-
PDF
- Split View
-
Views
-
Cite
Cite
Kanhua Yin, Zhiqi Zhang, Yi Lin, Changfa Guo, Yongxin Sun, Ziwei Tian, Qiuchen Xie, Chunsheng Wang, Surgical management of aortic coarctation in adolescents and adults, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 3, March 2017, Pages 430–435, https://doi.org/10.1093/icvts/ivw353
Close - Share Icon Share
Abstract
OBJECTIVES: Coarctation of the aorta (CoA) in adolescents and adults is often complicated by other cardiac or aortic problems and may carry additional surgical difficulties. Limited studies have reported the surgical outcomes of CoA repair in this particular patient population. We reviewed our contemporary experience of open surgical management of CoA in adolescents and adults.
METHODS: From 2008 to 2016, a total of 60 adolescents and adults (mean age, 32 years) underwent CoA repair at our centre. Of this cohort, 43 patients only underwent CoA repair (isolated group), while the remaining 17 underwent other concomitant cardiac procedures (concomitant group). Ascending-to-descending aortic bypass was the preferred repair technique in the concomitant group. Aortic valve replacement and a Bentall/David procedure were the two most frequently combined procedures.
RESULTS: There were no in-hospital deaths in the isolated group compared with three in the concomitant group (0% vs 17.6%, P = 0.029). The median intensive care unit stay (1 vs 2 days, P<0.01) and postoperative hospital stay (6 vs 9 days, P<0.01) were significantly longer in the concomitant group. Postoperative respiratory failure occurred more frequently in the concomitant group (0% vs 17.6%, P = 0.029). The isolated group had better overall survival during follow-up (P = 0.037). The 5-year overall survival rate was 98% in the isolated group and 82% in the concomitant group.
CONCLUSIONS: Surgical management of coarctation in adolescents and adults can achieve similar satisfactory outcomes as their neonatal counterparts, but the complexity of the concomitant procedures is a risk factor for this particular patient population.
Introduction
Coarctation of the aorta (CoA) accounts for 5–8% of all congenital heart diseases, and the prevalence of the isolated form is approximately 3 per 10 000 live births [1]. If untreated, patients usually develop hypertension, heart failure and other related cardiovascular diseases and die in their 30s to 40s [2]. Since surgical repair of CoA was first described in the mid-1940s, it has continued to be a major treatment option for patients with CoA. A recent large multiple-centre cohort from the Society of Thoracic Surgeons Congenital Heart Surgery Database reported that the overall mortality due to surgery was 2.4% and that postoperative complications occurred in 36% of all patients [3]. Long-term follow-up data showed the actuarial survival rates in patients with CoA repair reached 93.3%, 86.4% and 73.5% at 10, 20 and 30 years after surgery, respectively [4].
While most patients with CoA are treated early, untreated aortic coarctation in adolescents and adults represents a different cohort of patients. Compared to their neonatal or infant counterparts, these patients may have less severe stenosis of the aorta (thus bypassing early intervention) but present with extensive collateral arterials as well as more comorbidities such as chronic hypertension, cerebral artery aneurysm, aortic dilation and heart valve disorders, all of which represent additional difficulties for open surgery. Limited data regarding the outcomes of CoA repair in adolescents and/or adults have been published [5–8]. The objective of this study is to review our single-centre contemporary experience of open surgical repair of CoA in this particular patient population.
Materials and Methods
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval number: B2016-023). Demographic information, operative details and postoperative data were collected retrospectively from medical records and the Zhongshan Hospital Electronic Health Record System.
Study population
From May 2008 to January 2016, a total of 60 consecutive patients underwent open repair of CoA at the Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai, China. Of the 60 patients, 10 were adolescents (12–18 years old) and the rest were adults (> 18 years old). The mean age of this cohort was 32 years (range: 12–65 years). We classified the patients into either the isolated CoA repair group (isolated group, n = 43) or the CoA repair with concomitant cardiac procedure group (concomitant group, n = 17). The isolated group included patients who underwent CoA repair only or CoA repair with patent ductus arteriosus ligation. The concomitant group included patients who had other significant cardiac or aortic abnormalities such as cardiac valvular disorders, congenital anomalies and aortic aneurysms/dissections, all of which require single-stage or two-stage operations to correct both the CoA and concomitant cardiopathies. The patients’ demographic and preoperative data are summarized and compared in Table 1.
| Characteristic . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 32.0 ± 13.8 | 32.3 ± 14.9 | 31.5 ± 10 .8 | 0 .846 |
| Range | 12 –65 | 12 –65 | 14 –52 | |
| Male | 39 (65.0) | 25 (58.1) | 14 (82.4) | 0 .076 |
| Preoperative SBP (mmHg) | 162.1 ± 28.3 | 160.3 ± 24.4 | 166.6 ± 36.0 | 0 .529 |
| Preoperative DBP (mmHg) | 85.0 ± 15.2 | 88.3 ± 13.4 | 76.7 ± 16.1 | 0 .007* |
| Comorbidities | ||||
| Hypertension | 53 (88.3) | 40 (93.0) | 13 (76.5) | 0 .092 |
| Stroke | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Coronary artery disease | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Infectious endocarditis | 2 (3.3) | 0 (0) | 2 (11.8) | 0 .077 |
| Aortic valve disease | 15 (25.0) | 0 (0) | 15 (88.2) | <0 .001* |
| Mitral valve disease | 4 (6.7) | 0 (0) | 4 (23.5) | 0 .005* |
| Aortic dissection | 1 (1.9) | 0 (0) | 1 (5.9) | 0 .283 |
| Associated anomalies | ||||
| BAV | 23 (38.3) | 12 (27.9) | 11 (64.7) | 0 .008* |
| Dilated thoracic aorta | 19 (31.7) | 9 (20 .9) | 10 (58.8) | 0 .004* |
| Aortic size, mm | 47.5 ± 9.1 | 41.8 ± 3.1 | 52.7 ± 9.6 | 0 .008* |
| PLSVC | 6 (10 .0) | 4 (9.3) | 2 (11.8) | 1 .000 |
| VSD | 4 (6.7) | 1 (2.3) | 3 (17.6) | 0 .065 |
| ASD/PFO | 3 (5.0) | 1 (2.3) | 2 (11.8) | 0 .191 |
| PDA | 2 (3.7) | 2 (4.7) | 0 (0) | 1 .000 |
| Turner syndrome | 1 (1.9) | 1 (2.3) | 0 (0) | 1 .000 |
| Previous cardiac operation | ||||
| Prior CoA repair | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Others | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Characteristic . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 32.0 ± 13.8 | 32.3 ± 14.9 | 31.5 ± 10 .8 | 0 .846 |
| Range | 12 –65 | 12 –65 | 14 –52 | |
| Male | 39 (65.0) | 25 (58.1) | 14 (82.4) | 0 .076 |
| Preoperative SBP (mmHg) | 162.1 ± 28.3 | 160.3 ± 24.4 | 166.6 ± 36.0 | 0 .529 |
| Preoperative DBP (mmHg) | 85.0 ± 15.2 | 88.3 ± 13.4 | 76.7 ± 16.1 | 0 .007* |
| Comorbidities | ||||
| Hypertension | 53 (88.3) | 40 (93.0) | 13 (76.5) | 0 .092 |
| Stroke | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Coronary artery disease | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Infectious endocarditis | 2 (3.3) | 0 (0) | 2 (11.8) | 0 .077 |
| Aortic valve disease | 15 (25.0) | 0 (0) | 15 (88.2) | <0 .001* |
| Mitral valve disease | 4 (6.7) | 0 (0) | 4 (23.5) | 0 .005* |
| Aortic dissection | 1 (1.9) | 0 (0) | 1 (5.9) | 0 .283 |
| Associated anomalies | ||||
| BAV | 23 (38.3) | 12 (27.9) | 11 (64.7) | 0 .008* |
| Dilated thoracic aorta | 19 (31.7) | 9 (20 .9) | 10 (58.8) | 0 .004* |
| Aortic size, mm | 47.5 ± 9.1 | 41.8 ± 3.1 | 52.7 ± 9.6 | 0 .008* |
| PLSVC | 6 (10 .0) | 4 (9.3) | 2 (11.8) | 1 .000 |
| VSD | 4 (6.7) | 1 (2.3) | 3 (17.6) | 0 .065 |
| ASD/PFO | 3 (5.0) | 1 (2.3) | 2 (11.8) | 0 .191 |
| PDA | 2 (3.7) | 2 (4.7) | 0 (0) | 1 .000 |
| Turner syndrome | 1 (1.9) | 1 (2.3) | 0 (0) | 1 .000 |
| Previous cardiac operation | ||||
| Prior CoA repair | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Others | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
The results are expressed as the mean ± SD and n (%).
SBP: systolic blood pressure; DBP: diastolic blood pressure; BAV: bicuspid aortic valve; PLSVC: persistent left superior vena cava; VSD: ventricular septal defect; ASD: atrial septal defect; PFO: patent foramen ovale; PDA: patent ductus arteriosus; CoA: coarctation of the aorta.
Indicates statistical significance.
| Characteristic . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 32.0 ± 13.8 | 32.3 ± 14.9 | 31.5 ± 10 .8 | 0 .846 |
| Range | 12 –65 | 12 –65 | 14 –52 | |
| Male | 39 (65.0) | 25 (58.1) | 14 (82.4) | 0 .076 |
| Preoperative SBP (mmHg) | 162.1 ± 28.3 | 160.3 ± 24.4 | 166.6 ± 36.0 | 0 .529 |
| Preoperative DBP (mmHg) | 85.0 ± 15.2 | 88.3 ± 13.4 | 76.7 ± 16.1 | 0 .007* |
| Comorbidities | ||||
| Hypertension | 53 (88.3) | 40 (93.0) | 13 (76.5) | 0 .092 |
| Stroke | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Coronary artery disease | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Infectious endocarditis | 2 (3.3) | 0 (0) | 2 (11.8) | 0 .077 |
| Aortic valve disease | 15 (25.0) | 0 (0) | 15 (88.2) | <0 .001* |
| Mitral valve disease | 4 (6.7) | 0 (0) | 4 (23.5) | 0 .005* |
| Aortic dissection | 1 (1.9) | 0 (0) | 1 (5.9) | 0 .283 |
| Associated anomalies | ||||
| BAV | 23 (38.3) | 12 (27.9) | 11 (64.7) | 0 .008* |
| Dilated thoracic aorta | 19 (31.7) | 9 (20 .9) | 10 (58.8) | 0 .004* |
| Aortic size, mm | 47.5 ± 9.1 | 41.8 ± 3.1 | 52.7 ± 9.6 | 0 .008* |
| PLSVC | 6 (10 .0) | 4 (9.3) | 2 (11.8) | 1 .000 |
| VSD | 4 (6.7) | 1 (2.3) | 3 (17.6) | 0 .065 |
| ASD/PFO | 3 (5.0) | 1 (2.3) | 2 (11.8) | 0 .191 |
| PDA | 2 (3.7) | 2 (4.7) | 0 (0) | 1 .000 |
| Turner syndrome | 1 (1.9) | 1 (2.3) | 0 (0) | 1 .000 |
| Previous cardiac operation | ||||
| Prior CoA repair | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Others | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Characteristic . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 32.0 ± 13.8 | 32.3 ± 14.9 | 31.5 ± 10 .8 | 0 .846 |
| Range | 12 –65 | 12 –65 | 14 –52 | |
| Male | 39 (65.0) | 25 (58.1) | 14 (82.4) | 0 .076 |
| Preoperative SBP (mmHg) | 162.1 ± 28.3 | 160.3 ± 24.4 | 166.6 ± 36.0 | 0 .529 |
| Preoperative DBP (mmHg) | 85.0 ± 15.2 | 88.3 ± 13.4 | 76.7 ± 16.1 | 0 .007* |
| Comorbidities | ||||
| Hypertension | 53 (88.3) | 40 (93.0) | 13 (76.5) | 0 .092 |
| Stroke | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Coronary artery disease | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
| Infectious endocarditis | 2 (3.3) | 0 (0) | 2 (11.8) | 0 .077 |
| Aortic valve disease | 15 (25.0) | 0 (0) | 15 (88.2) | <0 .001* |
| Mitral valve disease | 4 (6.7) | 0 (0) | 4 (23.5) | 0 .005* |
| Aortic dissection | 1 (1.9) | 0 (0) | 1 (5.9) | 0 .283 |
| Associated anomalies | ||||
| BAV | 23 (38.3) | 12 (27.9) | 11 (64.7) | 0 .008* |
| Dilated thoracic aorta | 19 (31.7) | 9 (20 .9) | 10 (58.8) | 0 .004* |
| Aortic size, mm | 47.5 ± 9.1 | 41.8 ± 3.1 | 52.7 ± 9.6 | 0 .008* |
| PLSVC | 6 (10 .0) | 4 (9.3) | 2 (11.8) | 1 .000 |
| VSD | 4 (6.7) | 1 (2.3) | 3 (17.6) | 0 .065 |
| ASD/PFO | 3 (5.0) | 1 (2.3) | 2 (11.8) | 0 .191 |
| PDA | 2 (3.7) | 2 (4.7) | 0 (0) | 1 .000 |
| Turner syndrome | 1 (1.9) | 1 (2.3) | 0 (0) | 1 .000 |
| Previous cardiac operation | ||||
| Prior CoA repair | 3 (5.0) | 2 (4.7) | 1 (5.9) | 1 .000 |
| Others | 2 (3.3) | 2 (4.7) | 0 (0) | 1 .000 |
The results are expressed as the mean ± SD and n (%).
SBP: systolic blood pressure; DBP: diastolic blood pressure; BAV: bicuspid aortic valve; PLSVC: persistent left superior vena cava; VSD: ventricular septal defect; ASD: atrial septal defect; PFO: patent foramen ovale; PDA: patent ductus arteriosus; CoA: coarctation of the aorta.
Indicates statistical significance.
Surgical technique
A variety of surgical techniques are available to manage CoA and can be classified as either in situ management or extra-anatomical bypass. In our department, the choices of techniques were determined by the length of the coarctation segment, extent of collaterals and surgeon’s preference. There were several general rules that we applied: in situ techniques were often used in patients with relatively fewer collateral arteries, end-to-end anastomosis was primarily used in patients with a very short coarctation segment (< 1 cm), and for CoA concomitant with other major cardiac lesions, repair techniques were largely dependent on concomitant procedures and were addressed on a case-by-case basis. Table 2 summarizes our repair techniques for CoA. Patch angioplasty (13/43, 30%), left subclavian artery to descending aorta bypass (11/43, 26%) and proximal-to-distal coarctation bypass (7/43, 16%) accounted for >70% of the isolated CoA cases. Most patients in the concomitant cardiac procedure group (15/17, 88%) were treated with extra-anatomical bypass; among these patients, ascending-to-descending aortic bypass was the most preferred treatment option (9/17, 53%).
| CoA repair techniques . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . |
|---|---|---|---|
| In situ | |||
| Resection and end-to-end anastomosis | 2 | 2 | 0 |
| Resection and interposition graft | 7 | 7 | 0 |
| Patch angioplasty | 15 | 13 | 2 |
| Extra-anatomical bypass | |||
| Ascending-to-descending aorta | 12 | 3 | 9 |
| Left subclavian artery to descending aorta | 13 | 11 | 2a |
| Bypass tube graft | 11 | 7 | 4 |
| CoA repair techniques . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . |
|---|---|---|---|
| In situ | |||
| Resection and end-to-end anastomosis | 2 | 2 | 0 |
| Resection and interposition graft | 7 | 7 | 0 |
| Patch angioplasty | 15 | 13 | 2 |
| Extra-anatomical bypass | |||
| Ascending-to-descending aorta | 12 | 3 | 9 |
| Left subclavian artery to descending aorta | 13 | 11 | 2a |
| Bypass tube graft | 11 | 7 | 4 |
CoA: coarctation of the aorta.
One patient underwent concomitant aortic arch replacement within the left posterior approach in one stage, and another patient underwent a second-stage aortic valve replacement through median sternotomy following CoA repair.
| CoA repair techniques . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . |
|---|---|---|---|
| In situ | |||
| Resection and end-to-end anastomosis | 2 | 2 | 0 |
| Resection and interposition graft | 7 | 7 | 0 |
| Patch angioplasty | 15 | 13 | 2 |
| Extra-anatomical bypass | |||
| Ascending-to-descending aorta | 12 | 3 | 9 |
| Left subclavian artery to descending aorta | 13 | 11 | 2a |
| Bypass tube graft | 11 | 7 | 4 |
| CoA repair techniques . | Total series (n = 60) . | Isolated group (n = 43) . | Concomitant group (n = 17) . |
|---|---|---|---|
| In situ | |||
| Resection and end-to-end anastomosis | 2 | 2 | 0 |
| Resection and interposition graft | 7 | 7 | 0 |
| Patch angioplasty | 15 | 13 | 2 |
| Extra-anatomical bypass | |||
| Ascending-to-descending aorta | 12 | 3 | 9 |
| Left subclavian artery to descending aorta | 13 | 11 | 2a |
| Bypass tube graft | 11 | 7 | 4 |
CoA: coarctation of the aorta.
One patient underwent concomitant aortic arch replacement within the left posterior approach in one stage, and another patient underwent a second-stage aortic valve replacement through median sternotomy following CoA repair.
Each patient in the concomitant cardiac procedure group underwent one to three concomitant procedures. The concomitant procedures are detailed in Table 3. Aortic valve replacement (AVR, n = 8) and a Bentall/David procedure (n = 7) were two frequently combined procedures. Descending aorta replacement was performed to treat a patient with type B aortic dissection secondary to coarctation-related aortic dilation. Cardiopulmonary bypass (CPB) was used in all 17 patients. Most concomitant group patients (14/17, 82%) underwent a single-stage operation to correct multiple abnormalities. Another three underwent first-stage CoA repair and a subsequent second-stage operation after 2–5 months. The decision of using single- or two-stage operations was dependent on the patients’ clinical status and the surgeon’s preference.
| Concomitant procedures (patient number = 17)a . | |
|---|---|
| AVR | 8 |
| MVR/MVr | 4 |
| Bentall/David procedure | 7 |
| Descending aorta replacement | 1 |
| VSD repair | 2 |
| ASD/PFO repair | 2 |
| DCRV correction | 1 |
| Concomitant procedures (patient number = 17)a . | |
|---|---|
| AVR | 8 |
| MVR/MVr | 4 |
| Bentall/David procedure | 7 |
| Descending aorta replacement | 1 |
| VSD repair | 2 |
| ASD/PFO repair | 2 |
| DCRV correction | 1 |
Each patient underwent one to three concomitant procedures.
AVR: aortic valve replacement; MVR: mitral valve replacement; MVr: mitral valve repair; VSD: ventricular septal defect; ASD: atrial septal defect; PFO: patent foramen ovale; DCRV: double-chambered right ventricle.
| Concomitant procedures (patient number = 17)a . | |
|---|---|
| AVR | 8 |
| MVR/MVr | 4 |
| Bentall/David procedure | 7 |
| Descending aorta replacement | 1 |
| VSD repair | 2 |
| ASD/PFO repair | 2 |
| DCRV correction | 1 |
| Concomitant procedures (patient number = 17)a . | |
|---|---|
| AVR | 8 |
| MVR/MVr | 4 |
| Bentall/David procedure | 7 |
| Descending aorta replacement | 1 |
| VSD repair | 2 |
| ASD/PFO repair | 2 |
| DCRV correction | 1 |
Each patient underwent one to three concomitant procedures.
AVR: aortic valve replacement; MVR: mitral valve replacement; MVr: mitral valve repair; VSD: ventricular septal defect; ASD: atrial septal defect; PFO: patent foramen ovale; DCRV: double-chambered right ventricle.
Regarding the operative approach, isolated CoA repair was performed through a left posterolateral incision in the fourth or fifth intercostal space. In contrast, all the patients in the concomitant group who underwent a one-stage operation experienced a standard median sternotomy. Bicaval and ascending aorta cannulations were routinely performed for the ascending-to-descending aortic bypass procedure. The posterior pericardium was opened to expose the descending aorta, and then the distal anastomosis was made on the descending aorta with a partial clamp. The proximal anastomosis was normally completed before releasing the cross-clamp [7].
Follow-up
Follow-up was conducted by a combination of outpatient office visits and phone calls. Patients received a postoperative transthoracic echocardiogram and/or computed tomography scan within the first 3 months and annually thereafter. Because a large percentage of our patients lived in other cities far away from our centre, they were usually followed up by local medical centres, and their information was collected via regular telephone follow-up.
Statistical analysis
Normally distributed data are expressed as the mean ± SD, and non-normally distributed data are expressed as medians. Statistical comparisons of the means were conducted with Student’s t-test. The Wilcoxon rank sum test was used when two sample variances differed. Either Pearson’s chi-squared test or Fisher’s exact test (when the count in any cell of a contingency table was less than required) was used on the categorical variables. The Kaplan–Meier method was used to estimate the survival curve. All tests were two-tailed, and a P-value <0.05 was considered statistically significant. SPSS for Windows 19.0 (Chicago, IL) was used for the statistical analysis.
Results
Early outcomes
Postoperative outcomes are summarized in Table 4. No in-hospital deaths occurred in the isolated CoA group, but three patients in the concomitant cardiac procedure group died perioperatively. One patient, who received a previous correction of CoA 19 years ago, had a re-coarctation and a thoracic aortic aneurysm. He received a single-stage ascending-to-descending aortic bypass concomitant with the Bentall procedure and died of severe bleeding and secondary renal failure on postoperative day 11. Another patient had rheumatic heart disease with severe mitral valve stenosis and regurgitation, moderate to severe aortic stenosis and insufficiency, and a double-chambered right ventricle. She underwent double valve replacement, correction of the double-chambered right ventricle and ascending-to-descending aortic bypass. Although the complex surgery was performed smoothly, she suffered from severe hypoxaemia leading to prolonged mechanical ventilation support. Despite a tracheotomy and an aggressive pulmonary recovery treatment, she died of respiratory failure and sepsis on postoperative day 50. The third patient was diagnosed with mitral valve prolapse, severe mitral valve regurgitation and CoA. She underwent mitral valve replacement and ascending-to-descending aortic bypass but suffered from sudden hypoxaemia in the intensive care unit (ICU) and rapidly died on postoperative day 5.
| Characteristic . | Total series (n=60) . | Isolated group (n=43) . | Concomitant group (n=17) . | P-value . |
|---|---|---|---|---|
| In-hospital mortality | 3 (5.0) | 0 (0) | 3 (17.6) | 0.029* |
| Late death | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Postoperative ICU stay, days, median | 1 | 1 | 2 | <0.001* |
| Range | 1–50 | 1–22 | 1–50 | |
| Postoperative hospital stay, days, median | 6 | 6 | 9 | <0.001* |
| Range | 4–40 | 4–40 | 5–30 | |
| Complications | ||||
| Stroke | 0 (0) | 0 (0) | 0 (0) | – |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | – |
| Renal failure | 2 (3.3) | 0 (0) | 2 (11.8) | 0.077 |
| Respiratory failure | 3 (5.0) | 0 (0) | 3 (17.6) | 0.020* |
| Paraplegia | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Recurrent laryngeal nerve injury | 0 (0) | 0 (0) | 0 (0) | – |
| Bleeding requiring re-exploration | 1 (1.7) | 0 (0) | 1 (5.9) | 0.283 |
| Chylothorax | 2 (3.3) | 1 (2.3) | 1 (5.9) | 0.490 |
| Reoperation | ||||
| AVR | 2 (3.3) | 2 (4.7) | 0 (0) | 1.000 |
| Long-term hypertension control | Detailed in the results section | |||
| Characteristic . | Total series (n=60) . | Isolated group (n=43) . | Concomitant group (n=17) . | P-value . |
|---|---|---|---|---|
| In-hospital mortality | 3 (5.0) | 0 (0) | 3 (17.6) | 0.029* |
| Late death | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Postoperative ICU stay, days, median | 1 | 1 | 2 | <0.001* |
| Range | 1–50 | 1–22 | 1–50 | |
| Postoperative hospital stay, days, median | 6 | 6 | 9 | <0.001* |
| Range | 4–40 | 4–40 | 5–30 | |
| Complications | ||||
| Stroke | 0 (0) | 0 (0) | 0 (0) | – |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | – |
| Renal failure | 2 (3.3) | 0 (0) | 2 (11.8) | 0.077 |
| Respiratory failure | 3 (5.0) | 0 (0) | 3 (17.6) | 0.020* |
| Paraplegia | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Recurrent laryngeal nerve injury | 0 (0) | 0 (0) | 0 (0) | – |
| Bleeding requiring re-exploration | 1 (1.7) | 0 (0) | 1 (5.9) | 0.283 |
| Chylothorax | 2 (3.3) | 1 (2.3) | 1 (5.9) | 0.490 |
| Reoperation | ||||
| AVR | 2 (3.3) | 2 (4.7) | 0 (0) | 1.000 |
| Long-term hypertension control | Detailed in the results section | |||
Results expressed as n (%).
ICU: intensive care unit; AVR: aortic valve replacement.
Indicates statistical significance.
| Characteristic . | Total series (n=60) . | Isolated group (n=43) . | Concomitant group (n=17) . | P-value . |
|---|---|---|---|---|
| In-hospital mortality | 3 (5.0) | 0 (0) | 3 (17.6) | 0.029* |
| Late death | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Postoperative ICU stay, days, median | 1 | 1 | 2 | <0.001* |
| Range | 1–50 | 1–22 | 1–50 | |
| Postoperative hospital stay, days, median | 6 | 6 | 9 | <0.001* |
| Range | 4–40 | 4–40 | 5–30 | |
| Complications | ||||
| Stroke | 0 (0) | 0 (0) | 0 (0) | – |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | – |
| Renal failure | 2 (3.3) | 0 (0) | 2 (11.8) | 0.077 |
| Respiratory failure | 3 (5.0) | 0 (0) | 3 (17.6) | 0.020* |
| Paraplegia | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Recurrent laryngeal nerve injury | 0 (0) | 0 (0) | 0 (0) | – |
| Bleeding requiring re-exploration | 1 (1.7) | 0 (0) | 1 (5.9) | 0.283 |
| Chylothorax | 2 (3.3) | 1 (2.3) | 1 (5.9) | 0.490 |
| Reoperation | ||||
| AVR | 2 (3.3) | 2 (4.7) | 0 (0) | 1.000 |
| Long-term hypertension control | Detailed in the results section | |||
| Characteristic . | Total series (n=60) . | Isolated group (n=43) . | Concomitant group (n=17) . | P-value . |
|---|---|---|---|---|
| In-hospital mortality | 3 (5.0) | 0 (0) | 3 (17.6) | 0.029* |
| Late death | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Postoperative ICU stay, days, median | 1 | 1 | 2 | <0.001* |
| Range | 1–50 | 1–22 | 1–50 | |
| Postoperative hospital stay, days, median | 6 | 6 | 9 | <0.001* |
| Range | 4–40 | 4–40 | 5–30 | |
| Complications | ||||
| Stroke | 0 (0) | 0 (0) | 0 (0) | – |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | – |
| Renal failure | 2 (3.3) | 0 (0) | 2 (11.8) | 0.077 |
| Respiratory failure | 3 (5.0) | 0 (0) | 3 (17.6) | 0.020* |
| Paraplegia | 1 (1.7) | 1 (2.3) | 0 (0) | 1.000 |
| Recurrent laryngeal nerve injury | 0 (0) | 0 (0) | 0 (0) | – |
| Bleeding requiring re-exploration | 1 (1.7) | 0 (0) | 1 (5.9) | 0.283 |
| Chylothorax | 2 (3.3) | 1 (2.3) | 1 (5.9) | 0.490 |
| Reoperation | ||||
| AVR | 2 (3.3) | 2 (4.7) | 0 (0) | 1.000 |
| Long-term hypertension control | Detailed in the results section | |||
Results expressed as n (%).
ICU: intensive care unit; AVR: aortic valve replacement.
Indicates statistical significance.
Both the ICU stay time and postoperative hospital stay time in the concomitant cardiac procedure group were significantly longer than those of the isolated CoA group (ICU stay: median, 2 vs 1 day, P < 0.001; postoperative hospital stay: median, 9 vs 6 days, P < 0.001). Postoperative complications occurred more frequently in the concomitant cardiac procedure group than the isolated CoA group. Two patients in the concomitant group suffered from renal failure: one case described earlier was because of hypovolaemia, and another patient was treated successfully with bedside haemodialysis. Respiratory failure was found in three patients in the concomitant cardiac procedure group. All of these patients underwent tracheotomy, and one patient ultimately had a successful recovery. One isolated CoA repair patient who underwent a redo-left subclavian artery to descending aorta bypass developed paraplegia postoperatively.
Late outcomes
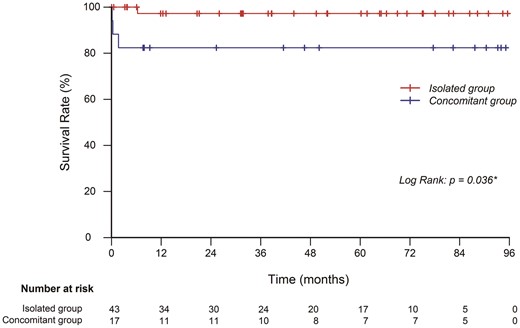
There were 45 patients in this cohort who had a follow-up of longer than 12 months (mean, 59.0 months). At the last follow-up for these patients, normotensive status was achieved in 32 patients (71%) without medication. The remaining 13 patients required one to three antihypertensive drugs to stabilize their blood pressure. There was no significant difference in postoperative hypertension control among patients who underwent different CoA repair techniques.
Two patients with isolated CoA repair required additional intervention due to valve disease during follow-up. Both patients underwent proximal-to-distal coarctation bypass at their first operation combined with bicuspid aortic valve (BAV). One 53-year-old man underwent AVR because of severe aortic regurgitation 3 years after the correction of CoA. The second patient, a 25-year-old man, suffered from infection endocarditis 7 years after CoA repair and underwent AVR after the acute infection was successfully controlled. Both patients recovered uneventfully and were alive at the last follow-up (February 2016).
Discussion
Coarctation in adolescent or adult patients is often complicated by an aortic aneurysm or dissection, heart valve diseases and other cardiovascular comorbidities. Concomitant cardiac or aortic problems may carry additional operative difficulties and increased surgical risk. Charlton-Ouw et al. [5] at the University of Texas at Houston reported a series of 29 adult patients with CoA, most of whom underwent open repair with a resection and interposition graft technique. The medical group achieved no in-hospital deaths, and three patients died during follow-up (10.3%). Roselli et al. [6] from the Cleveland Clinic published a large series of 110 adolescent and adult patients with CoA who were managed by open, endovascular or hybrid treatment. No in-hospital deaths were reported among the 40 patients who underwent open repair. Said et al. [7] from the Mayo Clinic shared their experience implementing the ascending-to-descending aortic bypass technique since 1985. Among 80 consecutive patients, there were no early deaths and 5 late deaths (6.25%).
In our series, the overall mortality rate was 6.67% (4/60), which is comparable to other studies. Of note, all three patients who died perioperatively were in the concomitant group, resulting a relatively high in-hospital mortality (0% vs 17.6%, P = 0.029) but acceptable overall survival (82.4% at 5 years). These results suggest that surgical management of adolescents and adults with coarctation could achieve similar satisfactory outcomes as their neonatal counterparts; however, the complexity of the concomitant procedures could be a risk factor for this particular patient population. Clinically, we believe that the causes of death in these three patients were not directly associated with the surgical correction of CoA. The complexity of the concomitant procedure and the prolonged CPB and aortic clamping time may be the causes of postoperative complications as well as subsequent death.
Aortic valve disease and aortic aneurysm or dissection were the two most frequent cardiovascular comorbidities in our cohort and were associated with a high incidence of BAV (23/60, 38%). Limited surgical options are available for those patients in whom both CoA and other cardiac pathologies need correction. Our primary approach was an ascending-to-descending aortic bypass with concomitant aortic valve procedure for single-stage management of these patients. As mentioned earlier, Said et al. [7] have described this technique in detail and achieved positive outcomes at the Mayo Clinic. Arakelyan et al. [8] from Russia also reported a 52 patient cohort. These clinicians applied the bypass technique using a right thoracotomy approach (other centres commonly use a left thoracotomy approach) and also achieved satisfactory outcomes. In our cohort, we found that ascending-to-descending aortic bypass is a safe solution to surgically treat complex CoA; however, this solution carries a relatively high incidence of postoperative respiratory complications. Three concomitant group patients, all of whom underwent ascending-to-descending aortic bypass, suffered from respiratory failure postoperatively, which resulted a significantly high respiratory failure rate in the concomitant group (0% vs 17.6%, P = 0.02). It is difficult to claim that this technique is a direct cause of respiratory failure as these three patients also underwent other major cardiac procedures that may have influenced lung function postoperatively. However, a similar surgical technique, aortic valve bypass (i.e. left ventricle apex to descending aorta bypass), was reported to have a high incidence of postoperative pulmonary complications such as prolonged ventilation, acute respiratory distress syndrome and pneumonia [9]. This finding suggests that the placement of bypass tube, whether between ascending and descending aorta or between the cardiac apex and descending aorta, may affect respiratory function and cause complications. Future studies are needed to identify the exact mechanisms involved.
Three patients with complex CoA underwent two-stage operations in our cohort compared to the remaining 14 patients who received a single-stage treatment. The second-stage procedures in these three patients were AVR, AVR + ventricular septal defect (VSD) repair and Bentall + mitral valve repair. All three had uneventful recoveries from both operations and were still alive at their last follow-up. Due to the small patient number in the two-stage operation group, it is difficult for us to compare the outcomes between single-stage and two-stage operation at this point. Walters et al. [10] compared single-stage with two-stage repair of patients with CoA and VSD and demonstrated that the postoperative complications and hospital mortality were similar between the two groups.
One patient in our cohort who received redo-left subclavian artery to descending aorta bypass developed paraplegia later during recovery. We believe that this occurrence might be associated with a thromboembolic event due to unsatisfactory anticoagulation. Paraplegia is a rare but devastating complication after CoA repair. The protection of the collateral arteries and dedicated monitoring of postoperative coagulation are of great importance.
Although it has been demonstrated that surgical correction can constantly reduce established hypertension in patients with CoA, persisting hypertension is still a common complication among adolescent and adult patients, as age at the time of surgery is an important risk factor [11, 12]. In the current study, >70% of patients who had a follow-up longer than 12 months achieved normotensive pressure without medication, suggesting optimal outcomes after surgery even for adult patients. Regarding the impact of different surgical repair techniques, a bypass procedure may not be as efficient as other techniques (e.g. interpositional grafting or patch repair) in relieving gradients. However, it is still a feasible technique if performing a concomitant intracardiac procedure. We hypothesize that because of long-term obstruction, the arteriole system of adult patients with CoA may have pathological changes leading to pertinacious hypertension, which is independent of the CoA repair techniques. This finding is consistent with the findings of our study, which suggested that the incidence of postoperative hypertension (i.e. patients who required antihypertensive drugs) were similar among the different groups of repair techniques.
Currently, endovascular therapy—either stent implantation or balloon angioplasty—is widely accepted as an alternative to surgery and is even considered a first-line treatment for adolescent and adult patients with CoA. However, in developing countries such as China, vascular stent- and catheter-related hybrid treatments are often not available in the national healthcare insurance systems and are therefore beyond the reach of most patients with CoA. These patients are often offered open repair instead. In addition, accumulating evidence has shown that balloon angioplasty is associated with a high rate of recurrent construction and late aneurysm formation [13, 14]. For stent placement, the COAST trial demonstrated that stenting can achieve favourable outcomes, but ∼13% of enrolled patients have undergone reintervention before publication of their intermediate outcomes [15, 16]. Therefore, surgical repair is still an important and not yet antiquated treatment option for older patients with CoA.
ACKNOWLEDGEMENTS
The authors would like to thank Difan Zheng (Fudan University Shanghai Cancer Centre) for his assistance in statistical analysis and Rui Zhang (Fudan University Huashan Hospital) for her critical reading of the article.
Funding
This work was supported by the Program of Shanghai Subject Chief Scientist [14XD1401000 to C.W.] and the Fudan University Zhongshan Hospital Youth Research Fund [2016ZSQN27 to Z.Z. and 2016ZSQN24 to Y.L.].
Conflict of interest: none declared.
REFERENCES
Author notes
These authors contributed equally to this work.
- aorta
- aortic coarctation
- aortic valve replacement
- adolescent
- adult
- coenzyme a
- follow-up
- objective (goal)
- hospital mortality
- newborn
- intensive care unit
- surgical procedures, operative
- survival rate
- heart
- bypass
- postoperative respiratory distress
- surgical outcome
- ascending aortic graft with valve suspension and coronary reconstruction with valve sparing aortic annulus remodeling




