-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Perry, Elizabeth David, Broadus Atkins, Gary Raff, Novel application of a percutaneous left ventricular assist device as a bridge to transplant in a paediatric patient with severe heart failure due to viral myocarditis, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 3, March 2017, Pages 474–476, https://doi.org/10.1093/icvts/ivw387
Close - Share Icon Share
Abstract
A 13-year obese female with suspected viral myocarditis presented with acute decompensated heart failure. Due to her body habitus, she was a poor candidate for immediate heart transplantation. A peripherally inserted left ventricular assist device (LVAD) was implanted via the right axillary artery. Following device insertion the patient experienced rapid improvement in symptoms. The LVAD provided effective left ventricular unloading for 50 days, promoting myocardial recovery and maintaining excellent patient performance status. The device placement strategy allowed for a high level of activity including completion of school-work and participation in a weight loss program. The patient achieved a 28-pound weight loss, thus improving candidacy for transplantation. Removal of the device was well tolerated and post-removal echocardiography revealed an improvement in the left ventricular ejection fraction (LVEF) from 21% at baseline to 38% after device removal. This case represents a successful application of a peripherally inserted LVAD as a bridge to transplant in a pediatric patient with severe heart failure due to suspected viral myocarditis. For select patients with this condition, a transaxillary LVAD should be considered as a therapeutic option as it is well tolerated and provides effective left ventricle unloading to promote myocardial recovery and maintain performance status.
Introduction
CTSNet Video Content: http://www.ctsnet.org/article/novel-application-percutaneous-left-ventricular-assist-device-bridge-transplant-pediatric (Reproduced with permission from CTSNet. Copyright: CTSNet 2015).
Case
A 13-year female presented to an outside institution with progressive dyspnoea for 2 weeks. An echocardiogram was performed and was significant for a LVEF of 22%. Viral myocarditis was suspected. Inotropic support was initiated for hypotension. She was then transferred to our institution for further evaluation and management.
Her medical history was significant for asthma and morbid obesity. Examination revealed a well-developed adolescent with a body mass index (BMI) of 42 (160 cm, 108 kg). The respiratory rate was 25 and she required 35% inhaled oxygen to maintain saturation >90%. Chest X-ray showed cardiomegaly and pulmonary congestion. Repeat echocardiogram confirmed a LVEF of 21% but preserved right ventricular function. Myocardial magnetic resonance imaging (MRI) was significant for active myocardial inflammation.
The patient continued to decompensate, becoming hemodynamically unstable with minimal activity despite maximal intravenous inotropic support. She was determined to be a poor candidate for immediate heart transplantation due to her BMI. Regarding mechanical circulatory support, she appeared to be an appropriate candidate for left ventricular assist alone as her myocardial dysfunction was largely confined to the left ventricle. Because the myocardial MRI showed active inflammation we were optimistic that functional recovery was still possible. We recognized the importance of sustained physical activity as part of an aggressive weight loss regimen to improve her candidacy for transplant, should this be required. We therefore proceeded with implantation of a transaxillary Impella 5.0 LVAD as this would accomplish effective left ventricular unloading, avoid a sternotomy or thoracotomy, and permit a relatively short recovery and high-level of physical activity.
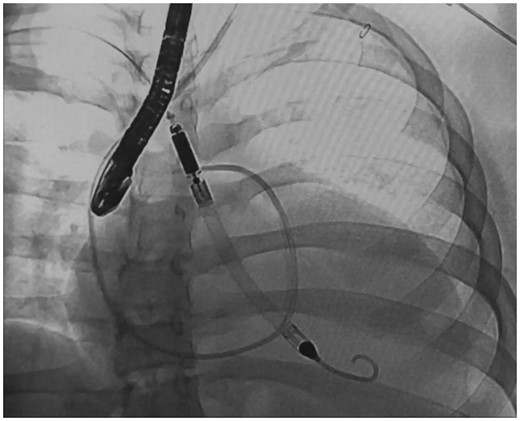
Insertion of Impella LVAD under fluoroscopy and echocardiography guidance
Her pain was minimal and she began ambulating on postoperative day 2. Intravenous inotropes were rapidly weaned and she was optimized on oral medication. Anticoagulation consisted of heparin solution (50 units/ml) via the device ‘purge’ catheter. Her postoperative course was unremarkable for several weeks and she was able to participate in school-work and an exercise program. Serial surveillance echocardiography persistently demonstrated improved ventricular function. On post-implant day 51 she developed hematuria. She then returned to the operating room for removal of the device and ligation of the axillary graft. Postoperative echocardiography demonstrated a LVEF of 38% and no evidence of device related cardiac injury. Her hematuria rapidly resolved. She continued to do well clinically and was discharged to home 9 days later. During the course of the hospitalization she achieved a 13-kg weight loss. She did well for several months at home but later developed recurring heart failure symptoms for which she was able to undergo readmitted and then underwent successful heart transplantation 6 months after Impella removal.
Discussion
This case represents a successful application of a transaxillary LVAD in a pediatric patient with viral myocarditis. There are several disadvantages associated with this device. These include the potential for hemolysis, infection, acute renal dysfunction, vascular complications, mitral valve injury and aortic valve injury [2]. Established weaning protocols have not been developed and there is a paucity of data related to extended use of this device. Despite these drawbacks there are clearly many advantages of using this peripheral, temporary LVAD for patients with acute viral myocarditis. transaxillary placement avoids a sternotomy or thoracotomy and allows for excellent mobility and activity. The device provides effective ventricular unloading for most patients to allow for myocardial recovery. The incidence of Impella related complications is low [3]. Importantly, peripheral insertion of the device has minimal impact on future procedures such as durable device implant or transplant.
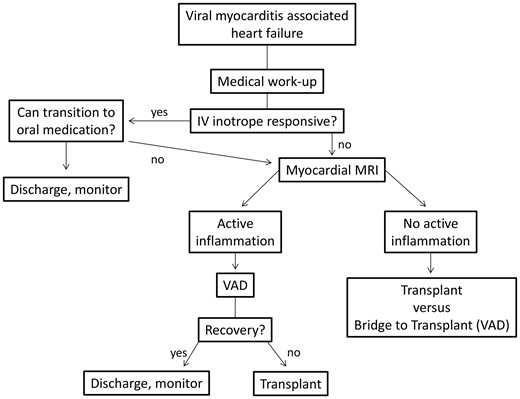
Decision-making algorithm for management of acute viral myocarditis induced heart failure
In conclusion, for select patients with viral myocarditis associated severe heart failure, a transaxillary Impella 5.0 LVAD should be considered as a therapeutic option as it is well tolerated and provides effective left ventricular unloading to promote myocardial recovery and maintain performance status.
Conflict of interest: none declared.
REFERENCES
Author notes
This presentation was published on CTSNet on 30 June 2015. It has been selected as the winner of the 2015 CTSNet Resident Video Competition. The editorial staff of CTSNet have nominated it as a ‘Best of CTSNet’ submission. A written patient consent has been obtained for this video publication.
- myocardium
- obesity
- left ventricular ejection fraction
- heart transplantation
- echocardiography
- viral myocarditis
- heart failure
- left ventricle
- weight reduction
- ventricular assist device
- axillary artery
- device removal
- karnofsky performance status
- pediatrics
- transplantation
- medical devices
- implantation procedure
- acute decompensated heart failure
- assessment of body build




