9 | 0.0001 |
| RPAI (mm/kg), mean ± SD | 1.83 ± 0.31 | 1.82 ± 0.33 | 0.95 |
| LPAI (mm/kg), mean ± SD | 1.56 ± 0.28 | 1.83 ± 0.60 | 0.17 |
| PATI (mm/kg), mean ± SD | 4.04 ± 1.40 | 4.17 ± 0.82 | 0.80 |
| . | HLHS (n = 14) . | Biventricular (n = 9) . | P-value . |
|---|---|---|---|
| Mean age (months) | 3.0 | 0.9 | |
| Mean weight (kg) | 4.2 | 3.1 | |
| P-RPAI (mm/kg), mean ± SD | 1.41 ± 0.46 | 1.88 ± 0.68 | 0.06 |
| P-LPAI (mm/kg), mean ± SD | 3.10 ± 0.60 | 3.06 ± 0.64 | 0.60 |
| P-PDAI (mm/kg), mean ± SD | 4.66 ± 1.00 | 5.03 ± 1.02 | 0.41 |
| PA angle (°),mean ± SD | 34.7 ± 11.2 | 69.3 ± 17.9 | 0.0001 |
| RPAI (mm/kg), mean ± SD | 1.83 ± 0.31 | 1.82 ± 0.33 | 0.95 |
| LPAI (mm/kg), mean ± SD | 1.56 ± 0.28 | 1.83 ± 0.60 | 0.17 |
| PATI (mm/kg), mean ± SD | 4.04 ± 1.40 | 4.17 ± 0.82 | 0.80 |
HLHS: hypoplastic left heart syndrome; LPAI: the diameter index of the left pulmonary arterial branch; PA angle: the angle of the right and left pulmonary arterial branches; PDA: patent ductus arteriosus; P-LPAI: the distance index between the pulmonary valve and the base of the left pulmonary arterial branch; P-PDAI: the distance index between the pulmonary valve and the base of the PDA; P-RPAI: the distance index between the pulmonary valve and the base of the right pulmonary arterial branch; PATI: the diameter index of the pulmonary arterial trunk; RPAI: the diameter index of the right pulmonary arterial branch; SD: standard deviation.
Preoperative great vessel geometry in HLHS-specific anatomical malformations
| . | HLHS (n = 14) . | Biventricular (n = 9) . | P-value . |
|---|---|---|---|
| Mean age (months) | 3.0 | 0.9 | |
| Mean weight (kg) | 4.2 | 3.1 | |
| P-RPAI (mm/kg), mean ± SD | 1.41 ± 0.46 | 1.88 ± 0.68 | 0.06 |
| P-LPAI (mm/kg), mean ± SD | 3.10 ± 0.60 | 3.06 ± 0.64 | 0.60 |
| P-PDAI (mm/kg), mean ± SD | 4.66 ± 1.00 | 5.03 ± 1.02 | 0.41 |
| PA angle (°),mean ± SD | 34.7 ± 11.2 | 69.3 ± 17.9 | 0.0001 |
| RPAI (mm/kg), mean ± SD | 1.83 ± 0.31 | 1.82 ± 0.33 | 0.95 |
| LPAI (mm/kg), mean ± SD | 1.56 ± 0.28 | 1.83 ± 0.60 | 0.17 |
| PATI (mm/kg), mean ± SD | 4.04 ± 1.40 | 4.17 ± 0.82 | 0.80 |
| . | HLHS (n = 14) . | Biventricular (n = 9) . | P-value . |
|---|---|---|---|
| Mean age (months) | 3.0 | 0.9 | |
| Mean weight (kg) | 4.2 | 3.1 | |
| P-RPAI (mm/kg), mean ± SD | 1.41 ± 0.46 | 1.88 ± 0.68 | 0.06 |
| P-LPAI (mm/kg), mean ± SD | 3.10 ± 0.60 | 3.06 ± 0.64 | 0.60 |
| P-PDAI (mm/kg), mean ± SD | 4.66 ± 1.00 | 5.03 ± 1.02 | 0.41 |
| PA angle (°),mean ± SD | 34.7 ± 11.2 | 69.3 ± 17.9 | 0.0001 |
| RPAI (mm/kg), mean ± SD | 1.83 ± 0.31 | 1.82 ± 0.33 | 0.95 |
| LPAI (mm/kg), mean ± SD | 1.56 ± 0.28 | 1.83 ± 0.60 | 0.17 |
| PATI (mm/kg), mean ± SD | 4.04 ± 1.40 | 4.17 ± 0.82 | 0.80 |
HLHS: hypoplastic left heart syndrome; LPAI: the diameter index of the left pulmonary arterial branch; PA angle: the angle of the right and left pulmonary arterial branches; PDA: patent ductus arteriosus; P-LPAI: the distance index between the pulmonary valve and the base of the left pulmonary arterial branch; P-PDAI: the distance index between the pulmonary valve and the base of the PDA; P-RPAI: the distance index between the pulmonary valve and the base of the right pulmonary arterial branch; PATI: the diameter index of the pulmonary arterial trunk; RPAI: the diameter index of the right pulmonary arterial branch; SD: standard deviation.
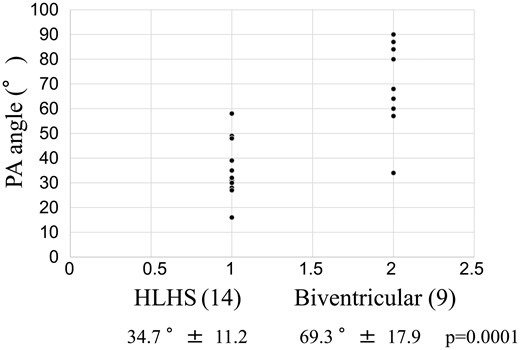
The angle of the right and left pulmonary arterial branches before the Stage II procedure. HLHS: hypoplastic left heart syndrome.
Postoperative great vessel geometry after pulmonary artery trunk-saving method
Significant postoperative aortopulmonary space was noted in the PATS group. Compared with controls, dimensions were larger for arch height at 1.87 ± 0.65 vs 3.05 ± 0.78 mm/kg (P = 0.02), in arch width at 1.51 ± 0.27 vs 2.76 ± 0.73 mm/kg (P = 0.003) and in arch angle at 94 ± 10 vs 107 ± 13 mm/kg (P = 0.07; Table 2). Pulmonary branch dimensions did not differ between the PATS and control groups but trended to larger dimensions: RPAI 0.83 ± 0.12 vs 0.71 ± 0.22 mm/kg (P = 0.32) and LPAI 0.61 ± 0.17 vs 0.56 ± 0.89 mm/kg (P = 0.61). Angiography after the Norwood procedure showed a long ascending aorta and smooth aortic arch angle in PATS patients compared with conventional Norwood patients (Fig. 6).
| . | Norwood (n = 6) . | PATS (n = 5) . | P-value . |
|---|---|---|---|
| Mean age (months) | 13.9 | 5.9 | |
| Mean weight (kg) | 8.0 | 5.2 | |
| Postoperation (months) | 13.6 | 1.1 | |
| Arch height (mm/kg), mean ± SD | 1.87 ± 0.65 | 3.05 ± 0.78 | 0.02 |
| Arch width (mm/kg), mean ± SD | 1.51 ± 0.27 | 2.76 ± 0.73 | 0.003 |
| Arch angle (°), mean ± SD | 94 ± 10 | 107 ± 13 | 0.07 |
| RPAI (mm/kg), mean ± SD | 0.71 ± 0.22 | 0.83 ± 0.12 | 0.32 |
| LPAI (mm/kg), mean ± SD | 0.56 ± 0.89 | 0.61 ± 0.17 | 0.61 |
| PATI (mm/kg), mean ± SD | 2.96 ± 0.79 | 3.13 ± 0.30 | 0.66 |
| . | Norwood (n = 6) . | PATS (n = 5) . | P-value . |
|---|---|---|---|
| Mean age (months) | 13.9 | 5.9 | |
| Mean weight (kg) | 8.0 | 5.2 | |
| Postoperation (months) | 13.6 | 1.1 | |
| Arch height (mm/kg), mean ± SD | 1.87 ± 0.65 | 3.05 ± 0.78 | 0.02 |
| Arch width (mm/kg), mean ± SD | 1.51 ± 0.27 | 2.76 ± 0.73 | 0.003 |
| Arch angle (°), mean ± SD | 94 ± 10 | 107 ± 13 | 0.07 |
| RPAI (mm/kg), mean ± SD | 0.71 ± 0.22 | 0.83 ± 0.12 | 0.32 |
| LPAI (mm/kg), mean ± SD | 0.56 ± 0.89 | 0.61 ± 0.17 | 0.61 |
| PATI (mm/kg), mean ± SD | 2.96 ± 0.79 | 3.13 ± 0.30 | 0.66 |
Arch angle: the angle between the 2 centre line of the ascending aorta and descending aorta; Arch height: the distance index between the inferior margin of the aortic arch and the pulmonary valve; Arch width: the distance index between the posterior margin of the pulmonary trunk and the anterior margin of the descending aorta at the level of the left main bronchus; LPAI: the diameter index of the left pulmonary arterial branch; PATI: the diameter index of the pulmonary arterial trunk; PATS: pulmonary artery trunk-saving method; RPAI: the diameter index of the right pulmonary arterial branch; SD: standard deviation.
| . | Norwood (n = 6) . | PATS (n = 5) . | P-value . |
|---|---|---|---|
| Mean age (months) | 13.9 | 5.9 | |
| Mean weight (kg) | 8.0 | 5.2 | |
| Postoperation (months) | 13.6 | 1.1 | |
| Arch height (mm/kg), mean ± SD | 1.87 ± 0.65 | 3.05 ± 0.78 | 0.02 |
| Arch width (mm/kg), mean ± SD | 1.51 ± 0.27 | 2.76 ± 0.73 | 0.003 |
| Arch angle (°), mean ± SD | 94 ± 10 | 107 ± 13 | 0.07 |
| RPAI (mm/kg), mean ± SD | 0.71 ± 0.22 | 0.83 ± 0.12 | 0.32 |
| LPAI (mm/kg), mean ± SD | 0.56 ± 0.89 | 0.61 ± 0.17 | 0.61 |
| PATI (mm/kg), mean ± SD | 2.96 ± 0.79 | 3.13 ± 0.30 | 0.66 |
| . | Norwood (n = 6) . | PATS (n = 5) . | P-value . |
|---|---|---|---|
| Mean age (months) | 13.9 | 5.9 | |
| Mean weight (kg) | 8.0 | 5.2 | |
| Postoperation (months) | 13.6 | 1.1 | |
| Arch height (mm/kg), mean ± SD | 1.87 ± 0.65 | 3.05 ± 0.78 | 0.02 |
| Arch width (mm/kg), mean ± SD | 1.51 ± 0.27 | 2.76 ± 0.73 | 0.003 |
| Arch angle (°), mean ± SD | 94 ± 10 | 107 ± 13 | 0.07 |
| RPAI (mm/kg), mean ± SD | 0.71 ± 0.22 | 0.83 ± 0.12 | 0.32 |
| LPAI (mm/kg), mean ± SD | 0.56 ± 0.89 | 0.61 ± 0.17 | 0.61 |
| PATI (mm/kg), mean ± SD | 2.96 ± 0.79 | 3.13 ± 0.30 | 0.66 |
Arch angle: the angle between the 2 centre line of the ascending aorta and descending aorta; Arch height: the distance index between the inferior margin of the aortic arch and the pulmonary valve; Arch width: the distance index between the posterior margin of the pulmonary trunk and the anterior margin of the descending aorta at the level of the left main bronchus; LPAI: the diameter index of the left pulmonary arterial branch; PATI: the diameter index of the pulmonary arterial trunk; PATS: pulmonary artery trunk-saving method; RPAI: the diameter index of the right pulmonary arterial branch; SD: standard deviation.
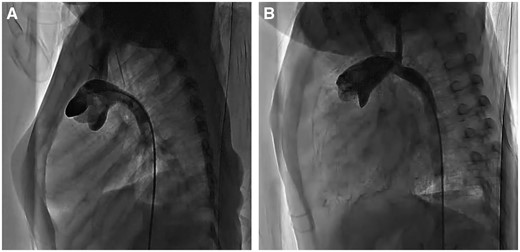
Angiogram of the aorta after the Norwood operation. (A) Conventional Norwood procedure; (B) pulmonary artery trunk-saving procedure.
DISCUSSION
In 1980, Norwood et al. [1] reported the first successful surgical palliation for HLHS. The first stage of this operation is commonly called the Norwood procedure. Aortic arch reconstruction techniques have changed from the original description, which involved creating a neoaorta using the original proximal pulmonary artery anastomosed to the ascending aorta and proximal aortic arch. In 1986, Jonas et al. [8] adopted a different technique that involved patching the proximal descending aorta beyond the junction with the ductus arteriosus, arch and ascending aorta with a pulmonary homograft. This technique was widely adopted as the standard method of arch reconstruction. In 1995, Fraser and Mee [9] reported a technique based on reconstructing the neoaorta without patch augmentation because of the possible disadvantages with the homograft, such as lack of growth, degeneration and calcification.
Reconstruction of the aortic arch is a crucial part of the Norwood procedure and is occasionally associated with postoperative left branch pulmonary artery or airway stenosis as well as aortic recoarctation [10]. Allograft patching is frequently used to enlarge the aorta, and appropriate tailoring of the patch is essential to avoid excessive dilatation of the ascending aorta and compression of the left pulmonary artery [6, 7]. In Japan, autologous tissue-only aortic reconstruction, as in the original Norwood procedure [1, 11], is common because allografts are not covered by national health insurance and almost unavailable through traditional means. In the Norwood procedure, the pulmonary trunk is divided just underneath the RPA branch and connected to the ascending aorta; therefore, the space remaining for the left pulmonary hilum is usually very small. This smaller space may result in LPA or bronchial stenosis because the pulmonary trunk is very short and the amount of autologous neoaortic tissue is insufficient to create adequate space for the left PA and its branches. We developed a new procedure (PATS) to resolve these issues and performed the procedure in 5 patients. This method involves dividing the RPA and LPA branches and saving the PA trunk length to create a space for the left pulmonary hilum; pulmonary arterial branches are anastomosed end-to-end. Because the new aorta consists of autologous tissue only, we can anticipate aortic growth and more natural haemodynamic blood flow. Also we adopted and developed the hybrid procedure to manage HLHS patients, Norwood plus bidirectional Glenn as a second-stage palliation after bilateral pulmonary artery bands [12–14].
The anatomical malformations of HLHS and its variants and the aortopulmonary space and aortic arch angle after the Norwood procedure have been studied rarely [10], although they are very important anatomical features that relate to postoperative morbidity. Also, only a limited number of previous reports have examined the extrinsic compression of the bronchus and PA branch that results from the neoaorta following the Norwood procedure.
Pulmonary artery trunk-saving method based on hypoplastic left heart syndrome-specific anatomical malformations
Poirier et al. [5] reported that tension-free autologous tissue-only aortic arch reconstruction is possible by longitudinally splitting the medial descending aorta and using as much MPA length as possible, which further reduces tension on the anastomosis. In the present study, because of the HLHS-specific anatomical malformations, the MPA length was insufficient because the RPA was branched adjacent to the MPA valve and the distance between the MPA valve and RPA was only 1.41 ± 0.46 mm/kg [the distance index between the pulmonary valve and the base of the right pulmonary branch (P-RPAI)]. Using the PATS method, the RPA and LPA branches are cut at the base of the branch to increase the length of the MPA, and defects in the pulmonary trunk are closed directly to reduce the diameter of the pulmonary trunk. In our HLHS patients, the RPA and LPA branches were branched adjacently on the dorsal aspect of the MPA trunk, and the PA branch angle was very small at 34.7 ± 11.2°. Using PATS, the pulmonary branches can be anastomosed end-to-end without tension.
García-Hernández et al. [15] reported that 2 patients who underwent aortic arch reconstruction using autologous great vessel tissue-only in the Norwood procedure died from respiratory insufficiency with extrinsic compression of the PA and left main bronchus by the neoaorta. Bichell et al. [4] reported that in Norwood patients undergoing autologous arch reconstruction with or without patch augmentation, LPA stenosis requiring plasty occurred in 10 of 13 (77%) patients. Clinical studies show that abnormal PA architecture is an independent risk factor predicting poor Fontan outcome [16, 17], and the Fontan pathway, notably including pulmonary arterial distortion and hypoplasia, required late PA reconstruction in >50% of patients [18]. Our PATS-related postoperative data showed that the distance between the pulmonary valve and the aortic arch (arch height) and between the ascending aorta and the descending aorta (arch width) are long enough to avoid extrinsic compression of the left PA and airway by the neoaorta.
Limitations
The results of this retrospective study are limited by the differences in the age of CT examination. CT measurement and great vessel shape were analysed almost 1 month after the Norwood operation in PATS patients and before the Fontan operation in conventional Norwood patients. Although measurement data were corrected by body weight as index, further and upcoming studies are necessary to assess the potential of aorta and PAs to grow adequately. A further limitation to the present study is that a small number of patients, and larger series with longer follow-up are needed.
CONCLUSION
We modified the Norwood procedure and developed a new technique called PATS to create a larger space between the ascending and descending aorta. HLHS-specific anatomical malformations suggested that PATS may avoid stenosis of the left PA and bronchus and that PATS can be a useful alternative in HLHS patients.
Conflict of interest: none declared.
REFERENCES
- aorta
- aortic arch
- hypoplastic left heart syndrome
- pulmonary artery
- congenital abnormality
- descending aorta
- lung
- repair of single ventricle with aortic outflow obstruction and aortic arch hypoplasia (hypoplastic left heart syndrome) (eg, norwood procedure)
- reconstructive surgical procedures
- trunk structure




