-
PDF
- Split View
-
Views
-
Cite
Cite
Naoko Ose, Hajime Maeda, Masayoshi Inoue, Eiichi Morii, Yasushi Shintani, Hiroshi Matsui, Hirohito Tada, Tositeru Tokunaga, Kenji Kimura, Yasushi Sakamaki, Yukiyasu Takeuchi, Kenjiro Fukuhara, Hiroshi Katsura, Teruo Iwasaki, Meinoshin Okumura, for Thoracic Surgery Study Group of Osaka University, Results of treatment for thymic neuroendocrine tumours: multicentre clinicopathological study, Interactive CardioVascular and Thoracic Surgery, Volume 26, Issue 1, January 2018, Pages 18–24, https://doi.org/10.1093/icvts/ivx265
Close - Share Icon Share
Abstract
A thymic neuroendocrine tumour (TNET) is rare, and few comprehensive reports of treatment results have been presented. To clarify the clinicopathological characteristics of TNET in affected patients, outcomes were retrospectively examined using cases accumulated in a multicentre survey.
Thirty patients (25 men and 5 women) who underwent surgical resection or biopsy procedures at 10 institutions of the Thoracic Surgery Study Group of Osaka University (TSSGO) between January 1986 and June 2015 and pathologically diagnosed with TNET were enrolled.
The examined tumours were classified as typical carcinoid in 7 patients, atypical carcinoid in 11 patients, large-cell neuroendocrine carcinoma in 3 patients and small-cell carcinoma in 9 patients, of which 2 underwent surgical biopsy procedures and 28 surgical resection, with a macroscopic complete resection procedure performed in 27 patients. Induction therapy was performed in 2 patients and adjuvant therapy in 10 patients. Thirteen patients had recurrence, with distant metastasis, especially in bone and lung tissues, more frequent than local recurrence. Overall survival was 77% after 5 years and 35% after 10 years, whereas relapse-free survival was 48% and 29%, and cancer-specific survival was 90% and 48%, respectively. Overall survival was significantly better in patients who underwent macroscopic complete resection (P = 0.010). As for relapse-free survival patients, TNM Stage I or II (P = 0.011) and received adjuvant therapy patients (P = 0.042) showed good survival rates.
The prognosis of patients with TNET was favourable in those treated with macroscopic complete resection. Survival is promising even in patients with postoperative recurrence, following treatment utilized for pulmonary neuroendocrine tumour or gastroenteropancreatic neuroendocrine tumour.
INTRODUCTION
A thymic neuroendocrine tumour (TNET) is rare, with a rate of incidence among all thymic epithelial tumours of approximately 2–5% [1], whereas neuroendocrine tumours (NETs) of thymic origin account for only 0.4% [2]. Pulmonary NET (PNET) and TNETs are classified as low grade for typical carcinoids (TCs), intermediate grade for atypical carcinoids (ATCs) and high grade for large-cell neuroendocrine carcinoma and small-cell carcinoma tumours [3], with the most common histological type of TNET reported to be ATC [1]. Several prognostic factors have been reported for patients with a TNET, including tumour size [4] and histological type [5], as well as others. These tumours have a high risk of metastasis to lymph nodes or distant organs. Furthermore, chemotherapy and radiotherapy treatments are less sensitive, thus complete surgical resection is a critical factor for determining prognosis [6], though reports of TNET treatment results are limited [1, 2, 5, 7–16], among which few describe cases of chemotherapy or radiotherapy. Although adjuvant therapy may be useful [7], no standard regimen has been established and the same drugs utilized for PNET are employed [14].
For the purpose of clarifying the clinicopathological characteristics and outcomes of patients with a TNET, we performed a multicentre retrospective study of those treated at facilities participating in the (TSSGO).
MATERIALS AND METHODS
Patients
Thirty patients (25 men and 5 women, mean age 54.3 ± 11.6 years) who underwent surgical resection or surgical biopsy procedures at 10 TSSGO institutions between January 1986 and June 2015 and pathologically diagnosed with TNET were enrolled. Patients who could not undergo rediagnosis because of a lack of paraffin block tumour specimens were ineligible. Of the 36 initially registered patients, 6 patients were excluded because of another diagnosis after central histopathological examination findings: 5 patients with a squamous cell carcinoma and 1 patient with no tumour.
Staging was based on Masaoka/Koga classification and the American Joint Committee on Cancer/ Union for international cancer control (AJCC/UICC) TNM Classification of Malignant Tumours, Eighth Edition [17]. The primary end-point was overall survival (OS), whereas relapse-free survival (RFS), cancer-specific survival (CSS), treatment contents and recurrence site were the secondary end-points. Recurrence was defined as the appearance of a new lesion after the operation, whereas local recurrence had no evidence of distant metastasis and included cut-end recurrence, mediastinal/hilar lymph node metastasis and dissemination. The analyses were conducted retrospectively and based on reviews of medical records. This study was initially approved by the Institutional Review Board of Toneyama Hospital (project approval no. 1343) on February 2014 and thereafter by each of the participating institutions.
Surgical procedure
Standard resection was defined as the resection of the tumour, thymus and adjacent organ with invasion. Lymph node dissection was performed in all patients, but the extent of resection varied depending on the facility and surgeon.
Histopathological diagnosis
The central histopathological examination was performed by a single pathologist (E.M.) using the 2015 World Health Organization classification of tumours of the thymus. A typical microscopic pattern is shown in Fig. 1. Specimens that had not been stained from all the 36 patients were collected, and rediagnosis was performed using findings from the primary tumours in 35 patients and recurrence in 1 patient. The obtained tumour tissues were fixed in 10% formalin, embedded in paraffin, cut into 4-μm-thick sections and routinely stained with haematoxylin and eosin.
![Typical histopathological findings for each type of thymic neuroendocrine tumour [haematoxylin and eosin staining (×100)]. LCNEC: large-cell neuroendocrine carcinoma.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icvts/26/1/10.1093_icvts_ivx265/1/m_ivx265f1.jpeg?Expires=1750237990&Signature=VO3WowhupXgQ0KrQFvDOBvtAxn08sNTNd6p9RCSipK8vkWZq49UbenN6MaKDEttxAvLLThNlofOPS4ALnWNvh0eK-axfxTmaEF~MWUXMKltCozawU0fOn-dSXHj9aOHEWqw~Qi2OzQ6mASJgZQT4ATn-iR2i7UydADqNwamlMbuWIBNRtrlsuF8~KTjN5Gs~QujJWEAlhKDzPOhSB1NXIA85txhPeff5Srs2f1EWwS3sNKslQpJimoctOLV1Ob2qaT2I55b59wff~kjA~HUuSYGvQo5MhTE-S0VAbJ~i76MI-pyrzuQxqW7W4ZwOYTkJ9qfcz4-wXHavocINkTxghg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Typical histopathological findings for each type of thymic neuroendocrine tumour [haematoxylin and eosin staining (×100)]. LCNEC: large-cell neuroendocrine carcinoma.
For immunohistochemical analysis, serial 4-μm-thick sections were deparaffinized and rehydrated. Sections were then heated in a pressure chamber at 125°C for 30 s, followed by 90°C for 10 s in buffer at pH 6.0 for antigen retrieval and stained using a HISTOSTAINER (Nichirei Biosciences Inc., Tokyo, Japan) with CD56, synaptophysin and chromogranin A (Nichirei Biosciences Inc.) to evaluate neuroendocrine differentiation as well as Ki-67 (Nichirei Biosciences Inc.) to evaluate cell proliferation.
A TC shows a low mitotic rate (<2 mitoses per 10 high-power fields) and no areas of necrosis, whereas an ATC exhibits a proliferation rate of 2–10 mitoses per high-power field, areas of punctate necrosis or both and a neuroendocrine carcinoma shows a high mitotic rate (>10 per 10 high-power fields) and extensive areas of necrosis.
Statistical analysis
Values were represented as mean ± standard deviation. OS rate was calculated from the date of surgery until the time of death from any cause or last visit (censored OS) using the Kaplan–Meier method. RFS was defined as the period from the date of surgery until recurrence, death from any cause or last visit without evidence of recurrence (censored RFS), whereas CSS was calculated from the date of surgery until the time of death from TNET or last visit (censored CSS). A log-rank test was used to assess differences between the subgroups. A P-value of <0.05 was considered statistically significant. Hazard ratio and confidence limits were estimated for each variable using the Cox univariate model. Multivariate analysis was not done because of the small size of the study cohort. All analyses were performed using the JMP12.0.1 statistical software package (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. Histopathological type was TC in 7 patients, ATC in 11 patients, large-cell neuroendocrine carcinoma in 3 patients and small-cell carcinoma in 9 patients. Twenty-five patients were men and 5 were women, and their mean age was 54.3 ± 11.6 (range 17–80) years. Eleven patients had symptoms: chest pain in 7 patients and cough in 4 patients. Nineteen asymptomatic patients were incidentally found during a routine medical check-up (n = 13) or follow-up examination for another disease (n = 6). Furthermore, multiple endocrine neoplasia 1 was complicated in 3 patients, and 1 of these 3 patients had paraneoplastic syndrome. A preoperative diagnosis of TNET was obtained based on percutaneous core biopsy findings in 7 (23.3%) patients, though a different diagnosis was made in 3 (thymoma in 2 and thymic carcinoma in 1) patients. The mean tumour size was 7.4 ± 4.1 (range 1–18) cm. It was classified as low grade for Ki67 index, which means less than 5%, in only 1 patient.
| . | Patients (n = 30) . |
|---|---|
| Gender (male/female) | 25/5 |
| Age | 54.3 ± 11.6 (17–80) |
| Symptoms | |
| None | 19 |
| Positive | 11 |
| Chest pain | 7 |
| Cough | 4 |
| Oedema | 1 |
| Paraneoplastic syndrome | 1 |
| MEN1 | 3 |
| Histological diagnosis | |
| Typical carcinoid | 7 |
| Atypical carcinoid | 11 |
| LCNEC | 3 |
| Small-cell carcinoma | 9 |
| Masaoka stage | |
| 1/2/3/4a/4b | 4/8/10/1/7 |
| WHO classification | |
| T1a/T1b/T2/T3/T4 | 11/5/1/13/0 |
| N0/N1/N2 | 24/3/3 |
| M0/M1a/M1b | 27/1/2 |
| Stage 1/2/3a/3b/4a/4b | 13/1/8/0/3/5 |
| . | Patients (n = 30) . |
|---|---|
| Gender (male/female) | 25/5 |
| Age | 54.3 ± 11.6 (17–80) |
| Symptoms | |
| None | 19 |
| Positive | 11 |
| Chest pain | 7 |
| Cough | 4 |
| Oedema | 1 |
| Paraneoplastic syndrome | 1 |
| MEN1 | 3 |
| Histological diagnosis | |
| Typical carcinoid | 7 |
| Atypical carcinoid | 11 |
| LCNEC | 3 |
| Small-cell carcinoma | 9 |
| Masaoka stage | |
| 1/2/3/4a/4b | 4/8/10/1/7 |
| WHO classification | |
| T1a/T1b/T2/T3/T4 | 11/5/1/13/0 |
| N0/N1/N2 | 24/3/3 |
| M0/M1a/M1b | 27/1/2 |
| Stage 1/2/3a/3b/4a/4b | 13/1/8/0/3/5 |
LCNEC: large-cell neuroendocrine carcinoma; MEN1: multiple endocrine neoplasia 1; WHO: World Health Organization.
| . | Patients (n = 30) . |
|---|---|
| Gender (male/female) | 25/5 |
| Age | 54.3 ± 11.6 (17–80) |
| Symptoms | |
| None | 19 |
| Positive | 11 |
| Chest pain | 7 |
| Cough | 4 |
| Oedema | 1 |
| Paraneoplastic syndrome | 1 |
| MEN1 | 3 |
| Histological diagnosis | |
| Typical carcinoid | 7 |
| Atypical carcinoid | 11 |
| LCNEC | 3 |
| Small-cell carcinoma | 9 |
| Masaoka stage | |
| 1/2/3/4a/4b | 4/8/10/1/7 |
| WHO classification | |
| T1a/T1b/T2/T3/T4 | 11/5/1/13/0 |
| N0/N1/N2 | 24/3/3 |
| M0/M1a/M1b | 27/1/2 |
| Stage 1/2/3a/3b/4a/4b | 13/1/8/0/3/5 |
| . | Patients (n = 30) . |
|---|---|
| Gender (male/female) | 25/5 |
| Age | 54.3 ± 11.6 (17–80) |
| Symptoms | |
| None | 19 |
| Positive | 11 |
| Chest pain | 7 |
| Cough | 4 |
| Oedema | 1 |
| Paraneoplastic syndrome | 1 |
| MEN1 | 3 |
| Histological diagnosis | |
| Typical carcinoid | 7 |
| Atypical carcinoid | 11 |
| LCNEC | 3 |
| Small-cell carcinoma | 9 |
| Masaoka stage | |
| 1/2/3/4a/4b | 4/8/10/1/7 |
| WHO classification | |
| T1a/T1b/T2/T3/T4 | 11/5/1/13/0 |
| N0/N1/N2 | 24/3/3 |
| M0/M1a/M1b | 27/1/2 |
| Stage 1/2/3a/3b/4a/4b | 13/1/8/0/3/5 |
LCNEC: large-cell neuroendocrine carcinoma; MEN1: multiple endocrine neoplasia 1; WHO: World Health Organization.
Treatment outcomes
The details of treatments and outcomes for all cases are summarized in Table 2. Surgical resection was performed in 28 patients and only a biopsy in 2 patients. The surgical approach utilized was a median sternotomy in 23 patients, thoracotomy in 3 patients, median sternotomy with thoracotomy in 2 patients and thoracoscopic surgery in 2 patients. Macroscopic complete resection (MCR) was done in 27 patients, including 1 patient with macroscopic resection of dissemination. The 1 patient who did not undergo MCR was surgically treated even though bone metastasis was evident. The mean diameter of the resected tumour was 7.4 ± 4.1 (range 1.5–18) cm. Induction therapy was performed in 2 patients and adjuvant therapy in 10 patients. The median follow-up period was 100.5 (15–342) months. Adjuvant therapy was performed in 10 of the present patients. Of these, 6 patients underwent radiotherapy, 3 patients underwent chemoradiotherapy and 1 patient was administered somatostatin analogue. Thirteen patients experienced relapse following resection. Bone metastasis was most frequently seen in 7 patients, followed by pleural dissemination in 6 patients, metastasis to a lung in 5 patients, metastasis to mediastinal and cervical lymph nodes in 1 patient and cut-end recurrence in 1 patient (overlapping cases included). For 2 patients with pleural dissemination and 1 patient with stump recurrence, surgical resection and radiotherapy were performed, and the pathological diagnosis in these patients was the same as the primary tumour in 2 comparable patients. Radiotherapy was performed for patients with bone or lymph node metastasis. Chemotherapy was administered in 5 patients and somatostatin analogue therapy in 3 patients (1 ATC, 1 TC and 1 small-cell carcinoma), whereas second-line treatment was performed in 5 patients. Six patients died of primary disease and 3 of other causes. Eight patients were alive with recurrence and 13 were free from relapse at the time of the final analysis.
| No . | Age/gender . | Histological diagnosis . | Size (cm) . | Operation . | MCR . | Pathological TNM stage . | Induction therapy . | Adjuvant therapy . | Survival (months) . | Recurrence . | Recurrence site . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | TC | 2 | ST | Yes | 1 | 8 | Alive | ||||
| 2 | 67/M | TC | 10 | ST | Yes | 1 | RT | 38 | Dead* | |||
| 3 | 36/M | TC | 2.3 | ST | Yes | 1 | 59 | Alive | ||||
| 4 | 58/M | TC | 8 | ST | No | 4b | 1 | Alive | ||||
| 5 | 42/M | TC | 13 | ST + lung + parathyroid | Yes | 3a | Somatostatin analogue | 74 | Distant + local | Bone, dissemination | Alive | |
| 6 | 55/M | TC | 12.5 | ST + PC | Yes | 2 | RT | 208 | Distant | Bone | Alive | |
| 7 | 50/M | TC | 7 | ST + lung + BCV | Yes | 3a | CRT | 111 | Local | Dissemination | Dead | |
| 8 | 67/F | ATC | 2.9 | Biopsy | No | 4b | 22 | Distant | Lung, bone | Dead | ||
| 9 | 17/F | ATC | 3 | ST | Yes | 1 | 17 | Alive | ||||
| 10 | 50/M | ATC | 1.5 | ST | Yes | 1 | 25 | Alive | ||||
| 11 | 63/M | ATC | 2 | ST | Yes | 1 | 43 | Alive | ||||
| 12 | 63/M | ATC | 7.2 | ST | Yes | 1 | RT | 100 | Distant + local | Lung, bone, dissemination | Alive | |
| 13 | 65/M | ATC | 4.5 | ST | Yes | 1 | 48 | Alive | ||||
| 14 | 48/M | ATC | 13.5 | ST + lung | Yes | 3a | CRT | 43 | Distant | Bone | Dead | |
| 15 | 48/F | ATC | ST + lung | Yes | 4a | CRT | 99 | Local | Dissemination | Dead | ||
| 16 | 61/M | ATC | 11.5 | ST + PC + lung + BCV | Yes | 3a | 84 | Alive | ||||
| 17 | 53/M | ATC | 18 | ST + PC + lung + PN | Yes | 4b | 113 | Local | Mediastinal lymph node | Alive | ||
| 18 | 49/M | ATC | 6.5 | ST + PC + lung + PN | Yes | 3a | 99 | Local | Recurrence in surgical margin | Alive | ||
| 19 | 80/F | LCNEC | 6.5 | ST | Yes | 4a | 71 | Dissemination | Alive | |||
| 20 | 57/F | LCNEC + Thymoma | 9 | ST + lung | Yes | 1 | RT | 30 | Distant | Lung | Alive | |
| 21 | 44/M | LCNEC | 7.8 | ST + PC + lung + PN | Yes | 3a | CDDP + VP16 + RT | 64 | Alive | |||
| 22 | 59/M | SCC | 5 | Biopsy | No | 3a | 14 | Distant | Bone | Dead* | ||
| 23 | 69/M | SCC | 7.5 | ST | Yes | 1 | 36 | Alive | ||||
| 24 | 53/M | SCC | 8.5 | ST | Yes | 1 | 36 | Alive | ||||
| 25 | 56/M | SCC | 9 | ST + pleura | Yes | 4a | RT | 176 | Distant + local | Lung, dissemination | Dead | |
| 26 | 57/M | SCC | 4.5 | ST | Yes | 1 | 60 | Alive | ||||
| 27 | 48/M | SCC | 5.8 | ST | Yes | 1 | 15 | Alive | ||||
| 28 | 62/M | SCC | 3.4 | ST + lung | Yes | 3a | CDDP + DTX | 28 | Alive | |||
| 29 | 49/M | SCC | 13.5 | ST + PC + lung | Yes | 4b | RT | 26 | Distant | Bone, liver | Alive | |
| 30 | 59/M | SCC | 7.5 | ST + SVC + BCV | Yes | 4b | 35 | Distant | Lung | Alive |
| No . | Age/gender . | Histological diagnosis . | Size (cm) . | Operation . | MCR . | Pathological TNM stage . | Induction therapy . | Adjuvant therapy . | Survival (months) . | Recurrence . | Recurrence site . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | TC | 2 | ST | Yes | 1 | 8 | Alive | ||||
| 2 | 67/M | TC | 10 | ST | Yes | 1 | RT | 38 | Dead* | |||
| 3 | 36/M | TC | 2.3 | ST | Yes | 1 | 59 | Alive | ||||
| 4 | 58/M | TC | 8 | ST | No | 4b | 1 | Alive | ||||
| 5 | 42/M | TC | 13 | ST + lung + parathyroid | Yes | 3a | Somatostatin analogue | 74 | Distant + local | Bone, dissemination | Alive | |
| 6 | 55/M | TC | 12.5 | ST + PC | Yes | 2 | RT | 208 | Distant | Bone | Alive | |
| 7 | 50/M | TC | 7 | ST + lung + BCV | Yes | 3a | CRT | 111 | Local | Dissemination | Dead | |
| 8 | 67/F | ATC | 2.9 | Biopsy | No | 4b | 22 | Distant | Lung, bone | Dead | ||
| 9 | 17/F | ATC | 3 | ST | Yes | 1 | 17 | Alive | ||||
| 10 | 50/M | ATC | 1.5 | ST | Yes | 1 | 25 | Alive | ||||
| 11 | 63/M | ATC | 2 | ST | Yes | 1 | 43 | Alive | ||||
| 12 | 63/M | ATC | 7.2 | ST | Yes | 1 | RT | 100 | Distant + local | Lung, bone, dissemination | Alive | |
| 13 | 65/M | ATC | 4.5 | ST | Yes | 1 | 48 | Alive | ||||
| 14 | 48/M | ATC | 13.5 | ST + lung | Yes | 3a | CRT | 43 | Distant | Bone | Dead | |
| 15 | 48/F | ATC | ST + lung | Yes | 4a | CRT | 99 | Local | Dissemination | Dead | ||
| 16 | 61/M | ATC | 11.5 | ST + PC + lung + BCV | Yes | 3a | 84 | Alive | ||||
| 17 | 53/M | ATC | 18 | ST + PC + lung + PN | Yes | 4b | 113 | Local | Mediastinal lymph node | Alive | ||
| 18 | 49/M | ATC | 6.5 | ST + PC + lung + PN | Yes | 3a | 99 | Local | Recurrence in surgical margin | Alive | ||
| 19 | 80/F | LCNEC | 6.5 | ST | Yes | 4a | 71 | Dissemination | Alive | |||
| 20 | 57/F | LCNEC + Thymoma | 9 | ST + lung | Yes | 1 | RT | 30 | Distant | Lung | Alive | |
| 21 | 44/M | LCNEC | 7.8 | ST + PC + lung + PN | Yes | 3a | CDDP + VP16 + RT | 64 | Alive | |||
| 22 | 59/M | SCC | 5 | Biopsy | No | 3a | 14 | Distant | Bone | Dead* | ||
| 23 | 69/M | SCC | 7.5 | ST | Yes | 1 | 36 | Alive | ||||
| 24 | 53/M | SCC | 8.5 | ST | Yes | 1 | 36 | Alive | ||||
| 25 | 56/M | SCC | 9 | ST + pleura | Yes | 4a | RT | 176 | Distant + local | Lung, dissemination | Dead | |
| 26 | 57/M | SCC | 4.5 | ST | Yes | 1 | 60 | Alive | ||||
| 27 | 48/M | SCC | 5.8 | ST | Yes | 1 | 15 | Alive | ||||
| 28 | 62/M | SCC | 3.4 | ST + lung | Yes | 3a | CDDP + DTX | 28 | Alive | |||
| 29 | 49/M | SCC | 13.5 | ST + PC + lung | Yes | 4b | RT | 26 | Distant | Bone, liver | Alive | |
| 30 | 59/M | SCC | 7.5 | ST + SVC + BCV | Yes | 4b | 35 | Distant | Lung | Alive |
Dead* indicates death with other disease.
ATC: atypical carcinoid; BCV: brachiocephalic vein; CDDP: cisplatin; CRT: chemoradiotherapy; DTX: docetaxel; F: female; LCNEC: large-cell neuroendocrine carcinoma; M: male; MCR: macroscopic complete resection; PC: pericardium; PN: phrenic nerve; RT: radiotherapy; SCC: small-cell carcinoma; ST: standard thymectomy; SVC: superior vena cava; TC: typical carcinoid; VP-16: etoposide.
| No . | Age/gender . | Histological diagnosis . | Size (cm) . | Operation . | MCR . | Pathological TNM stage . | Induction therapy . | Adjuvant therapy . | Survival (months) . | Recurrence . | Recurrence site . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | TC | 2 | ST | Yes | 1 | 8 | Alive | ||||
| 2 | 67/M | TC | 10 | ST | Yes | 1 | RT | 38 | Dead* | |||
| 3 | 36/M | TC | 2.3 | ST | Yes | 1 | 59 | Alive | ||||
| 4 | 58/M | TC | 8 | ST | No | 4b | 1 | Alive | ||||
| 5 | 42/M | TC | 13 | ST + lung + parathyroid | Yes | 3a | Somatostatin analogue | 74 | Distant + local | Bone, dissemination | Alive | |
| 6 | 55/M | TC | 12.5 | ST + PC | Yes | 2 | RT | 208 | Distant | Bone | Alive | |
| 7 | 50/M | TC | 7 | ST + lung + BCV | Yes | 3a | CRT | 111 | Local | Dissemination | Dead | |
| 8 | 67/F | ATC | 2.9 | Biopsy | No | 4b | 22 | Distant | Lung, bone | Dead | ||
| 9 | 17/F | ATC | 3 | ST | Yes | 1 | 17 | Alive | ||||
| 10 | 50/M | ATC | 1.5 | ST | Yes | 1 | 25 | Alive | ||||
| 11 | 63/M | ATC | 2 | ST | Yes | 1 | 43 | Alive | ||||
| 12 | 63/M | ATC | 7.2 | ST | Yes | 1 | RT | 100 | Distant + local | Lung, bone, dissemination | Alive | |
| 13 | 65/M | ATC | 4.5 | ST | Yes | 1 | 48 | Alive | ||||
| 14 | 48/M | ATC | 13.5 | ST + lung | Yes | 3a | CRT | 43 | Distant | Bone | Dead | |
| 15 | 48/F | ATC | ST + lung | Yes | 4a | CRT | 99 | Local | Dissemination | Dead | ||
| 16 | 61/M | ATC | 11.5 | ST + PC + lung + BCV | Yes | 3a | 84 | Alive | ||||
| 17 | 53/M | ATC | 18 | ST + PC + lung + PN | Yes | 4b | 113 | Local | Mediastinal lymph node | Alive | ||
| 18 | 49/M | ATC | 6.5 | ST + PC + lung + PN | Yes | 3a | 99 | Local | Recurrence in surgical margin | Alive | ||
| 19 | 80/F | LCNEC | 6.5 | ST | Yes | 4a | 71 | Dissemination | Alive | |||
| 20 | 57/F | LCNEC + Thymoma | 9 | ST + lung | Yes | 1 | RT | 30 | Distant | Lung | Alive | |
| 21 | 44/M | LCNEC | 7.8 | ST + PC + lung + PN | Yes | 3a | CDDP + VP16 + RT | 64 | Alive | |||
| 22 | 59/M | SCC | 5 | Biopsy | No | 3a | 14 | Distant | Bone | Dead* | ||
| 23 | 69/M | SCC | 7.5 | ST | Yes | 1 | 36 | Alive | ||||
| 24 | 53/M | SCC | 8.5 | ST | Yes | 1 | 36 | Alive | ||||
| 25 | 56/M | SCC | 9 | ST + pleura | Yes | 4a | RT | 176 | Distant + local | Lung, dissemination | Dead | |
| 26 | 57/M | SCC | 4.5 | ST | Yes | 1 | 60 | Alive | ||||
| 27 | 48/M | SCC | 5.8 | ST | Yes | 1 | 15 | Alive | ||||
| 28 | 62/M | SCC | 3.4 | ST + lung | Yes | 3a | CDDP + DTX | 28 | Alive | |||
| 29 | 49/M | SCC | 13.5 | ST + PC + lung | Yes | 4b | RT | 26 | Distant | Bone, liver | Alive | |
| 30 | 59/M | SCC | 7.5 | ST + SVC + BCV | Yes | 4b | 35 | Distant | Lung | Alive |
| No . | Age/gender . | Histological diagnosis . | Size (cm) . | Operation . | MCR . | Pathological TNM stage . | Induction therapy . | Adjuvant therapy . | Survival (months) . | Recurrence . | Recurrence site . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | TC | 2 | ST | Yes | 1 | 8 | Alive | ||||
| 2 | 67/M | TC | 10 | ST | Yes | 1 | RT | 38 | Dead* | |||
| 3 | 36/M | TC | 2.3 | ST | Yes | 1 | 59 | Alive | ||||
| 4 | 58/M | TC | 8 | ST | No | 4b | 1 | Alive | ||||
| 5 | 42/M | TC | 13 | ST + lung + parathyroid | Yes | 3a | Somatostatin analogue | 74 | Distant + local | Bone, dissemination | Alive | |
| 6 | 55/M | TC | 12.5 | ST + PC | Yes | 2 | RT | 208 | Distant | Bone | Alive | |
| 7 | 50/M | TC | 7 | ST + lung + BCV | Yes | 3a | CRT | 111 | Local | Dissemination | Dead | |
| 8 | 67/F | ATC | 2.9 | Biopsy | No | 4b | 22 | Distant | Lung, bone | Dead | ||
| 9 | 17/F | ATC | 3 | ST | Yes | 1 | 17 | Alive | ||||
| 10 | 50/M | ATC | 1.5 | ST | Yes | 1 | 25 | Alive | ||||
| 11 | 63/M | ATC | 2 | ST | Yes | 1 | 43 | Alive | ||||
| 12 | 63/M | ATC | 7.2 | ST | Yes | 1 | RT | 100 | Distant + local | Lung, bone, dissemination | Alive | |
| 13 | 65/M | ATC | 4.5 | ST | Yes | 1 | 48 | Alive | ||||
| 14 | 48/M | ATC | 13.5 | ST + lung | Yes | 3a | CRT | 43 | Distant | Bone | Dead | |
| 15 | 48/F | ATC | ST + lung | Yes | 4a | CRT | 99 | Local | Dissemination | Dead | ||
| 16 | 61/M | ATC | 11.5 | ST + PC + lung + BCV | Yes | 3a | 84 | Alive | ||||
| 17 | 53/M | ATC | 18 | ST + PC + lung + PN | Yes | 4b | 113 | Local | Mediastinal lymph node | Alive | ||
| 18 | 49/M | ATC | 6.5 | ST + PC + lung + PN | Yes | 3a | 99 | Local | Recurrence in surgical margin | Alive | ||
| 19 | 80/F | LCNEC | 6.5 | ST | Yes | 4a | 71 | Dissemination | Alive | |||
| 20 | 57/F | LCNEC + Thymoma | 9 | ST + lung | Yes | 1 | RT | 30 | Distant | Lung | Alive | |
| 21 | 44/M | LCNEC | 7.8 | ST + PC + lung + PN | Yes | 3a | CDDP + VP16 + RT | 64 | Alive | |||
| 22 | 59/M | SCC | 5 | Biopsy | No | 3a | 14 | Distant | Bone | Dead* | ||
| 23 | 69/M | SCC | 7.5 | ST | Yes | 1 | 36 | Alive | ||||
| 24 | 53/M | SCC | 8.5 | ST | Yes | 1 | 36 | Alive | ||||
| 25 | 56/M | SCC | 9 | ST + pleura | Yes | 4a | RT | 176 | Distant + local | Lung, dissemination | Dead | |
| 26 | 57/M | SCC | 4.5 | ST | Yes | 1 | 60 | Alive | ||||
| 27 | 48/M | SCC | 5.8 | ST | Yes | 1 | 15 | Alive | ||||
| 28 | 62/M | SCC | 3.4 | ST + lung | Yes | 3a | CDDP + DTX | 28 | Alive | |||
| 29 | 49/M | SCC | 13.5 | ST + PC + lung | Yes | 4b | RT | 26 | Distant | Bone, liver | Alive | |
| 30 | 59/M | SCC | 7.5 | ST + SVC + BCV | Yes | 4b | 35 | Distant | Lung | Alive |
Dead* indicates death with other disease.
ATC: atypical carcinoid; BCV: brachiocephalic vein; CDDP: cisplatin; CRT: chemoradiotherapy; DTX: docetaxel; F: female; LCNEC: large-cell neuroendocrine carcinoma; M: male; MCR: macroscopic complete resection; PC: pericardium; PN: phrenic nerve; RT: radiotherapy; SCC: small-cell carcinoma; ST: standard thymectomy; SVC: superior vena cava; TC: typical carcinoid; VP-16: etoposide.
Survival analysis
For all patients, the 5-year OS was 77% and 10-year OS was 35% (Fig. 2A), whereas the 5-year CSS was 90%. The 5- and 10-year RFS rates were 48% and 29%, respectively (Fig. 2A). In patients who underwent MCR, the 5- and 10-year OS rates were 83% and 38%, respectively, whereas the 5- and 10-year RFS rates were 53% and 32%, respectively (Fig. 2B). OS was significantly favourable for patients who underwent MCR, whereas histological type (Fig. 2C), tumour size and Ki67 index did not have significant effects. RFS was favourable for men, patients diagnosed in an early stage (Fig. 2D) and without adjuvant therapy.
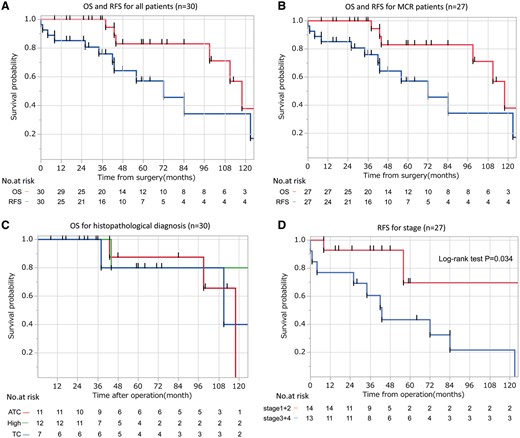
(A–D) The Kaplan–Meier survival curves according to (A) OS and RFS in all patients, (B) OS and RFS in patients who underwent MCR, (C) OS based on histopathological type and (D) RFS based on TNM stage. ATC: atypical carcinoid; OS: overall survival; RFS: relapse-free survival; MCR: macroscopic complete resection; TC: typical carcinoid.
In univariate analysis, OS was good for patients who underwent MCR (P = 0.010). As for RFS, patients classified as TNM Stage I or II (P = 0.011) and without adjuvant therapy (P = 0.042) showed good results. No significant differences were observed with regard to Masaoka stage, histological grade, tumour size or Ki67 index (Table 3).
| Variables . | Overall survival . | Relapse-free survival . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% confidence interval . | P-value . | Hazard ratio . | 95% confidence interval . | P-value . | |
| Gender | 0.16 | 0.068 | ||||
| Male | 1.00 | 1.000 | ||||
| Female | 3.97 | 0.520–24.1 | 3.45 | 0.90–11.3 | ||
| Histopathological type | 0.86 | 0.89 | ||||
| TC + ATC | 1.00 | 1.00 | ||||
| SCC + LCNEC | 0.861 | 0.119–4.23 | 1.07 | 0.359–2.96 | ||
| Resection status | 0.010 | NA | ||||
| MCR | 1.00 | |||||
| No MCR | 4.15 | 1.41–12.2 | ||||
| Masaoka stage | 0.57 | 0.16 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 1.85 | 0.263–36.6 | 2.36 | 0.720–10.5 | ||
| TNM stage | 0.15 | 0.011 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 3.95 | 0.660–75.2 | 5.26 | 1.41–34.0 | ||
| Tumour size | 0.68 | 0.35 | ||||
| <7cm | 1.00 | 1.00 | ||||
| ≥7cm | 1.51 | 0.181–12.6 | 1.73 | 0.567–6.39 | ||
| Ki67 index | 0.65 | 0.83 | ||||
| <10% | 1.00 | 1.00 | ||||
| ≥10% | 1.55 | 0.236–12.7 | 0.888 | 0.315–2.88 | ||
| Adjuvant therapya | 0.16 | 0.042 | ||||
| None | 1.00 | 1.00 | ||||
| Done | 3.93 | 0.615–76.2 | 3.34 | 1.05–12.6 | ||
| Variables . | Overall survival . | Relapse-free survival . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% confidence interval . | P-value . | Hazard ratio . | 95% confidence interval . | P-value . | |
| Gender | 0.16 | 0.068 | ||||
| Male | 1.00 | 1.000 | ||||
| Female | 3.97 | 0.520–24.1 | 3.45 | 0.90–11.3 | ||
| Histopathological type | 0.86 | 0.89 | ||||
| TC + ATC | 1.00 | 1.00 | ||||
| SCC + LCNEC | 0.861 | 0.119–4.23 | 1.07 | 0.359–2.96 | ||
| Resection status | 0.010 | NA | ||||
| MCR | 1.00 | |||||
| No MCR | 4.15 | 1.41–12.2 | ||||
| Masaoka stage | 0.57 | 0.16 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 1.85 | 0.263–36.6 | 2.36 | 0.720–10.5 | ||
| TNM stage | 0.15 | 0.011 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 3.95 | 0.660–75.2 | 5.26 | 1.41–34.0 | ||
| Tumour size | 0.68 | 0.35 | ||||
| <7cm | 1.00 | 1.00 | ||||
| ≥7cm | 1.51 | 0.181–12.6 | 1.73 | 0.567–6.39 | ||
| Ki67 index | 0.65 | 0.83 | ||||
| <10% | 1.00 | 1.00 | ||||
| ≥10% | 1.55 | 0.236–12.7 | 0.888 | 0.315–2.88 | ||
| Adjuvant therapya | 0.16 | 0.042 | ||||
| None | 1.00 | 1.00 | ||||
| Done | 3.93 | 0.615–76.2 | 3.34 | 1.05–12.6 | ||
Estimated only in MCR cases.
ATC: atypical carcinoid; LCNEC: large-cell neuroendocrine carcinoma; MCR: macroscopic complete resection; NA: not applicable; SCC: small-cell carcinoma; TC: typical carcinoid.
| Variables . | Overall survival . | Relapse-free survival . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% confidence interval . | P-value . | Hazard ratio . | 95% confidence interval . | P-value . | |
| Gender | 0.16 | 0.068 | ||||
| Male | 1.00 | 1.000 | ||||
| Female | 3.97 | 0.520–24.1 | 3.45 | 0.90–11.3 | ||
| Histopathological type | 0.86 | 0.89 | ||||
| TC + ATC | 1.00 | 1.00 | ||||
| SCC + LCNEC | 0.861 | 0.119–4.23 | 1.07 | 0.359–2.96 | ||
| Resection status | 0.010 | NA | ||||
| MCR | 1.00 | |||||
| No MCR | 4.15 | 1.41–12.2 | ||||
| Masaoka stage | 0.57 | 0.16 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 1.85 | 0.263–36.6 | 2.36 | 0.720–10.5 | ||
| TNM stage | 0.15 | 0.011 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 3.95 | 0.660–75.2 | 5.26 | 1.41–34.0 | ||
| Tumour size | 0.68 | 0.35 | ||||
| <7cm | 1.00 | 1.00 | ||||
| ≥7cm | 1.51 | 0.181–12.6 | 1.73 | 0.567–6.39 | ||
| Ki67 index | 0.65 | 0.83 | ||||
| <10% | 1.00 | 1.00 | ||||
| ≥10% | 1.55 | 0.236–12.7 | 0.888 | 0.315–2.88 | ||
| Adjuvant therapya | 0.16 | 0.042 | ||||
| None | 1.00 | 1.00 | ||||
| Done | 3.93 | 0.615–76.2 | 3.34 | 1.05–12.6 | ||
| Variables . | Overall survival . | Relapse-free survival . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% confidence interval . | P-value . | Hazard ratio . | 95% confidence interval . | P-value . | |
| Gender | 0.16 | 0.068 | ||||
| Male | 1.00 | 1.000 | ||||
| Female | 3.97 | 0.520–24.1 | 3.45 | 0.90–11.3 | ||
| Histopathological type | 0.86 | 0.89 | ||||
| TC + ATC | 1.00 | 1.00 | ||||
| SCC + LCNEC | 0.861 | 0.119–4.23 | 1.07 | 0.359–2.96 | ||
| Resection status | 0.010 | NA | ||||
| MCR | 1.00 | |||||
| No MCR | 4.15 | 1.41–12.2 | ||||
| Masaoka stage | 0.57 | 0.16 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 1.85 | 0.263–36.6 | 2.36 | 0.720–10.5 | ||
| TNM stage | 0.15 | 0.011 | ||||
| 1 + 2 | 1.00 | 1.00 | ||||
| 3 + 4 | 3.95 | 0.660–75.2 | 5.26 | 1.41–34.0 | ||
| Tumour size | 0.68 | 0.35 | ||||
| <7cm | 1.00 | 1.00 | ||||
| ≥7cm | 1.51 | 0.181–12.6 | 1.73 | 0.567–6.39 | ||
| Ki67 index | 0.65 | 0.83 | ||||
| <10% | 1.00 | 1.00 | ||||
| ≥10% | 1.55 | 0.236–12.7 | 0.888 | 0.315–2.88 | ||
| Adjuvant therapya | 0.16 | 0.042 | ||||
| None | 1.00 | 1.00 | ||||
| Done | 3.93 | 0.615–76.2 | 3.34 | 1.05–12.6 | ||
Estimated only in MCR cases.
ATC: atypical carcinoid; LCNEC: large-cell neuroendocrine carcinoma; MCR: macroscopic complete resection; NA: not applicable; SCC: small-cell carcinoma; TC: typical carcinoid.
Survival after recurrence in patients with postoperative relapse was 92% at 2 years and 25% at 5 years. Prognosis was more favourable in patients with only local recurrence or dissemination when compared with patients with distant metastasis (P = 0.049); however, the difference between with and without re-resection was not significant (P = 0.92).
DISCUSSION
Epidemiological findings have shown that a TNET occurs more frequently in men with a mean age of 50 years, and nearly all TNETs have been reported to be non-functional [2, 4, 18], which were confirmed by our findings. The most common histological type of TNET reported is ATC [1]. These characteristics differentiate TNET from gastroenteropancreatic NET (GEP-NET), the most commonly reported NETs. TNET is a rare disease for which standard treatment has yet to be established.
Currently, surgical resection is the first treatment choice, as in thymic carcinoma patients, with inoperative and recurrent cases treated according to the regimen used for PNET. Complete resection is considered to be an important prognostic factor for patients with a thymic carcinoma [19–21] and a TNET [2, 9, 11, 14–16], and the rates of OS and RFS of TNET patients have been reported to be quite similar to those of patients with thymic cancer [19, 22]. In this study, CSS for patients who underwent an MCR procedure was 94.1%, thus complete resection may be the first treatment choice for TNET.
The 5-year survival rate of TNET patients has been reported to range from 27% [8] to 91.6% [12] and 10-year survival from 0% [9] to 69.8% [12] (Table 4). However, reports in the last 10 years have noted better rates for 5-year survival ranging from 53% to 91.6% and for 10-year survival from 39% to 69.8% [2, 4, 11, 12–15], as also seen in this study. These improvements in survival are considered to be the result of enhancements of staging technique and perioperative management as well as progress in chemotherapy and radiotherapy.
| Reference . | Year . | No. . | 5 years (%) . | 10 years (%) . |
|---|---|---|---|---|
| Wick et al. [8] | 1982 | 15 | 27 | 7 |
| de Montpréville et al. [9] | 1996 | 14 | 31 | 0 |
| Fukai et al. [10] | 1999 | 15 | 33 | 7 |
| Moran and Suster [5] | 2000 | 80 | 29 | 10 |
| Tiffet et al. [7] | 2003 | 12 | 50 | NA |
| Cardillo et al. [12] | 2010 | 19 | 91.6 | 69.8 |
| Gaur et al. [2] | 2010 | 160 | 53 | NA |
| Cardillo et al. [4] | 2012 | 35 | 84.6 | 60.8 |
| Crona et al. [14] | 2013 | 28 | 79 | 41 |
| Song and Zhang [16] | 2014 | 22 | 45.5 | NA |
| Filosso et al. [15] | 2015 | 205 | 68 | 39 |
| Current series | 2016 | 30 | 81.7 | 52.5 |
| Reference . | Year . | No. . | 5 years (%) . | 10 years (%) . |
|---|---|---|---|---|
| Wick et al. [8] | 1982 | 15 | 27 | 7 |
| de Montpréville et al. [9] | 1996 | 14 | 31 | 0 |
| Fukai et al. [10] | 1999 | 15 | 33 | 7 |
| Moran and Suster [5] | 2000 | 80 | 29 | 10 |
| Tiffet et al. [7] | 2003 | 12 | 50 | NA |
| Cardillo et al. [12] | 2010 | 19 | 91.6 | 69.8 |
| Gaur et al. [2] | 2010 | 160 | 53 | NA |
| Cardillo et al. [4] | 2012 | 35 | 84.6 | 60.8 |
| Crona et al. [14] | 2013 | 28 | 79 | 41 |
| Song and Zhang [16] | 2014 | 22 | 45.5 | NA |
| Filosso et al. [15] | 2015 | 205 | 68 | 39 |
| Current series | 2016 | 30 | 81.7 | 52.5 |
NA: not applicable.
| Reference . | Year . | No. . | 5 years (%) . | 10 years (%) . |
|---|---|---|---|---|
| Wick et al. [8] | 1982 | 15 | 27 | 7 |
| de Montpréville et al. [9] | 1996 | 14 | 31 | 0 |
| Fukai et al. [10] | 1999 | 15 | 33 | 7 |
| Moran and Suster [5] | 2000 | 80 | 29 | 10 |
| Tiffet et al. [7] | 2003 | 12 | 50 | NA |
| Cardillo et al. [12] | 2010 | 19 | 91.6 | 69.8 |
| Gaur et al. [2] | 2010 | 160 | 53 | NA |
| Cardillo et al. [4] | 2012 | 35 | 84.6 | 60.8 |
| Crona et al. [14] | 2013 | 28 | 79 | 41 |
| Song and Zhang [16] | 2014 | 22 | 45.5 | NA |
| Filosso et al. [15] | 2015 | 205 | 68 | 39 |
| Current series | 2016 | 30 | 81.7 | 52.5 |
| Reference . | Year . | No. . | 5 years (%) . | 10 years (%) . |
|---|---|---|---|---|
| Wick et al. [8] | 1982 | 15 | 27 | 7 |
| de Montpréville et al. [9] | 1996 | 14 | 31 | 0 |
| Fukai et al. [10] | 1999 | 15 | 33 | 7 |
| Moran and Suster [5] | 2000 | 80 | 29 | 10 |
| Tiffet et al. [7] | 2003 | 12 | 50 | NA |
| Cardillo et al. [12] | 2010 | 19 | 91.6 | 69.8 |
| Gaur et al. [2] | 2010 | 160 | 53 | NA |
| Cardillo et al. [4] | 2012 | 35 | 84.6 | 60.8 |
| Crona et al. [14] | 2013 | 28 | 79 | 41 |
| Song and Zhang [16] | 2014 | 22 | 45.5 | NA |
| Filosso et al. [15] | 2015 | 205 | 68 | 39 |
| Current series | 2016 | 30 | 81.7 | 52.5 |
NA: not applicable.
Tumour size, histological grade, paraneoplastic symptoms, Masaoka stage, TNM stage, surgical resection and Ki67 index have been reported to be prognostic factors [2, 4, 14, 15]. In this study, OS was favourable only in patients who underwent MCR. However, the number of patients investigated was low, and those factors were not concluded to be prognostic factors. Some reports have noted that histopathological type is not a prognostic factor. This may be a characteristic of TNET, because patients with high-grade NET did not exhibit worse prognosis than those with low-/intermediate-grade NET, in contrast to PNET or GEP-NET. Also, TNM classification [17] may be useful for forecasting prognosis of TNET patients, because RFS was favourable for patients diagnosed in an early TNM stage.
Adjuvant therapy was performed in 10 of the present patients, with 9 patients eventually suffering from recurrence, thus it remains unknown whether such therapy is effective. OS was not significantly different between with and without adjuvant therapy, though RFS was better in patients who did not undergo treatment. We considered that patients who received adjuvant therapy were in a more advanced stage. However, the site of recurrence in all these patients was distant or pleural dissemination, not local; hence, radiotherapy was likely effective for controlling local recurrence. Three patients were treated with adjuvant chemoradiotherapy in the 1990s, when the regimen consisted of cisplatin, vindesine and mitomycin C, and each developed distant metastasis and recurrence. In contrast, in the most recent 10-year period, none of the patients were treated with postoperative adjuvant chemotherapy, as effectiveness is controversial.
In our previous study, 1 patient diagnosed with large-cell neuroendocrine carcinoma based on preoperative computed tomography-guided biopsy results showed remarkable response to induction chemoradiotherapy with cisplatin plus etoposide and had a long-term RFS period [23]. Since chemotherapy or radiotherapy may be effective, especially for high-grade TNET, we considered that a preoperative biopsy examination should be performed for patients suspected to have a malignant thymic tumour, which is difficult to completely remove, for the purpose of complete resection and selection of an appropriate chemotherapeutic regimen. However, it is necessary to collect an adequately sized sample, because accurate pathohistological diagnosis is expected to be difficult, as the diagnosis based on the preoperative biopsy was changed in 3 patients in the present series.
Distant metastasis, especially in bone and lung tissues, was more prevalent than local recurrence, which is similar to PNET. A regimen including a platinum-containing drug along with a third-generation anticancer drug (docetaxel, etoposide and CPT-11) has been used in recent patients with high-grade TNET, whereas somatostatin analogue therapy is used for low-/intermediate-grade patients. Crona et al. [14] reported that temozolomide treatment resulted in partial response and a stable disease condition. Recently, several new drugs for low-/intermediate-grade GEP-NET have been introduced, such as sunitinib [24] and streptozocin [25], whereas everolimus has also been shown effective for PNET [26, 27]. Good effects for TNET as well can be expected from these drugs.
Limitations
On the basis of our results, no conclusion could be drawn regarding the effects of tumour size, Masaoka stage, or Ki67 index on prognosis, because of the limited number of cases analysed. On the other hand, a diagnosis of TNET was definitive based on current criteria, because all patients were rediagnosed using pathological findings. In this multicentre study, we found that histopathological diagnosis was difficult, though we were able to clarify outcomes in patients who received a variety of different therapies. Because of the rarity of TNET, it will be necessary to continue accumulating cases from several institutions.
CONCLUSION
The prognosis of TNET patients was favourable in patients who underwent MCR, and we concluded that MCR is an important prognostic factor, the same as in thymic cancer patients. Distant metastasis in bone and lung tissues was found to be more prevalent than local recurrence, similar to PNET. Cancer-bearing survival is promising even in patients with postoperative recurrence, following treatment utilized for PNET or GEP-NET.
ACKNOWLEDGEMENTS
We thank all institutions that have joined this study: Osaka University, National Hospital Organization Kinki-chuo Chest Medical Center, Osaka City Medical Hospital, Osaka Prefectural Medical Center for Respiratory and Allergic Diseases, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka Police Hospital, Rinku General Medical Center, JCHO Osaka Hospital, Takarazuka City Hospital and Toneyama Hospital.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 7th Annual Meeting of International Thymic Malignancy Interest Group, San Francisco, CA, USA, 16 September 2016.
- biopsy
- cancer
- carcinoid tumor
- carcinoma
- objective (goal)
- neoadjuvant therapy
- surgical procedures, operative
- survival rate
- neoplasms
- thoracic surgery procedures
- adjuvant therapy
- lung parenchyma
- metastasis, distant
- pulmonary neuroendocrine neoplasms
- excision
- thymic neuroendocrine tumor
- gastro-enteropancreatic neuroendocrine tumor
- large cell neuroendocrine carcinoma




